Abstract
Study Objectives:
Depression is a risk factor for medication non-compliance. We aimed to identify if depression is associated with poorer adherence during home-based autotitrating continuous positive airway pressure (autoPAP) titration.
Design:
Mixed retrospective-observational study.
Setting:
Academic center.
Participants:
Two-hundred forty continuous positive airway pressure-naïve obstructive sleep apnea (OSA) patients.
Measurements:
Patients underwent approximately 1 week of home-based autoPAP titration with adherence data downloaded from the device. Electronic hospital records were reviewed in a consecutive manner for inclusion. Three areas of potential predictors were examined: (i) demographics and clinical factors, (ii) disease severity, and (iii) device-related variables. Depression and anxiety were assessed using the Hospital Anxiety and Depression Scale (HADS). Scores on the subscales were categorized as normal or clinical diagnoses of depression (≥ 8) and anxiety (≥ 11). The primary outcome variable was the mean hours of autoPAP used per night.
Results:
Patients were diagnosed with OSA by either attended polysomnography (n = 73, AHI 25.5[15.1-41.5]) or unattended home oximetry (n = 167, ODI3 34.0[22.4-57.4]) and had home-based autoPAP titration over 6.2 ± 1.2 nights. Mean autoPAP use was 4.5 ± 2.4 hours per night. Multiple linear regression analysis revealed that depression and lower 95th percentile pressures significantly predicted lesser hours of autoPAP use (R2 = 0.19, p < 0.001). Significantly milder OSA in those requiring lower pressures may have confounded the relationship between 95th percentile pressure and autoPAP use.
Conclusion:
Depression was independently associated with poorer adherence during home-based autoPAP titration. Depression may be a potential target for clinicians and future research aimed at enhancing adherence to autoPAP therapy.
Citation:
Law M; Naughton M; Ho S; Roebuck T; Dabscheck E. Depression may reduce adherence during CPAP titration trial. J Clin Sleep Med 2014;10(2):163-169.
Keywords: Obstructive sleep apnea, continuous positive airway pressure, adherence, depression
Obstructive sleep apnea (OSA) is a common condition, characterised by repetitive collapse of the upper airway resulting in snoring, episodic oxygen desaturations, sleep arousal, and excessive daytime sleepiness.1 Patients with untreated OSA are at an increased risk for hypertension, motor vehicle accidents, and possibly coronary artery disease and stroke.2–5 Treatment of OSA by continuous positive airway pressure therapy (CPAP) has been shown to at least partially reduce these risks.3,6 However, poor adherence to CPAP therapy remains the greatest obstacle to effective treatment of OSA, with few well-established predictors for CPAP adherence.7
Depression may play an important role in non-adherence, with a recent meta-analysis finding that depression is associated with poor compliance to medications across a range of chronic conditions.8 The literature to date has been inconclusive, with significant study heterogeneity and small sample sizes limiting interpretation of results and comparison across studies. One postal survey of 178 established CPAP users found an association between depression scores and non-compliance to therapy; however, relied on non-objective self-reported CPAP use.9 Smaller studies have reported no association between depressive symptoms and long-term CPAP adherence.10–14
To our knowledge, a study into the effect of depression on adherence to the first trial of therapy during home-based autotitrating CPAP (autoPAP) therapy has not been reported. This method of CPAP titration is well established as an acceptable alternative to laboratory-based manual titration, producing comparable patient outcomes.15,16 This has seen the rise in popularity of home-based autoPAP titration, given the long wait times at sleep institutions and rising burden of disease. Mean wait times from referral to laboratory-based CPAP implementation can be protracted, ranging from 7 to 8 months in Australia, to 14 months in the United Kingdom, to 24 months in Canada.17 Despite this, adherence to autoPAP therapy has not been extensively studied.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Poor adherence to CPAP therapy remains the greatest obstacle to effective treatment of OSA. Depression is a known risk factor for medication non-compliance. The literature to date surrounding depression and CPAP adherence has been inconclusive.
Study Impact: This study found a significant independent association between depression and poor autoPAP adherence and is the first study to report such a finding using an objective measure of CPAP machine usage. Depression may be a potential target for clinicians and future research aimed at enhancing adherence to autoPAP therapy.
Early adherence during the one week of home-based autoPAP titration has been shown to closely predict future adherence to fixed-pressure CPAP therapy.18 Likewise, studies have shown that long-term adherence may be identified as early as the third and fourth day of treatment.19,20 Therefore we aimed to identify if depression is independently associated with adherence during home-based autoPAP titration, clearly a vulnerable period for non-adherence. The recognition of such an association could identify a reversible determinant of non-adherence to autoPAP therapy and may direct future studies aiming to enhance adherence to this therapy.
METHODS
This was a retrospective-observational study at the Alfred Sleep Disorders Service. Electronic hospital records were reviewed in a consecutive manner. All CPAP-naïve patients with a new diagnosis of OSA referred for home-based CPAP titration using an autoPAP device were eligible to be included in this analysis. Patients with another comorbid sleep disordered breathing diagnosis were excluded from this analysis (e.g., central sleep apnea with Cheyne-Stokes respiration or obesity hypoventilation). This study was approved by the institution's human research ethics committee.
