Abstract
Study Objectives:
Temporomandibular pain disorders (TMD) and myofascial pain were linked to increased prevalence of insomnia and obstructive sleep apnea (OSA) on clinical grounds. However, the literature lacks an accurate polysomnographic (PSG) characterization of sleep abnormalities associated with TMD, given that prior studies included small or uncontrolled samples of TMD patients. The present investigation aims to objectively evaluate measures of sleep and respiratory disturbance in a large representative sample of TMD cases in comparison with matched controls.
Methods:
Sleep, respiration, and limb movements were measured using a 2-night attended PSG protocol in 170 women—124 TMD cases with myofascial pain and 46 demographically matched controls. The second night data were compared between the groups using ANCOVAs. In TMD cases, the relationship between pain ratings and sleep parameters was analyzed using multiple regressions.
Results:
In comparison to healthy controls, TMD cased evidenced a significant increase in stage N1 sleep (12.2% ± 7.6% vs. 9.2% ± 5.0%, p = 0.03), which was only mild relative to normative values. TMD cases also demonstrated mild but significant elevations in arousals associated with all types of respiratory events (6.0/h ± 6.1 vs. 3.5/h ± 3.3 p = 0.02) and in respiratory effort related arousals (RERAs, 4.3/h ± 4.3 vs. 2.6/h ± 2.7, p = 0.02). Myofascial pain predicted a lower sleep efficiency (p = 0.01), more frequent awakenings (p = 0.04), and higher RERA index (p = 0.04) among TMD cases.
Conclusions:
Myofascial pain in TMD is associated with mild elevation in sleep fragmentation and increased frequency of RERA events. Further research is required to evaluate the clinical significance of these findings.
Citation:
Dubrovsky B; Raphael KG; Lavigne GJ; Janal MN; Sirois DA; Wigren PE; Nemelivsky LV; Klausner JJ; Krieger AC. Polysomnographic investigation of sleep and respiratory parameters in women with temporomandibular pain disorders. J Clin Sleep Med 2014;10(2):195-201.
Keywords: Temporomandibular disorders, myofascial pain, sleep disturbance, respiratory effort related arousals, upper airway resistance
Temporomandibular disorders (TMD), most often characterized by painful musculoskeletal signs and symptoms in the masticatory region, is a pain syndrome estimated to affect approximately 5% to 15% of the population, predominantly women.1–4 Subjective sleep disturbance has been consistently reported in TMD patients.5,6 Two earlier polysomnographic (PSG) studies failed to demonstrate objective differences in sleep or respiratory measures between TMD cases with myofascial pain and control participants free from myofascial pain and signs of TMD; however, the small sample sizes and restrictive selection criteria limit their interpretability.7,8
BRIEF SUMMARY
Current Knowledge/Study Rationale: A relationship between temporomandibular pain disorders (TMD) and sleep and respiratory disturbance has been suggested; however, large-scale controlled polysomnographic studies are lacking. The present investigation used two-night polysomnography to measure sleep architecture and respiration in a large sample of women with TMD and demographically matched controls.
Study Impact: TMD cases, relative to controls, evidenced a higher percentage of stage N1 sleep and a higher respiratory effort related arousal index. However, overall sleep and respiratory disturbances were only mild in the TMD group. The clinical significance of these findings is presently unclear and merits further investigation.
A recent investigation employing PSG recordings and clinical sleep interviews in an uncontrolled sample of TMD cases showed that TMD is associated with primary sleep disorders, such as insomnia and obstructive sleep apnea (OSA): nearly 36% of TMD cases met diagnostic criteria for insomnia, and over 28% met criteria for OSA.9 In the same sample of TMD cases, sleep efficiency on PSG was directly related to a measure of pain threshold, suggesting an association between pain and sleep disturbance in TMD.9,10 Additionally, a high prevalence of TMD was reported in clinical patients with mild to moderate OSA referred for a clinical dental evaluation, lending further support to the association between TMD and OSA.11 It was argued that a 28% rate of OSA diagnosis in a sample of TMD patients composed mainly of young females with relatively low body mass index (BMI) lends support to the possibility of elevated risk of OSA in TMD, requiring further evaluation in a large scale study.9
However, mean sleep architecture and continuity measured by polysomnography were within normative ranges, including sleep efficiency, sleep onset latency, rapid eye movement (REM) sleep latency, and sleep stage distribution.9,10 Further, in this sample of TMD cases, the mean apnea-hypopnea index (AHI) was only borderline at 5.1 events per hour.9,10 While the diagnosis of OSA in these participants was based on established criteria for scoring and interpreting the respiratory disturbance index (RDI) using standard laboratory PSG, which includes respiratory effort related arousals (RERAs) in addition to apneas and hypopneas,12,13 the degree of sleep disordered breathing among TMD cases who met the criteria for OSA was predominantly mild.9,10 Moreover, no control group was employed.9,10 Therefore, the question of whether TMD and non-TMD populations are systematically different on objective measures of sleep and sleep disordered breathing remains unanswered.
