Abstract
To gain some insight into the mechanisms by which plant cells die as a result of abiotic stress, we exposed tobacco (Nicotiana tabacum) Bright-Yellow 2 cells to heat shock and investigated cell survival as a function of time after heat shock induction. Heat treatment at 55°C triggered processes leading to programmed cell death (PCD) that was complete after 72 h. In the early phase, cells undergoing PCD showed an immediate burst in hydrogen peroxide (H2O2) and superoxide (O2·-) anion production. Consistently, death was prevented by the antioxidants ascorbate (ASC) and superoxide dismutase (SOD). Actinomycin D and cycloheximide, inhibitors of transcription and translation, respectively, also prevented cell death, but with a lower efficiency. Induction of PCD resulted in gradual oxidation of endogenous ASC; this was accompanied by a decrease in both the amount and the specific activity of the cytosolic ASC peroxidase (cAPX). A reduction in cAPX gene expression was also found in the late PCD phase. Moreover, changes of cAPX kinetic properties were found in PCD cells. Production of ROS in PCD cells was accompanied by early inhibition of glucose (Glc) oxidation, with a strong impairment of mitochondrial function as shown by an increase in cellular NAD(P)H fluorescence, and by failure of mitochondria isolated from cells undergoing PCD to generate membrane potential and to oxidize succinate in a manner controlled by ADP. Thus, we propose that in the early phase of tobacco Bright-Yellow 2 cell PCD, ROS production occurs, perhaps because of damage of the cell antioxidant system, with impairment of the mitochondrial oxidative phosphorylation.
In plants, programmed cell death (PCD) is responsible for removal of redundant, misplaced, or damaged cells, and, thus, contributes significantly to both development and maintenance of these multicellular organisms. Activation of PCD in plants takes place during a variety of processes including differentiation of tracheary elements (Fukuda, 2000; Yu et al., 2002) and female gametophytes (Wu and Cheun, 2000), the development of cereal endosperm and aleurone cells (Young and Gallie, 2000; Fath et al., 2002), responses to external stimuli such as the hypersensitive reaction against pathogen attacks (Lamb and Dixon, 1997; Beers and McDowell, 2001) and severe abiotic stresses (Koukalovà et al., 1997; Weaver et al., 1998; Rao and Davis, 2001). At present, however, how signaling pathways lead plant cells toward death via apoptosis and how death occurs are far from being elucidated. Mammalian and plant PCD processes share several morphological and biochemical features, including cytoplasm shrinkage, nuclear condensation, DNA laddering, expression of caspase-like proteolytic activity, and release of cytochrome c from mitochondria (Balk et al., 1999; Sun et al., 1999; Kim et al., 2003). Nonetheless, in distinction from mammals (for instance, see Atlante et al., 2003a, 2003b), how plant PCD takes place remains somewhat obscure. In this context, the role of the reactive oxygen species (ROS) in plants merits special attention because ROS can exert two opposite effects: They can activate pathways aimed at saving the cell from demise (Pastore et al., 1999, 2000; Di Cagno et al., 2001; Mittler, 2002; Vranova et al., 2002) but can also impair the cellular redox balance, thus triggering PCD, perhaps with mitochondria playing a pivotal role in integrating cellular stress and regulating PCD (Jones, 2000; Lam et al., 2001; Tiwari et al., 2002). These ROS-dependent responses could be dependent on localization, timing, and level of ROS production in response to different stimuli (Mittler, 2002; De Gara et al., 2003) and on the occurrence of cell scavenger systems. Among the latter, ascorbate (ASC) plays a crucial role (Noctor and Foyer, 1998; Smirnoff, 2000; De Gara, 2003), it being both a direct scavenger and electron donor of ASC peroxidase, a key enzyme for scavenging hydrogen peroxide (H2O2) in plant cells (Asada, 1992; Shigeoka et al., 2002).