Subjects
Over 22 months, 321 patients at the Alfred Sleep Disorders and Ventilatory Failure Service, underwent home-based CPAP titration using an autoPAP device. As per this center's regular clinical practice, these patients were offered approximately a one-week trial on autoPAP to establish optimal fixed pressures for long-term treatment and examine the patient's subjective benefit from therapy. Two hundred fifty-nine patients were eligible for inclusion. AutoPAP machine downloads were missing from 19 patient medical records; thus data from 240 patients were included in the final analysis.
Diagnosis
All patients were diagnosed with OSA by either attended polysomnography (PSG), or unattended home oximetry study. PSGs were conducted using Compumedics E Series equipment (Melbourne, Australia) with the following signals recorded: electrocardiogram, electroencephalogram, electromyogram, electrooculogram, arterial oxygen saturation using pulse oximetry, nasal airflow, chest wall and abdomen movement, body position, and snoring via microphone, all in accordance with 2007 American Academy of Sleep Medicine (AASM) guidelines.21 Oximetry within the PSG was conducted using the Novametrix 520A pulse oximeter (Philips Respironics, Murrysville, PA) with a 2-s averaging time and 0.125 Hz signal acquisition. The apnea-hypopnea index (AHI) was computed from the number of apneas and hypopneas as defined by the 2007 AASM Alternative criteria.21 Six patients had their care transferred from other centers, and their diagnostic data from external PSGs were included in the analysis. Overnight home oximetry was conducted using the Masimo Rad 5 oximeter (Masimo, Irvine, CA) with a 2-s averaging time and 0.5 Hz signal acquisition. This center previously been involved in a study in which diagnosis and management of OSA by oximetry has been found to be similar to in-hospital PSG.22 Oximetry was reserved for patients with a high pretest probability for OSA and low likelihood of comorbid sleep pathology. More specifically, where Cheyne-Stokes respiration was suspected, a PSG was ordered; where obesity hypoventilation was suspected, an ABG was ordered; if hypercapnia was found, the patient was excluded from this study. A sleep scientist counselled all patients as to the correct use of these machines to ensure valid results. An oxygen desaturation was defined as a decrease > 3% from baseline arterial saturation,23 and the oxygen desaturation index (ODI3; oxygen desaturations per hour of recording time) was computed according to this definition. The results of PSGs and home oximetry studies were scored by sleep scientists and reviewed and reported by sleep physicians. PSGs and home oximetry studies were diagnostic for OSA if the AHI or ODI3 was > 5/hour.21 At our institution, there are no AHI or ODI3 criteria to commence CPAP. Commencement of CPAP was at the discretion of the treating physician.
AutoPAP Titration
All patients underwent home-based CPAP titration using an autoPAP device and were issued a ResMed S8 Autoset Spirit II (ResMed, Sydney, NSW, Australia) for approximately 1 week. As part of routine clinical care, patients received an explanation, training, and mask fitting, including a 1-h trial on autoPAP to become accustomed to the machine. Explanation, training, and mask fitting was performed by the same sleep nurse with expertise in this area. Optional humidifier and nasal or full-face mask were issued according to preference and comfort. Patients were instructed to use autoPAP every night for as long as possible. At the end of the titration period, machine computerized data was downloaded and reviewed by sleep physicians.
Data Variables
Potential determinants of autoPAP adherence were examined across 3 areas: (i) baseline demographics and clinical factors—age, sex, body mass index (BMI), subjective sleepiness as measured by the Epworth Sleepiness Scale (ESS), depression and anxiety as defined by the Hospital Anxiety and Depression Scale (HADS) score, comorbidities (hypertension, hypercholesterolemia, ischemic heart disease, congestive cardiac failure, cerebrovascular disease, or diabetes mellitus); (ii) disease severity—AHI (from PSGs) or ODI3 (from home oximetry studies), minimum SpO2 recorded, and percentage of time spent < SpO2 90% (%); (iii) device-related factors (downloaded from autoPAP machines)—95th percentile pressure (cm H2O), median mask leak (L/min of airflow), and residual AHI.
Hospital Anxiety and Depression Scale (HADS)
All patients undergoing home-based autoPAP titration were invited to complete the HADS24 prior to starting therapy. The HADS is a 14-item self-reporting questionnaire used commonly in research and clinical practice as a screening instrument for depression and anxiety. This instrument was designed to rate the severity of depression and anxiety in medically ill patients, assessing only psychological and cognitive symptoms (excluding somatic symptoms potentially attributable to comorbid medical conditions). It contains 7 questions probing for depression symptoms (HADS depression subscale; HADS-D) and anxiety symptoms (HADS anxiety subscale; HADS-A) each. Each question is scored from 0 to 3, with a total score up to 21 for each subscale.
For the purposes of this study, all patients with a HADS-D score ≥ 8 were defined as having clinical depression, and those with a HADS-A score ≥ 11 were defined as having clinical anxiety. These cutoff points were established in a preliminary study at the same center to give maximal sensitivity and specificity for identifying major depression according to the Mini International Neuropsychiatric Interview which uses the gold-standard Diagnostic and Statistical Manual IV (DSM-IV) criteria. A HADS-D score ≥ 8 has a sensitivity of 83.1% and a specificity of 83.3% for a major depressive episode; HADS-A score ≥ 11 has a sensitivity of 93.1% and a specificity of 84.7% for generalized anxiety and/or panic disorder.