The present investigation set out to determine whether PSG measures of sleep architecture, respiratory events and limb movements differ between a large, well-defined sample of women with TMD and demographically similar control participants. The evaluation of a possible association between PSG variables and myofascial pain measures in TMD cases was a secondary aim of the study. This investigation was part of a larger study of the association between TMD and sleep bruxism.14 It was hypothesized that TMD patients would evidence a greater objective sleep disturbance and a higher frequency of respiratory events than control participants.
METHODS
The research protocol was approved by the Institutional Review Board at the New York University (NYU) School of Medicine.
Participants
Recruitment, interviews and examinations took place at the New York University College of Dentistry (NYUCD). The PSG data were recorded at a sleep laboratory affiliated with the NYU School of Medicine. All participants signed the informed consent form. Only female participants were recruited, as TMD afflicts predominantly women.1 Participants were recruited solely on the basis of the presence (cases) or absence (controls) of a myofascial TMD; no consideration was given to the presence of other factors, such as bruxism. All TMD cases met Research Diagnostic Criteria for TMD (RDC/TMD) Group I (involving myofascial pain).15 The criteria include report of pain or ache in the facial area, as well as pain reported in response to palpation of ≥ 3 of the 20 facial muscle sites. More detailed descriptions of the RDC/TMD criteria used for the inclusion of TMD participants are reported elsewhere.14 Control participants were recruited from NYUCD dental clinics and acquaintances of participating cases. Excluded from participation were persons who were pregnant, habitually smoked after bedtime, habitually slept < 4 h per night or had < 4 h of sleep on the adaptation PSG night, had diabetes or peripheral neuropathy, had a neuropathic facial pain condition, had severe OSA (AHI ≥ 30 events/h of sleep) diagnosed previously or identified on the first study night, used positive air pressure (PAP) devices, wore a soft appliance for bruxism, had sustained a physical trauma to face, or had ongoing dental work. Cases and controls were matched for age, socioeconomic status, self-identified race, and self-identified Hispanic ethnicity.
Measures
Participants were interviewed by the clinical research coordinator regarding their pain experience using a standardized RDC/TMD pain history questionnaire. The questionnaire assessed the length of myofascial pain and pain-related functional disability (measured on a Likert scale from 0 to 10). It also included rating scales that measured myofascial pain at the time of the interview, the worst pain in the preceding 6 months, and average pain in the preceding 6 months (Likert scales with the 2 extremes defined in words: 0 is “no pain” and 10 is “pain as bad as it could be”). The mean of these 3 rating scales was defined as “characteristic pain intensity” (CPI).16 In addition, on the evening of each PSG, participants rated their myofascial pain at that moment in time (current) and the average pain in the preceding 24-h period on a similar scale. In the morning after each PSG, participants rated the average pain during the PSG night, as well as current pain. More detailed descriptions of pain measures are reported elsewhere.14 Control participants were free from myofascial pain. All participants also completed the Epworth Sleepiness Scale (ESS) questionnaire.17
Each participant was scheduled to complete all-night attended PSG recordings on 2 consecutive nights. On both nights the PSG montage included a 6-channel electroencephalogram (EEG), bilateral electrooculogram (EOG), submental electromyogram (chin EMG), bilateral anterior tibialis EMG, electrocardiogram (ECG) using a single modified lead II, rib cage and abdominal movement belts with piezoelectric sensors, nasal airflow pressure transducer and nasal/oral thermistor, oximetry, a snoring sensor, and a body position sensor. In addition, bilateral masseter EMG and bilateral temporalis EMG channels were used to detect bruxism events. A SomnoStarPro acquisition system (Viasys Healthcare, San Diego, CA) with sampling rates ranging from 50 Hz to 200 Hz was used. Audio and video signals were recorded continuously. The onset and offset times of nocturnal PSG recordings were determined from each participant's habitual bedtime and uptime, with the recordings running approximately from 22:30 to 07:00. To control for the first night effect, data from the first PSG night were considered an acclimation night and were not generally used in the analyses. They were, however, used for 3 TMD participants who declined to return for the second night, and for 6 TMD cases and 1 control participant whose data from the second PSG night were unusable due to technical difficulties.