In this work, we first developed a plant model system consisting of heat-shocked tobacco (Nicotiana tabacum) Bright-Yellow 2 (TBY-2) cells, in which PCD occurs in the absence of added PCD inducers. Then, we investigated events occurring in the early phase of PCD including ROS production, changes in the expression and activity of cytosolic ASC peroxidase (cAPX), and the alterations in cell oxidative metabolism that depends on mitochondrial function. We found that both cAPX function and mitochondrial energy metabolism are already impaired in the early stages of PCD.
RESULTS
Heat Shock-Induced PCD in Cultured TBY-2 Cells
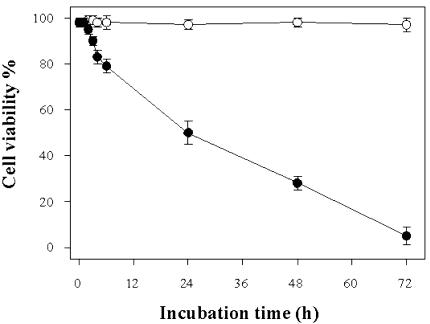
To ascertain whether and how heat shock can induce PCD in cultured cells of TBY-2, cells were kept for 10 min at temperatures ranging between 27°C (control cells) and 65°C, and cell viability was analyzed as a function of time after treatment. Cell viability was essentially unaffected after heating up to 45°C, whereas incubation at 55°C resulted in a progressive decrease in cell viability. The effect started after 2 h (cells in these conditions will be referred to as 2-h PCD cells). Viability of cells was 50% after 24 h (24-h PCD cells) and negligible after 72 h (Fig. 1). Incubation at 60°C and 65°C resulted in cell death in 24 h (data not shown).
Figure 1.
Time-dependent effect of heat shock on cell viability. One hundred milliliters of TBY-2 cell cultures was treated for 10 min at 55°C and returned to 27°C for a 72-h period. At indicated time intervals after heat treatment, viability of both control (○) and heat-shocked (•) cells was measured using trypan blue staining and examination by phase contrast microscopy. In both cases, the percentage (±se) of viable cells was counted in a population of at least 1,000 cells in six separate experiments.
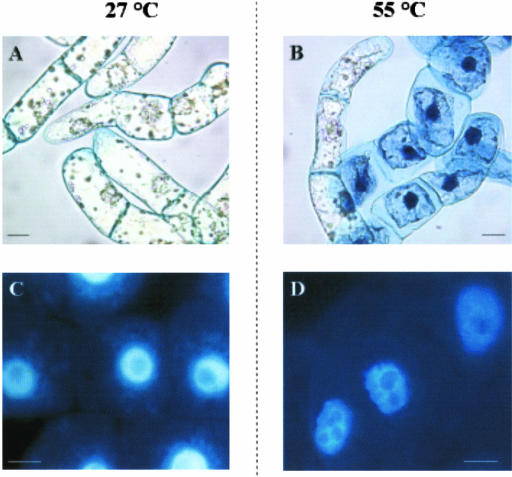
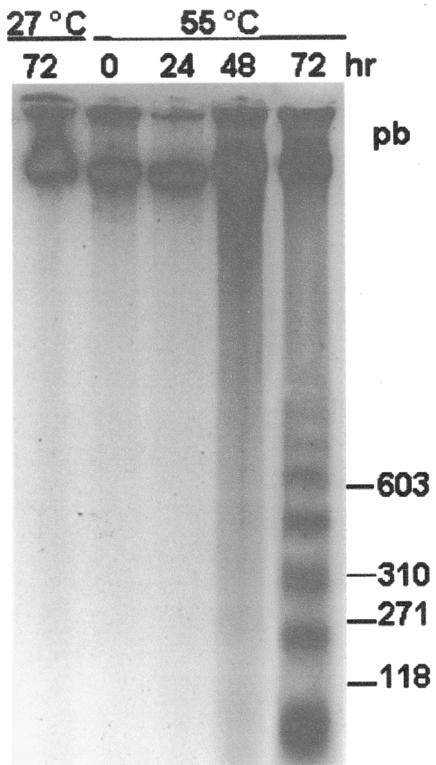
To distinguish whether cell death occurred by PCD or by necrosis, we investigated the occurrence of indicators of PCD including cytoplasm shrinkage, chromatin condensation, and DNA laddering. Cytoplasm shrinkage was found in most 24-h PCD cells (72% ± 7%; Fig. 2B) but only in 12% ± 2% and 2% ± 2% of dead cells heated at 60°C and 65°C respectively. As expected, control cells exhibited normal morphology (Fig. 2A). In parallel experiments, chromatin morphology was observed by DAPI staining. Nuclei of control cells exhibited a large central nucleolus surrounded by uniformly stained chromatin (Fig. 2C), whereas the chromatin had a granular appearance with lobated nuclei in cells shocked at 