Statistical Analysis
Multiple linear regression analysis was performed to build a multivariate predictive model for the outcome variable, mean hours of autoPAP use during home-based CPAP titration. An automatic backward elimination method was used in the final analysis. Data has been reported as mean and standard deviation (mean ± SD) or median and interquartile range (median [IQR]) as appropriate to tests of normality. Statistical significance was defined as p < 0.05 using two-tailed tests. Statistical analysis was performed using IBM SPSS Statistics Version 20.0 (IBM Corporation, Armonk, NY, USA).
Statistical analyses were performed on all available data. Due to the retrospective nature of the study, variables were occasionally unavailable. BMI and ESS score were unavailable for 14 (5.8%) and 23 (10.8%) patients, respectively.
RESULTS
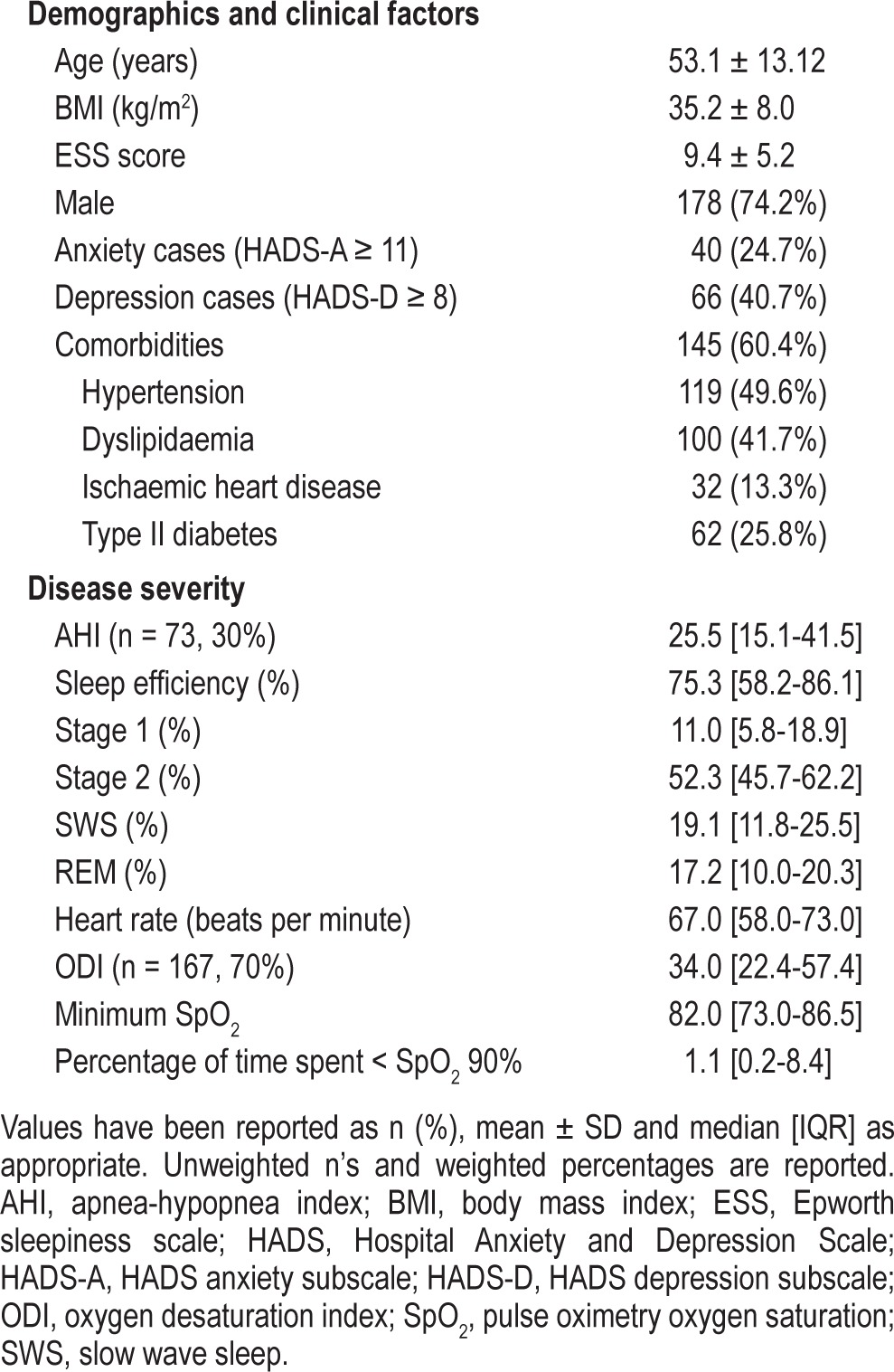
Two-hundred forty patients (age 53.1 ± 13.2 years; 74.2% male) with a BMI of 35.2 ± 8.0 kg/m2 underwent in-home autoPAP titration and were included in this analysis. This was a sample representative of moderate to severe OSA. Seventy percent of patients (n = 167) underwent diagnostic overnight oximetry study with a median ODI3 of 34.0 (22.4-57.4); 31% (n = 73) underwent full polysomnography with a median AHI of 25.5 (15.1-41.5). Therapeutic CPAP pressure based on 95th percentile pressure of autoPAP download was 10.4 ± 2.4 cm H2O. Patients had autoPAP titration for an average of 6.2 ± 1.2 nights, with machine use of 4.5 ± 2.4 h/night during this period. Mask leak (5.4 [2.4-10.8] L/min) and residual AHI (5.6 [3.1-9.8] events/h) across the sample was low.