PSG data were exported to Stellate Harmonie software (Natus, San Carlos, CA) for analysis, where manual scoring of sleep stages, arousals, apneas, hypopneas, RERAs, and periodic limb movements (PLMs) was done by 2 scorers in accordance with the American Academy of Sleep Medicine (AASM) 2007 Scoring Manual, using the recommended hypopnea definition.12 Both scorers had Ph.D. degrees and extensive training in sleep medicine, including the experience of scoring sleep records under a direct supervision of a physician board-certified in sleep. Adequate interscorer reliability was ascertained for the larger study and published elsewhere.14
The following variables were derived from the scored data: total sleep time (TST, min), sleep efficiency (SE = TST / time in bed * 100%), sleep onset latency (SOL, min), number of awakenings, number of sleep stage shifts into N1 from any other stage of sleep (stage N1 shifts), percentages of sleep stages N1, N2, N3, and REM sleep (time in a given sleep stage / TST * 100%), REM sleep latency (min), AHI, RERA index, RDI calculated as the number of apneas, hypopneas, and RERAs per hour of sleep,13 indices for spontaneous arousals, respiratory event-related arousals (arousals associated with all types of respiratory events), PLM-related arousals, and for all arousals combined, as well as a PLM index. Presence and severity of abnormal values were determined based on AASM recommended cutoffs.13,18,19 All indices were calculated as the number of events per hour of sleep.
Data Analysis
Independent-samples t tests were used to compare BMI, age, and ESS scores between TMD cases and control participants. Analysis of covariance (ANCOVA) was used to analyze group differences on all PSG measures of sleep architecture, arousal indices, respiratory events and PLMs, with BMI and age as covariates. In addition, because the majority of dependent variables had a skewed distribution, similar ANCOVA models were used to confirm raw score analysis after rank-transformation. As the pattern of results was essentially the same for both analyses, with some loss of power after the rank transformation, only the results of the analysis of raw scores are reported. To analyze the relationship between myofascial pain and sleep in TMD cases only, each PSG variable was treated as an outcome variable in parallel multiple regression models that contained the pain ratings as explanatory variables and adjusted for BMI and age. The following PSG variables were selected for this analysis based on the combined considerations of typically reported PSG parameters and the pattern of between-group differences: SE, SOL, REM sleep latency, stage N1%, number of awakenings, number of stage N1 shifts, respiratory arousal index, spontaneous arousal index, total arousal index, RERA index, RDI, and AHI. In addition, Fisher exact test was used to compare TMD cases and controls on the frequency of meeting exclusion criteria and medication use. Significance level of 0.05 was used for all analyses. The ratio of TMD cases to controls was planned to be approximately 2:1 in order to provide power of 80% to detect moderately sized effects between controls and any half of the cases that might be distinguished by variables related to aims of the parent study; power in excess of 80% was available for comparisons of all cases to controls in the present investigation.
RESULTS
Participant Characteristics
Among prospective TMD participants, 5 were excluded due to self-reported severe OSA and PAP use, 7 were excluded based on the self-report of obtaining < 4 h of sleep per night, and one was excluded due to both PAP use and short nocturnal sleep. Among prospective control participants, 2 were excluded due to self-reported short sleep times. No participants were excluded based on the results of the first night adaptation PSG study. Thus, 13/137 (9.5%) of cases and 2/48 (4.2%) of controls were excluded for reasons that would likely have increased PSG indices of disturbed sleep, but the exclusion rates were statistically similar between the groups (Fisher exact test, p = 0.36).
A total of 170 women (124 diagnosed with myofascial TMD, 46 controls) completed the study. No differences were observed between the groups on any measured demographic variable. Self-reported race was predominantly white (62.6%), black (14.4%), or “other” race (14.4%), with the other 8.6% comprised of several categories, including Asian, Native American, and biracial. Hispanic ethnicity was reported by 22.5%. Mean age was 39.2 years (standard deviation [SD] = 14.6, range 19-78), with a mean of 15 years of education (SD = 2.2, range 11-20). Both groups had a similar age composition (mean = 40.3 years, SD = 14.8 in TMD cases; mean = 36.1 years, SD = 13.5 in controls, p = 0.1). The mean BMI was borderline elevated and essentially identical in both groups (mean = 25.0 kg/m2, SD = 5.0 in TMD cases and mean = 25.0 kg/m2, SD = 5.5 in controls, p = 0.96). The mean ESS values were within normal limits and similar in both groups (mean = 6.5, SD = 4.3 in TMD cases; mean = 7.1, SD = 4.3 in controls, p = 0.39). The TMD cases reported moderate intensities of characteristic pain (mean = 5.2, SD = 1.7), relatively low levels of pain disability (mean = 1.8, SD = 2.2), and the onset of facial pain approximately 10.5 years before study entry (mean = 10.5 years, SD = 10.6; median = 7 years).