55°C (Fig. 2D). Further confirmation that 55°C heat shock does induce PCD was obtained by assaying DNA cleavage in oligo-nucleosomal units by cell death-specific endonucleases. DNA laddering was found in 48- and 72-h PCD cells, at which times 75% to 98% cells were already dead (Fig. 3). In cells treated at 60°C and 65°C, neither chromatin condensation nor DNA laddering were found (not shown), thus showing that cell death occurred by necrosis.
Figure 2.
Chromatin condensation and cytoplasm shrinkage induced by heat shock in TBY-2 cells. Cell suspension was subjected to heat shock and returned to growth temperature for 24 h. Aliquots of both control (27°C) and heat-shocked (55°C) cells were collected, stained with trypan blue, and visualized under phase contrast microscopy (A and B) or stained with 4,6-diamidino-2-phenylindole (DAPI) and analyzed by fluorescence microscopy (C and D). Pictures represent typical examples. Bar = 20 μm.
Figure 3.
Fragmentation DNA induced by heat shock in TBY-2 cells. Cell cultures were treated at 55°C and returned to 27°C for a 72-h period. At the indicated time intervals after heat shock, cells were collected, frozen in liquid nitrogen, and genomic DNA was extracted as described in “Materials and Methods.” Five hundred nanograms of genomic DNA was separated on a 1.8% (w/v) agarose gel and detected by staining the gel with ethidium bromide.
ROS Production in TBY-2 Cells Undergoing PCD
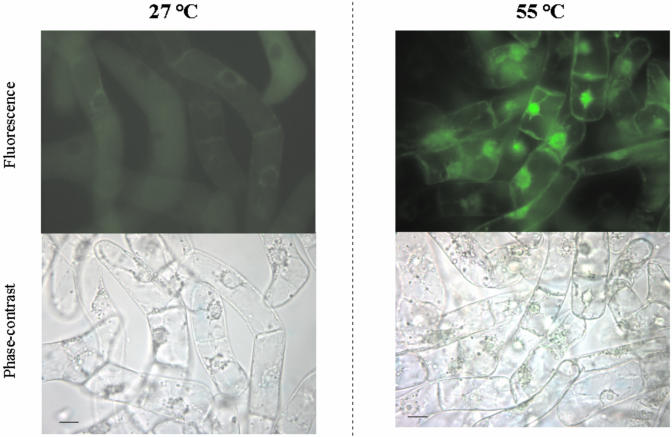
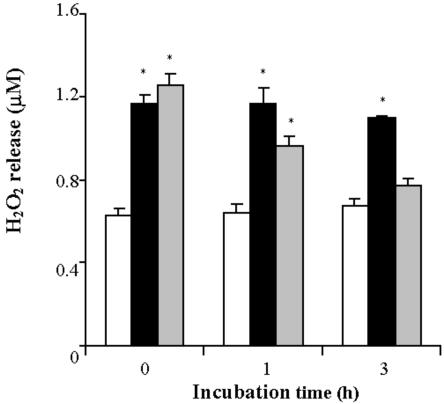
In the light of the crucial role played by ROS in PCD (Dat et al., 2003), we investigated production of ROS in heat-shocked TBY-2 cells by monitoring both H2O2 and superoxide anion (O2-.) production. Generation of H2O2, measured by following the fluorescence of the dye rhodamine produced from hydrorhodamine 123 (DHR123) in the presence of H2O2, occurred in the majority of cells immediately after heat shock, (0-h PCD cells) as shown by the bright fluorescence resulting from staining with DHR123 (Fig. 4), whereas production of ROS in control cells was negligible. Production of H2O2 was also investigated as a function of time (Fig. 5). As a result of heat shock, the level of H2O2 increased by about 200% with respect to the control cells and remained constant over the investigated time period. Pre-incubation with ASC failed to prevent the increase in H2O2 in 0-h PCD cells, but the H2O2 was gradually removed over a 3-h period. It should be noted that an immediate and very large (500% over control levels) production of H2O2 was found in cells heat shocked at 65°C (not shown).