Mood data were available for 67.5% (n = 162) of the sample, with 5.8% (n = 14) opting out of the mood screen and 26.7% (n = 64) not completing the HADS questionnaire due to a non-English speaking background, poor cognition, or administrative error. There were no significant differences across age, sex, BMI, ESS score, polysomnographic measures of OSA severity, or the use of autoPAP machines between patients who completed the HADS and those who did not complete the HADS. Depression and anxiety appear to be highly prevalent in this population, with 40.7% defined as depression cases and 24.7% defined as anxiety cases. Further descriptive data is available in Table 1.
Table 1.
Baseline characteristics of the sample (n = 240)
Regression Analysis
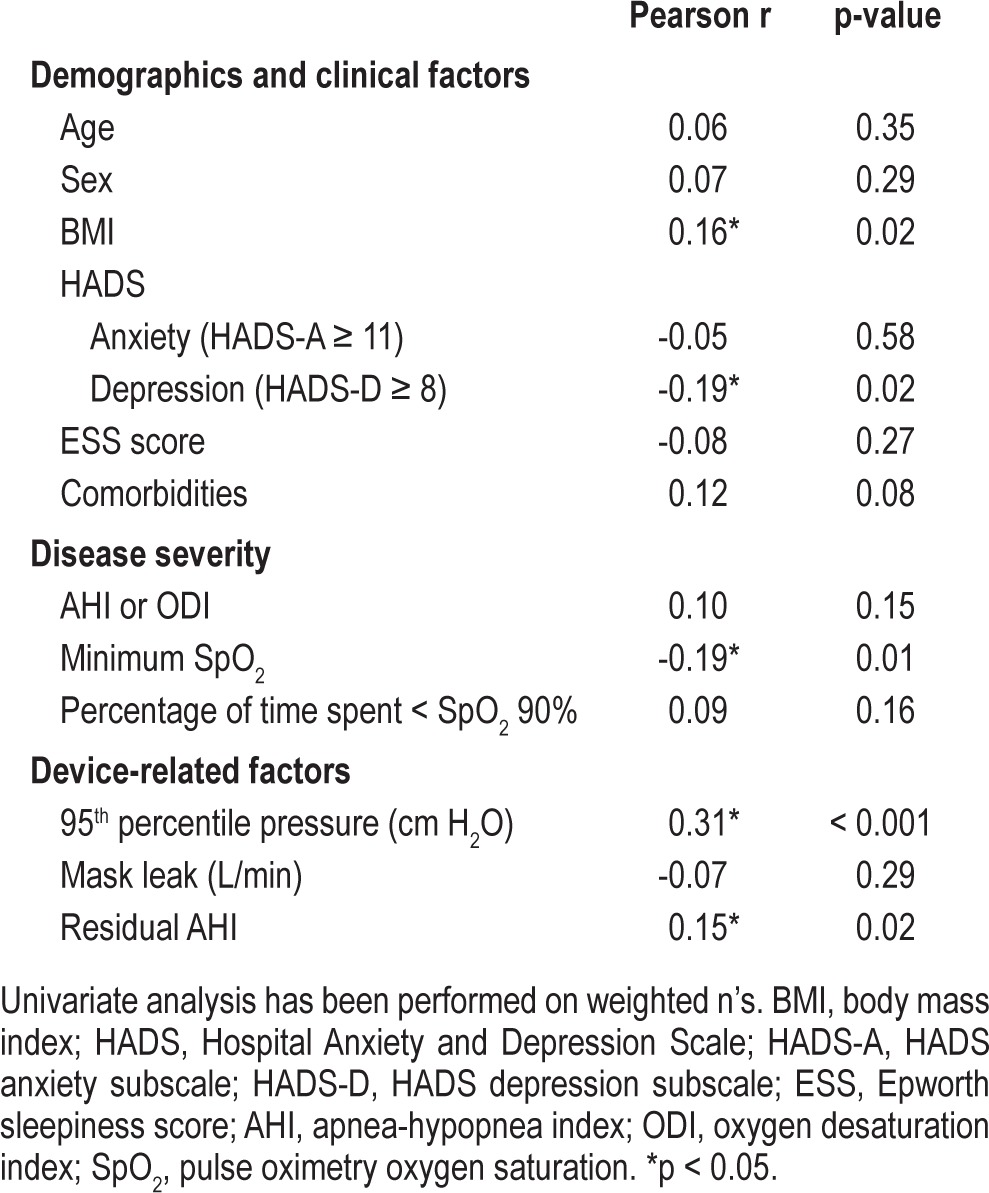
Univariate analyses identified an unadjusted association between BMI, minimum SpO2, depression, 95th percentile pressure, residual AHI, and autoPAP use during home-based titration. These results are presented in Table 2. Covariates with p < 0.2 (BMI, depression, presence of comorbidities, AHI or ODI3, minimum SpO2, percentage of time spent < SpO2 90%, 95th percentile pressure, and residual AHI) were retained for the multivariate analysis. Variables thought to exert influence on adherence such as age, sex, and ESS were forced as covariates into the multivariate analysis irrespective of the univariate analysis results. The final regression model was unchanged, and the original method and model has been reported.