Polysomnographic Data
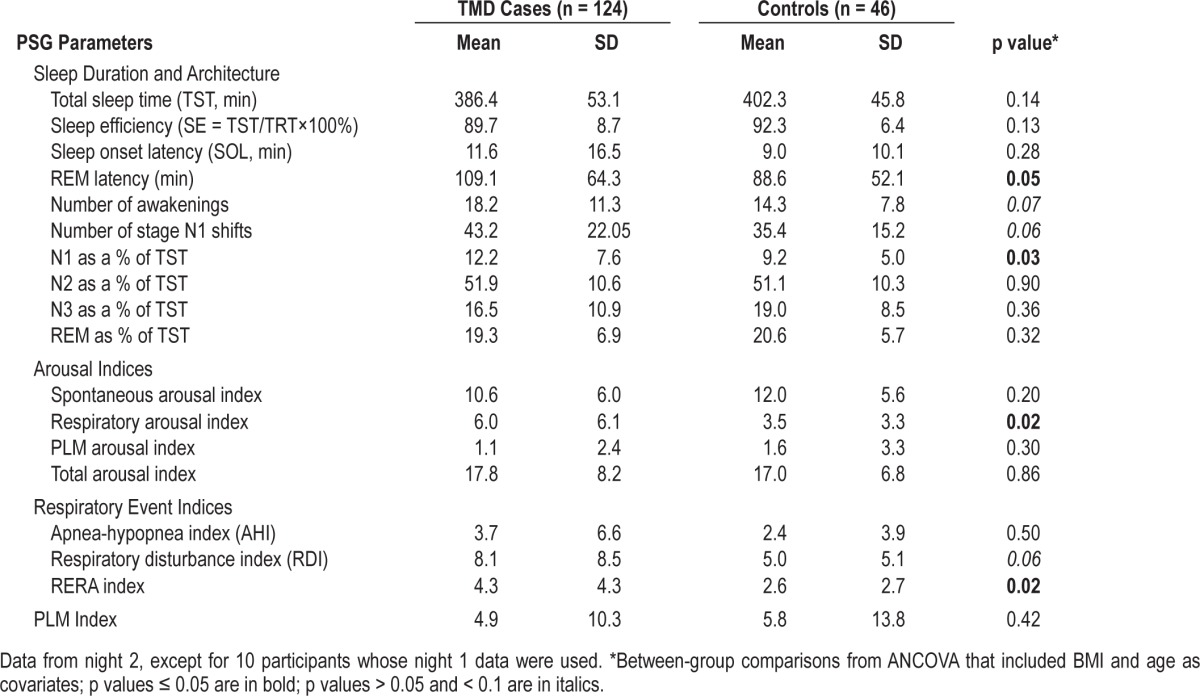
Based on self-reports, the days preceding the PSG nights were typical in terms of alcohol and caffeine consumption, as well as daytime fatigue and napping behavior. Table 1 shows descriptive statistics for all PSG measures and p values for between-group comparisons.
Table 1.
Mean and standard deviation (SD) values for polysomnographic variables in TMD cases and controls.
Sleep Architecture and Arousal Variables
As seen in Table 1, TMD cases had a significantly higher mean percentage of stage N1 sleep (p = 0.03) and tended towards a greater number of awakenings (p = 0.07) and a greater number of stage N1 shifts (p = 0.06). TMD cases also showed almost twice as many arousals associated with all types of respiratory events (apneas, hypopneas and RERA events) as controls (p = 0.02). Groups did not differ on indices of spontaneous arousals, PLM-related arousals, or total arousals. Mean REM sleep latency, although longer in TMD cases than controls, remained within normal limits in both groups.
Respiratory Events
Central apneas were similarly rare in both groups (mean < 0.4/h of sleep, SD < 0.6). Therefore, these events were aggregated with obstructive respiratory events for the purposes of reporting AHI and RDI. As shown in Table 1, the mean AHI values were similarly low in both groups (< 4/h of sleep). The mean RDI values were mildly elevated in both groups and tended to be more so among cases than controls (8.1/h of sleep vs. 5.0/h of sleep, p = 0.06). The mean RERA indices were low in both groups; however, significantly higher in TMD cases than controls (4.3/h of sleep vs. 2.6/h of sleep, p = 0.02). Thus, the evidence for the increased sleep disordered breathing among TMD cases is specific to increased frequency of RERAs.
Given the fact that scoring of a RERA event requires an EEG arousal from sleep, a multiple linear regression analysis was performed to evaluate whether the increased RERA index in TMD cases was due to differences in sleep fragmentation between groups. The analysis showed that the RERA index remained significantly predicted by TMD Status (B = 1.13, standard error = 0.43, p = 0.01) after adjusting the model for BMI, age, stage N1%, number of awakenings, number of stage N1 shifts, spontaneous arousal index, and total arousal index. Therefore, the increased frequency of RERAs among TMD cases appears statistically independent of sleep fragmentation measures, such as awakenings, total arousals and stage N1%.
PLMs
As shown in Table 1, the mean PLM indices and the mean indices for PLM-associated arousals were similarly low in both groups.
Pain
Myofascial Pain Ratings and PSG Variables in TMD Patients
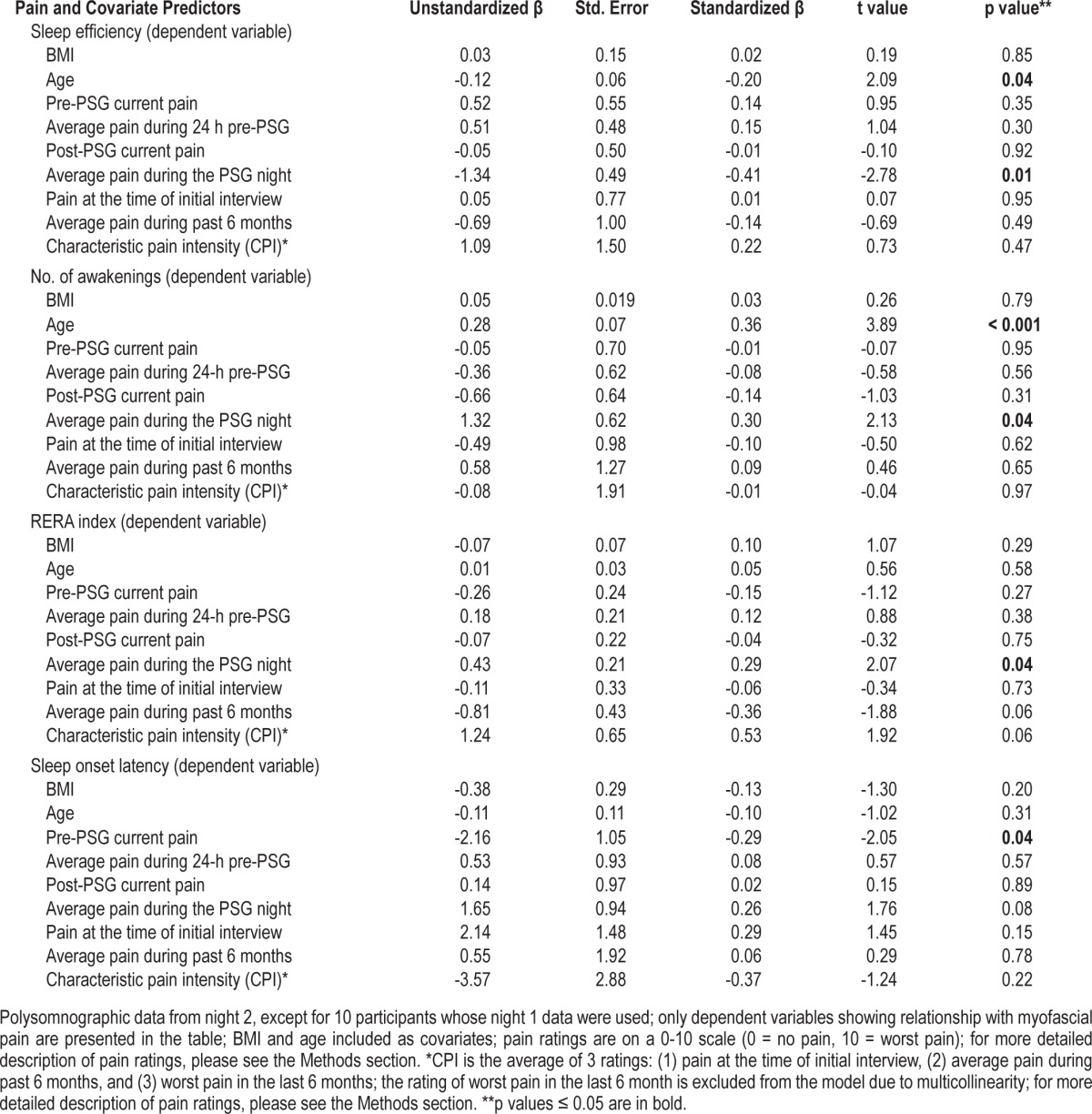
Table 2 summarizes the results of the multiple regression models for dependent PSG variables that had a significant relationship with at least one pain measure. Post-sleep ratings of the average pain during the PSG night were inversely related to SE (B = 1.34, standard error = 0.49, p = 0.01) and directly related to the number of awakenings (B = 1.32, standard error = 0.62, p = 0.04) and RERA index (B = 0.43, standard error = 0.21, p = 0.04). Pre-sleep ratings of current pain were inversely related to SOL (B = -2.16, standard error = 1.05, p = 0.04). However, higher pre-sleep ratings of current pain were associated with the use of opioid medications as a binary variable (r(118) = 0.22, p = 0.02), and the introduction of the opioid medication use as a covariate into the multiple regression model resulted in the loss of significant association between pre-sleep pain ratings and SOL.
Table 2.
Multiple regression models using ratings of myofascial pain to predict polysomnographic variables in TMD Cases (n = 124).