Table 2.
Univariate linear regression analysis between dependent variables and autoPAP use (mean hours of use per night)
An automatic backward elimination method was used to construct a multivariate model. Preliminary analyses were conducted to ensure there were no violations of normality, linearity, multicollinearity, and homoscedasticity. As median pressure and 95th percentile pressure were collinear (r = 0.898, p < 0.0001), multiple regressions were performed with the 95th percentile pressure variable due to greater clinical significance.
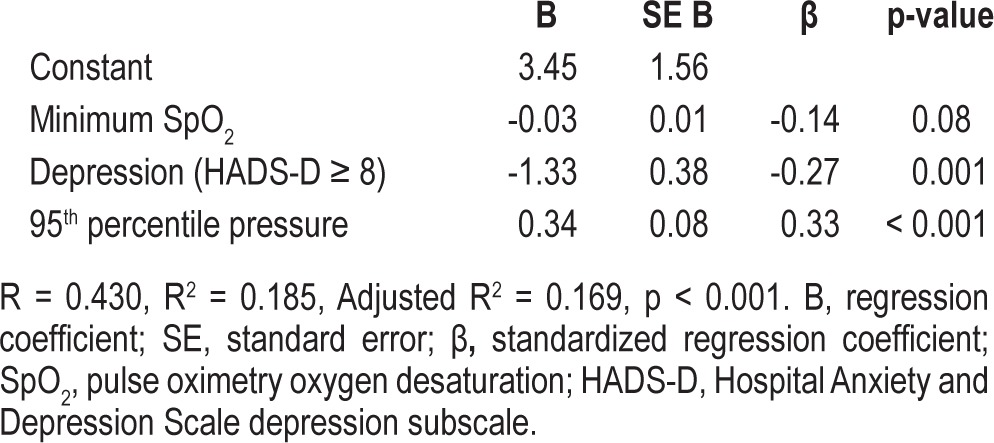
Multiple linear regression analysis constructed a three-variable model explaining 18.5% of total variance in autoPAP use (Table 3; R2 = 0.185, p < 0.01). Depression and lower 95th percentile pressures predicted for lower adherence. Minimum SpO2 did not make a statistically significant contribution to the model. Depression and 95th percentile pressure uniquely explained 7.0% (p = 0.001) and 10.2% (p < 0.001) of autoPAP use, respectively.
Table 3.
Predictors of autoPAP use, multivariate regression model
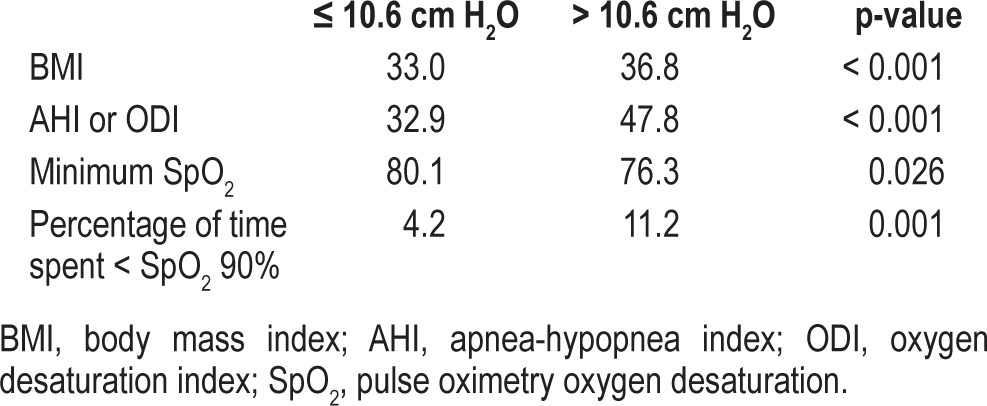
To further investigate the relationship between 95th percentile pressure and autoPAP adherence, a median split of 95th percentile pressure was used to dichotomize the sample into those requiring higher pressures (> 10.6 cm H2O) and those requiring lower pressures (≤ 10.6 cm H2O). Exploratory independent t-tests (Table 4) showed that those requiring higher pressures were more obese, and had more severe OSA (higher AHI during PSG or ODI3 during home oximetry, and longer duration with SpO2 < 90%), which may have confounded this finding.
Table 4.
Exploratory independent t-tests, comparison of those requiring higher (> 10.6 cm H2O) to lower (≤ 10.6 cm H2O) 95th percentile pressures
DISCUSSION
To our knowledge, this is the first study to find an independent association between depression and an objective measure of CPAP use. Our predictive model suggested depression and 95th percentile pressure explain 18.5% of the variance in autoPAP use. That is, depression and lower autoPAP pressures predicted poorer autoPAP adherence. These findings are similar to those reported by Campos-Rodriguez et al. in a large observational study of 708 women, reporting that the use of psycho-active medications and lower CPAP pressures predicted poorer CPAP adherence over 5 and 10 years.25
The literature regarding depression and CPAP adherence is inconclusive. A number of studies in small to moderately sized samples have found no association.11–14 Edinger et al. reported a combination of variables including depressive personality traits to explain 62% of the total variance of CPAP adherence in 28 male veterans.26 One postal survey of established CPAP users found an association between depressive symptoms and CPAP non-compliance.9 However both studies relied on non-objective, self-reported CPAP use, therefore introducing reporting bias.