Medication Use and Case-Control Differences
To analyze whether TMD pain is associated with the use of medications that may potentially influence between-group differences in PSG variables, TMD cases and controls were compared on each medication category using Fisher exact test. TMD cases more frequently used nonsteroidal anti-inflammatory drugs (NSAIDs; 77.4% vs. 15.2%, p < 0.001) and muscle relaxants (11.3% vs. 0%, p = 0.01). The use of other types of medications, including opioids, corticosteroids, antidepressants, benzodiazepines, and hypnotics, was generally low (< 8% in either group for each medication type) and not significantly different between the groups. When the use of NSAIDs was used as a covariate in addition to BMI and age, the pattern of case-control differences in PSG variables remained similar, with some loss of power (difference in stage N1%: p = 0.05; difference in RERA index: p = 0.05). As muscle relaxants were not used by any of the control participants, the 14 TMD participants using these medications were removed from the sample and the between-group comparisons were repeated. The results followed virtually the same pattern (difference in stage N1%: p = 0.03; difference in RERA index: p = 0.01). These analyses show that the medication use associated with TMD pain does not account for the intergroup differences in PSG variables.
DISCUSSION
The present investigation employed what, to our knowledge, is the largest reported sample of TMD cases with myofascial pain undergoing a two-night protocol using standard PSG recording. The results suggest that (i) disrupted sleep and (ii) increased frequency of RERA events without a detectable difference in the mean AHI values were more commonly seen in TMD cases than in demographically and BMI-matched control participants.
Sleep disturbance in TMD cases was evidenced by a higher percentage of stage N1 sleep and a tendency towards a greater number of awakenings and shifts into stage N1 sleep. TMD cases demonstrated essentially normal mean PSG values for other commonly used measures of sleep architecture, including TST, SE, SOL, percentages of stages N2, N3, and REM sleep and REM sleep latency. There was no difference between TMD cases and controls on PLM and related arousals indices. These findings are generally consistent with previous reports of essentially normal PSG sleep parameters in TMD.7–10 Although the between-group difference in stage N1 sleep percentage is small (12.2% in TMD cases vs. 9.2% in controls, Table 1), the mean value falls outside of the normal range20,21 in TMD cases but not in controls. The increase in stage N1% in TMD cases was present even after controlling for BMI and age, factors shown to influence stage N1%,20,21 which suggests that this increase is robust and potentially clinically relevant. However, as the mean percent of stage N1 sleep presently observed in TMD cases would be considered mildly elevated in clinical settings, objective sleep disturbance associated with TMD appears to be only mild.
Although the AHI was not different between the groups, TMD cases demonstrated a greater frequency of RERA events as well as arousals associated with all types of respiratory events. The increase in the RERA index was robust even after statistically controlling for possible contributions of BMI, age, and several measures of sleep fragmentation. Moreover, the RERA index in TMD cases was directly related to subjective ratings of myofascial pain during the PSG night. One possible interpretation of these findings is that upper airway resistance is increased in TMD, and therefore, sleep disordered breathing might be a source of sleep disturbance in TMD cases. Alternatively, pain-related decrease in the arousal threshold in TMD cases may set the stage for more frequent arousals during minor airway narrowing episodes that do not necessarily lead to arousals in controls.
In considering the possible relationship between TMD and sleep disordered breathing, it should be noted that the application of the recently updated criteria recommended by the AASM for the scoring of hypopneas22 might have resulted in re-categorizing some RERA events into hypopneas. However, the mean RDI was only mildly elevated in TMD cases (8.1/h of sleep, Table 1), which is consistent with previously published data.9,10 While the uncontrolled study of TMD cases reported a high prevalence of OSA on the bases of both PSG and clinical interviews,9 the absence of a clinical sleep interview in the present investigation does not allow the establishment of an OSA diagnosis. Therefore, the clinical significance of the elevated RERA frequency in TMD is presently unclear. The possibility nonetheless remains that the application of the standard diagnostic criteria13 emphasizing the clinical history in the context of mild RDI may result in intergroup differences in the categorization of sleep disordered breathing, which merits further investigation.
Although the mechanisms that may link sleep disordered breathing and TMD are unknown, some possibilities may be worth considering. First, it has been suggested that craniofacial variables may play a role in the etiology of sleep disordered breathing, specifically RERAs, especially in women and non-obese patients.23–25 Although the role of craniofacial features in TMD has been disputed,26,27 two empirical studies have reported an association between TMD and certain craniofacial abnormalities.28,29 The possibility that craniofacial variables may contribute to both myofascial pain and sleep disordered breathing in TMD, while highly speculative, is consistent with the present study showing higher frequency of RERAs in women with TMD whose mean BMI was essentially within normal limits. Second, the effect of myofascial pain on the central control of muscles involved in breathing, chewing, and swallowing has been hypothesized to provide a pathophysiological mechanism relating TMD to sleep disordered breathing.30
The alternative interpretation of the elevated RERA frequency as a consequence of a lower arousal threshold in TMD is consistent with the finding of an association between higher ratings of myofascial pain during the PSG night and decreased sleep efficiency, as well as more frequent awakenings and RERA events among TMD cases. The similarity of these findings in women with TMD to PSG evaluations of women with fibromyalgia showing increased sleep disruption and inspiratory flow limitation without elevated AHI underscores pain-related arousability as a possible cause of elevated RERA index in TMD.31,32 It has been suggested that a lower arousal threshold in response to respiratory stimuli may contribute to the etiology of sleep disordered breathing,33 and elevating the arousal threshold with a hypnotic may reduce the frequency of respiratory events.34 Therefore, the role of chronic pain conditions in lowering the arousal threshold to respiratory stimuli during sleep needs to be determined in future studies.
Unexpectedly, higher ratings of myofascial pain on the evening of PSG were associated with shorter sleep latencies in TMD cases. In a recent PSG study of migraine sufferers, shorter sleep latency was observed in the preictal phase relative to the interictal phase.35 Methodological differences notwithstanding, these two findings appear to suggest that an acute pain increase in a chronic pain condition may be related to shorter sleep latency.35 While no details on medication use among migraine sufferers were reported,35 in the present sample of women with TMD the relationship between pre-sleep pain ratings and sleep latency was apparently mediated by the use of opioid medications. Another hypothetical possibility is that pain-related fatigue in the context of chronic sleep deprivation may facilitate sleep onset.
Overall, the present data point towards the possibility of a complex interplay between myofascial pain, sleep continuity, and upper airway behavior. The mechanisms and clinical ramifications of this interplay require elucidation in future research.
Several important methodological characteristics of the present study should be noted. First, only women participated in the present study, which limits generalizability of the results. Second, as no clinical sleep interviews were used, clinical diagnoses of insomnia or OSA could not be established. Third, due to methodological demands of the larger study, individuals with severe OSA, using PAP devices, or reporting less than four hours of sleep per night were excluded from the study. These exclusion criteria likely resulted in underestimation of indices of sleep and respiratory disturbance in our sample. However, the exclusion rates were statistically similar between the groups. Therefore, while practical considerations may have reduced absolute estimates of sleep disturbance in these participants, relative measures (group comparisons) remain valid. And finally, the use of medications was not an exclusion criterion; however, no relationship was uncovered between medication use and intergroup differences in PSG variables. These limitations notwithstanding, the present study remains a well-controlled PSG investigation of the largest representative sample of TMD cases to date.
In conclusion, TMD cases with chronic myofascial pain have a mild degree of objective sleep disturbance and a mild increase in upper airway resistance during sleep, both of which appear to relate to acute levels myofascial pain at night. Further research is needed to help clarify clinical ramifications of sleep and respiratory disturbances in TMD, as well as elucidate the interrelationship between sleep and respiratory disturbance and myofascial pain.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported in part by grant R01 DE018569 from the National Institutes of Health, Bethesda, MD. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Janal MN, Raphael KG, Nayak S, Klausner J. Prevalence of myofascial temporomandibular disorder in US community women. J Oral Rehabil. 2008;35:801–9. doi: 10.1111/j.1365-2842.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 2.Isong U, Gansky SA, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orofac Pain. 2008;22:317–22. [PMC free article] [PubMed] [Google Scholar]
- 3.LeResche L, Sauders K, Von Korff MF, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69:153–60. doi: 10.1016/s0304-3959(96)03230-7. [DOI] [PubMed] [Google Scholar]
- 4.Bagis B, Ayaz EA, Turgut S, Durkan R, Özcan M. Gender difference in prevalence of signs and symptoms of temporomandibular joint disorders: a retrospective study on 243 consecutive patients. Int J Med Sci. 2012;9:539–44. doi: 10.7150/ijms.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yatani H, Studts J, Cordova M, Carlson CR, Okeson JP. Comparison of sleep quality and clinical and psychologic characteristics in patients with temporomandibular disorders. J Orofac Pain. 2002;16:221–8. [PubMed] [Google Scholar]