The discrepancy in these findings may be explained by the significant heterogeneity in the literature, limiting the interpretation and comparison of results. Studies differ in the primary selection of patients and sample size, the depression screening instrument used, and the definition of “adherence.” In particular, different instruments used to identify depression or “depressive symptoms” are a source of misleading variation between studies. For example, studies have used the Beck Depression Inventory, Centre of Epidemiological Studies Depression Scale, personality trait tests and the Hospital Anxiety and Depression Scale (HADS), the last of these generally finding more significant results. This difference is likely due to the different questionnaire constructs, with most sampling symptoms common to OSA and depression, thereby creating false positives. We chose to use the HADS in this study, as it avoids vegetative confounders and is the most suitable for use in OSA patients. More consistency in the choice of measures would allow for clearer interpretation and reliable comparisons across studies.
Several mechanisms may be responsible for the association between depression and poor CPAP adherence. Firstly, untreated depression-related fatigue may be confused with sleepiness from OSA. A number of studies have reported depressive symptoms making greater contributions to fatigue in OSA, than OSA severity.27–30 Fatigue and sleepiness are similar but distinctly different constructs, and the improvement of sleepiness on CPAP therapy may not be as salient if depression-related fatigue is present.29,31 Secondly, depression may sensitize patients to CPAP side effects (mask discomfort, air leak, claustrophobia, nasal congestion), with studies demonstrating that depressed individuals tend to report more symptoms regardless of the physiological severity of the condition.32 Depressed patients may therefore be less tolerant of side effects and abandon therapy early, compared to their non-depressed counterparts. These factors may be most relevant for patients who experience initial negative experiences with therapy, with these patients less likely to be adherent at one week and one month.13,18,33 CPAP adherence and symptom improvement may therefore be diminished by the presence of depression, and these results suggest that the consideration of untreated depression may be pertinent if a patient does not report improvement in sleepiness despite CPAP therapy.
Our model found that poorer autoPAP adherence was associated with lower 95th percentile pressure. Considering the potential for mask discomfort and mask leak at higher pressures, lower CPAP pressures may theoretically be more comfortable and confer better therapy adherence. Studies examining CPAP pressure and adherence have mostly been negative,20,34,35 with only one report of such an association.36 Our findings suggest that the relationship between 95th percentile pressure and autoPAP use may result from covariance with measures of OSA severity. These findings are parallel with the findings of McArdle et al., who found that long-term adherence was greater in patients with higher CPAP pressures, presumably due to greater OSA severity.37 An alternative explanation as to why lower CPAP pressures were associated with poorer autoPAP adherence is that sleep quality was inferior in the patients who required lower pressures. The nature of this study meant that sleep quality could not be assessed.
The relationship between OSA and depression is complex and probably bi-directional. Contrary to our findings, it could be argued that patients with depression and OSA are more likely to adhere to CPAP, as CPAP improves their symptoms of depression. However, Giles et al. in a meta-analysis concluded that the effects of CPAP on depression are inconclusive and require further study.38 Furthermore, pharmacological interventions in depression take several weeks to take effect,39 and adherence in this study was measured after just 7 days.
Given that our predictive model explained only 18.5% of autoPAP use during home-based titration over about week, understanding adherence is evidently a complex behavior with contributions from a number of factors. Other studies examining for multiple predictors of CPAP adherence have also found low explanatory power (4% to 25%).18,36,40–42 Few reliable determinants of CPAP adherence have been established, with polysomnographic severity and higher levels of baseline sleepiness (ESS score) the most consistent baseline correlate of ongoing CPAP use.37,43,44 Studies have mostly used univariate analyses, finding only weak to moderate correlations with these findings likely to vary with sample size, statistical power, and selection bias in clinical samples. Commonly explored anthropometric, symptomatic, or polysomnographic predictors have been found to only explain 4% to 25% of variance in CPAP use. As such, further exploration beyond biomedical predictors has been warranted. A patient's higher health value and greater internal locus of control has been shown to be important in CPAP adherence.12,26,36 Similarly, regular users of CPAP were more likely to have self-referred for assessment, in comparison to a partner-initiated referral.45 Social circumstances may also play a role, with major life events prior to beginning CPAP therapy predicting poorer adherence. In sum, a patient's decision to use CPAP is multifaceted, and our findings add to the growing literature examining cognitive and psychological factors in CPAP adherence.
The present study adds to the current literature addressing CPAP adherence, examining a wide-range of variables in a large multivariate analysis. Predictors of early adherence to CPAP therapy have not been extensively studied. However this first week is a critical time when non-adherence behavior is established. We believe that studying adherence during the first trial of therapy during home-based autoPAP titration provides the clearest picture. In particular, studies examining depression and adherence after initial CPAP titration, or in established CPAP users are open to selection bias, as depressed patients may have self-selected out of these groups by discontinuing CPAP therapy. Therefore, while the cross-sectional nature of our study does not allow us to confer a causal relationship, these findings provide preliminary understanding of the relationship between depression and CPAP adherence.
Limitations
There are limitations to this study. First, OSA was diagnosed with overnight pulse oximetry in 70% of the sample, with 30% undergoing traditional polysomnography. This practice reflects the regular clinical practice of this sleep institution; many healthcare systems are also adopting domiciliary sleep services to reduce wait times and expenses. Both groups were equal across baseline characteristics, with the exception of AHI and ODI3. The overnight oximetry group had a greater number of respiratory events per hour in comparison to those who had polysomnography. This is not unexpected given that patients with a high pre-test probability of moderate to severe OSA are referred for overnight oximetry. While these measures are not equal, they have been found to be comparable when combined with appropriate clinical assessment.22,23,46,47 Both polysomnography and overnight oximetry are acceptable methods of diagnosing OSA when used appropriately, and we believe our results remain clinically relevant. Secondly, we did not have mood data on 26.7% of the sample, due to the patient's non-English speaking background or administrative error. Only 5.8% of the sample elected not to participate in the mood screen. Age, sex, BMI, ESS score, OSA severity, and hours of autoPAP use did not differ between the group who completed the HADS and those who did not complete the HADS. Considering the random and unselected nature of the missing data, we believe that the potential for selection bias is minimal. Our results may in fact underestimate the association between depression and autoPAP adherence, as depressed patients may be biased in their responses or not respond to the questionnaire.
In addition, comorbid conditions such as insomnia and periodic limb movement disorder may contribute to the explanatory role of depression in this model due to reduced total sleep time. We did not separately record these conditions, nor investigate the effect of total sleep time on autoPAP use. It is therefore possible that patients with these comorbid conditions were less adherent due to reduced total sleep time. However, insomnia has been shown to have no impact on CPAP acceptance or 6-month compliance, despite being prevalent in OSA patients.48 In summary, the unselected and consecutive selection of cases reflects the demographics and referral pattern for a large sleep disorders referral center and should be generalizable to such a population.
CONCLUSION
In conclusion, this large multivariate analysis found a significant independent association between depression and poor autoPAP adherence. To our knowledge, this is the first study to report such a finding using an objective measure of CPAP machine use. Such an association, although implied in day-to-day practice, and well established in studies of medication adherence, has not been previously reported in CPAP therapy. The relationship between 95th percentile pressure and autoPAP adherence may have been confounded by OSA severity. Further research into other predictors of autoPAP adherence is required, as our model only explains a portion of the total variance in autoPAP use. Depression may be a potential target for clinicians and future research aimed at enhancing adherence to autoPAP therapy. Further research is required to determine if screening for and treating depression improves adherence and outcomes in this population.
DISCLOSURE STATEMENT
This was not an industry supported study. Work was performed at Alfred Hospital, Prahran, Victoria, Australia. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 7.Archbold KH, Parthasarathy S. Adherence to positive airway pressure therapy in adults and children. Curr Opin Pulm Med. 2009;15:585–90. doi: 10.1097/MCP.0b013e3283319b3f. [DOI] [PubMed] [Google Scholar]
- 8.Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26:1175–82. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjelsberg FN, Ruud EA, Stavem K. Predictors of symptoms of anxiety and depression in obstructive sleep apnea. Sleep Med. 2005;6:341–6. doi: 10.1016/j.sleep.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Wells RD, Freedland KE, Carney RM, Duntley SP, Stepanski EJ. Adherence, reports of benefits, and depression among patients treated with continuous positive airway pressure. Psychosom Med. 2007;69:449–54. doi: 10.1097/psy.0b013e318068b2f7. [DOI] [PubMed] [Google Scholar]
- 11.Poulet C, Veale D, Arnol N, et al. Psychological variables as predictors of adherence to treatment by continuous positive airway pressure. Sleep Med. 2009;10:993–9. doi: 10.1016/j.sleep.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Stepnowsky CJ, Jr., Bardwell WA, Moore PJ, Ancoli-Israel S, Dimsdale JE. Psychologic correlates of compliance with continuous positive airway pressure. Sleep. 2002;25:758–62. doi: 10.1093/sleep/25.7.758. [DOI] [PubMed] [Google Scholar]
- 13.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep. 2004;27:134–8. doi: 10.1093/sleep/27.1.134. [DOI] [PubMed] [Google Scholar]
- 14.Pieh C, Bach M, Popp R, et al. Insomnia symptoms influence CPAP compliance. Sleep Breath. 2013;17:99–104. doi: 10.1007/s11325-012-0655-9. [DOI] [PubMed] [Google Scholar]
- 15.Berry RB, Parish JM, Hartse KM. The use of auto-titrating continuous positive airway pressure for treatment of adult obstructive sleep apnea. An American Academy of Sleep Medicine review. Sleep. 2002;25:148–73. [PubMed] [Google Scholar]
- 16.Masa JF, Jimenez A, Duran J, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170:1218–24. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