- 6.Quartana PJ, Wickwire EM, Klick B, Grace E, Smith MT. Naturalistic changes in insomnia symptoms and pain in temporomandibular joint disorder: a cross-lagged panel analysis. Pain. 2010;149:325–31. doi: 10.1016/j.pain.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Camparis CM, Formigoni G, Teixeira MJ, Bittencourt LR, Tufik S, de Siqueira JT. Sleep bruxism and temporomandibular disorder: Clinical and polysomnographic evaluation. Arch Oral Biol. 2006;51:721–8. doi: 10.1016/j.archoralbio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Rossetti LM, Pereira de Araujo Cdos R, Rossetti PH, Conti PC. Association between rhythmic masticatory muscle activity during sleep and masticatory myofascial pain: a polysomnographic study. J Orofac Pain. 2008;22:190–200. [PubMed] [Google Scholar]
- 9.Smith MT, Wickwire EM, Grace EG, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32:779–90. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: Associations with pain inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13:1043–7. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunali PA, Almeida FR, Santos CD, et al. Prevalence of temporomandibular disorders in obstructive sleep apnea patients referred for oral appliance therapy. J Orofac Pain. 2009;23:339–44. [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson AL, Quan SF, for the American Academy of Sleep Medicine . Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 13.American Academy of Sleep Medicine. Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders. [Google Scholar]
- 14.Raphael KG, Sirois DA, Janal MN, et al. Sleep bruxism and myofascial temporomandibular disorders: A laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012;143:1223–31. doi: 10.14219/jada.archive.2012.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- 16.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–49. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Carskadon MA, Dement WC. Monitoring and staging human sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis: Elsevier Saunders; 2011. pp. 16–26. [Google Scholar]
- 19.Epstein LJ, Kriso D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 20.Sahlin C, Franklin KA, Stenlund H, Lindberg E. Sleep in women: Normal values for sleep stages and position and the effect of age, obesity, sleep apnea, smoking, alcohol and hypertension. Sleep Med. 2009;10:1025–30. doi: 10.1016/j.sleep.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 22.Berry RB, Brooks R, Gamaldo CE, et al. for the American Academy of Sleep Medicine . Darien, IL: American Academy of Sleep Medicine; 2012. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology And Technical Specifications. Version 2.0. www.aasmnet.org. [Google Scholar]
- 23.Guilleminault C, Stoohs R, Kim YD, Chervin R, Black J, Clerk A. Upper airway sleep-disordered breathing in women. Ann Intern Med. 1995;122:493–501. doi: 10.7326/0003-4819-122-7-199504010-00003. [DOI] [PubMed] [Google Scholar]
- 24.D'Ambrosio F, Proietti D, Masieri S, Roncacci A, Saponara M, Fabiani M. Cephalometric variables in the diagnosis of obstructive sleep apneas syndrome. In: Fabiani M, editor. Surgery for Snoring and Obstructive Sleep Apnea Syndrome. The Hague, The Netherlands: Kugler Publications; 2003. [Google Scholar]
- 25.Pépin JL, Guillot M, Tamisier R, Lévy P. The upper airway resistance syndrome. Respiration. 2012;83:559–66. doi: 10.1159/000335839. [DOI] [PubMed] [Google Scholar]
- 26.Klasser GD, Greene CS. The changing field of temporomandibular disorders: what dentists need to know. J Can Dent Assoc. 2009;75:49–53. [PubMed] [Google Scholar]
- 27.John MT. There is no conclusive evidence for a relationship between the structure of the lower face and temporomandibular disorders. J Evid Based Dent Pract. 2007;7:73–4. doi: 10.1016/j.jebdp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Hwang CJ, Sung SJ, Kim SJ. Lateral cephalometric characteristics of malocclusion patients with temporomandibular joint disorder symptoms. Am J Orthod Dentofacial Orthop. 2006;129:497–503. doi: 10.1016/j.ajodo.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Miller JR, Burgess JA, Critchlow CW. Association between mandibular retrognathia and TMJ disorders in adult females. J Public Health Dent. 2004;64:157–63. doi: 10.1111/j.1752-7325.2004.tb02746.x. [DOI] [PubMed] [Google Scholar]
- 30.Bethesda, MD: National Heart Lung and Blood Institute (US); Cardiovascular and sleep-related consequences of temporomandibular disorders [Internet] c2001 [updated 2013 May; cited 2013 July 13]. Available from: http://www.nhlbi.nih.gov/meetings/workshops/tmj_wksp.pdf. [Google Scholar]
- 31.Besteiro González JL, Suárez Fernández TV, Arboleya Rodríguez L, Muñiz J, Lemos Giráldez S, Alvarez Fernández A. Sleep architecture in patients with fibromyalgia. Psicothema. 2011;23:368–73. [PubMed] [Google Scholar]
- 32.Gold AR, Dipalo F, Gold MS, Broderick J. Inspiratory airflow dynamics during sleep in women with fibromyalgia. Sleep. 2004;27:459–66. doi: 10.1093/sleep/27.3.459. [DOI] [PubMed] [Google Scholar]
- 33.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 34.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–14. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engstrøm M, Hagen K, Bjørk MH, Stovner LJ, Gravdahl GB, Stjern M, Sand T. Sleep quality, arousal and pain thresholds in migraineurs: a blinded controlled polysomnographic study. J Headache Pain. 2013;14:12. doi: 10.1186/1129-2377-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