- 17.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 18.Valentin A, Subramanian S, Quan SF, Berry RB, Parthasarathy S. Air leak is associated with poor adherence to autoPAP therapy. Sleep. 2011;34:801–6. doi: 10.5665/SLEEP.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–4. [PubMed] [Google Scholar]
- 20.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson A. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 22.Antic NA, Buchan C, Esterman A, et al. A randomized controlled trial of nurse-led care for symptomatic moderate-severe obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:501–8. doi: 10.1164/rccm.200810-1558OC. [DOI] [PubMed] [Google Scholar]
- 23.Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. 2001;120:625–33. doi: 10.1378/chest.120.2.625. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nunez N, et al. Long-term CPAP compliance in females with obstructive sleep apnoea. Eur Respir J. 2013;42:1255–62. doi: 10.1183/09031936.00165812. [DOI] [PubMed] [Google Scholar]
- 26.Edinger JD, Carwile S, Miller P, Hope V, Mayti C. Psychological status, syndromatic measures, and compliance with nasal CPAP therapy for sleep apnea. Percept Mot Skills. 1994;78:1116–8. doi: 10.2466/pms.1994.78.3c.1116. [DOI] [PubMed] [Google Scholar]
- 27.Jackson ML, Stough C, Howard ME, et al. The contribution of fatigue and sleepiness to depression in patients attending the sleep laboratory for evaluation of obstructive sleep apnea. Sleep Breath. 2011;15:439–45. doi: 10.1007/s11325-010-0355-2. [DOI] [PubMed] [Google Scholar]
- 28.Stepnowsky CJ, Palau JJ, Zamora T, Ancoli-Israel S, Loredo JS. Fatigue in sleep apnea: the role of depressive symptoms and self-reported sleep quality. Sleep Med. 2011;12:832–7. doi: 10.1016/j.sleep.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Bardwell WA, Moore P, Ancoli-Israel S, Dimsdale JE. Fatigue in obstructive sleep apnea: driven by depressive symptoms instead of apnea severity? Am J Psychiatry. 2003;160:350–5. doi: 10.1176/appi.ajp.160.2.350. [DOI] [PubMed] [Google Scholar]
- 30.Bardwell WA, Norman D, Ancoli-Israel S, et al. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med. 2007;5:21–38. doi: 10.1207/s15402010bsm0501_2. [DOI] [PubMed] [Google Scholar]
- 31.Shen J, Barbera J, Shapiro CM. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63–76. doi: 10.1016/j.smrv.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54:216–26. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 33.Drake CL, Day R, Hudgel D, et al. Sleep during titration predicts continuous positive airway pressure compliance. Sleep. 2003;26:308–11. doi: 10.1093/sleep/26.3.308. [DOI] [PubMed] [Google Scholar]
- 34.Meurice JC, Dore P, Paquereau J, et al. Predictive factors of long-term compliance with nasal continuous positive airway pressure treatment in sleep apnea syndrome. Chest. 1994;105:429–33. doi: 10.1378/chest.105.2.429. [DOI] [PubMed] [Google Scholar]
- 35.Krieger J. Long-term compliance with nasal continuous positive airway pressure (CPAP) in obstructive sleep apnea patients and nonapneic snorers. Sleep. 1992;15:S42–6. doi: 10.1093/sleep/15.suppl_6.s42. [DOI] [PubMed] [Google Scholar]
- 36.Wild MR, Engleman HM, Douglas NJ, Espie CA. Can psychological factors help us to determine adherence to CPAP? A prospective study. Eur Respir J. 2004;24:461–5. doi: 10.1183/09031936.04.00114603. [DOI] [PubMed] [Google Scholar]
- 37.McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–14. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 38.Giles TL, Lasserson TJ, Smith BJ, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev (Online) 2006 doi: 10.1002/14651858.CD001106.pub2. CD001106. [DOI] [PubMed] [Google Scholar]
- 39.Frazer A, Benmansour S. Delayed pharmacological effects of antidepressants. Mol Psychiatry. 2002;7(Suppl 1):S23–8. doi: 10.1038/sj.mp.4001015. [DOI] [PubMed] [Google Scholar]
- 40.Bakker JP, O'Keeffe KM, Neill AM, Campbell AJ. Ethnic disparities in CPAP adherence in New Zealand: effects of socioeconomic status, health literacy and self-efficacy. Sleep. 2011;34:1595–603. doi: 10.5665/sleep.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen S, Smith S, Oei TP. Adherence to continuous positive airway pressure therapy in obstructive sleep apnoea sufferers: a theoretical approach to treatment adherence and intervention. Clin Psychol Rev. 2008;28:1355–71. doi: 10.1016/j.cpr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Ye L, Pack AI, Maislin G, et al. Predictors of continuous positive airway pressure use during the first week of treatment. J Sleep Res. 2012;21:419–26. doi: 10.1111/j.1365-2869.2011.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65:829–32. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 44.Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnoea/ hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 45.Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Respir Crit Care Med. 1999;159:1096–100. doi: 10.1164/ajrccm.159.4.9808008. [DOI] [PubMed] [Google Scholar]
- 46.Ling IT, James AL, Hillman DR. Interrelationships between body mass, oxygen desaturation, and apnea-hypopnea indices in a sleep clinic population. Sleep. 2012;35:89–96. doi: 10.5665/sleep.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magalang UJ, Dmochowski J, Veeramachaneni S, et al. Prediction of the apneahypopnea index from overnight pulse oximetry. Chest. 2003;124:1694–701. doi: 10.1378/chest.124.5.1694. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen XL, Chaskalovic J, Rakotonanahary D, Fleury B. Insomnia symptoms and CPAP compliance in OSAS patients: A descriptive study using Data Mining methods. Sleep Med. 2010;11:777–84. doi: 10.1016/j.sleep.2010.04.008. [DOI] [PubMed] [Google Scholar]