Figure 4.
Production of H2O2 in PCD-induced cells. TBY-2 cells were kept either at 27°C or taken immediately after heat shock at 55°C. The cells were stained with DHR123, and H2O2 production was visualized by fluorescent microscopy as described in “Materials and Methods.” Cells were photographed under both fluorescent field and phase contrast. Pictures represent typical examples. Bar = 20 μm.
Figure 5.
Release of H2O2 into the culture medium by TBY-2 cells. The concentration of H2O2 was measured in the absence (black columns) or in the presence of 0.4 mm ASC (gray columns) added to the culture medium before heat shock. The concentration of H2O2 in control cells (white columns) was also measured. At the indicated times, 1 mL of cell suspension (0.12 g of cells) was taken, and the concentration of H2O2 was determined in the medium as described in “Materials and Methods.” Values represent mean (±se) of five experiments. An asterisk indicates the values that are significantly different from the control (P < 0.01).
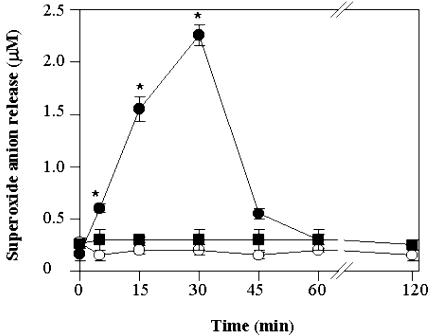
In another set of experiments (Fig. 6), generation of O2-. was measured in the culture medium as a function of time, both in control and in heat-shocked TBY-2 cells, and in the absence or presence of superoxide dismutase (SOD). The level of O2-. was found to increase enormously (up to 1,000%) over the first 30 min in 55°C shocked cells and then to drop to control levels by 1 h. The levels of O2·- superoxide production both in control cells and in cells pre-incubated with SOD were low and remained constant at all times.
Figure 6.
Release of O2-. into the culture medium by TBY-2 cells. The concentration of O2-. was measured in the absence (•) or in the presence of 75 units mL-1 superoxide dismutase (SOD; ▪) added to the culture medium before heat shock. The concentration of O2-. in control cells (○) was also measured. At the times indicated, 1 mL of cell suspension (0.12 g cells) was taken, and O2-. concentration was determined in the medium as described in “Materials and Methods.” Values represent mean (±se) of four experiments. An asterisk indicates the values that are significantly different from the control (P < 0.01).
Effects of Scavengers of ROS and Transcription/Translation Inhibitors on Heat-Induced PCD
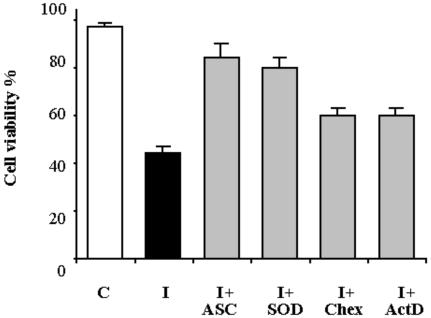
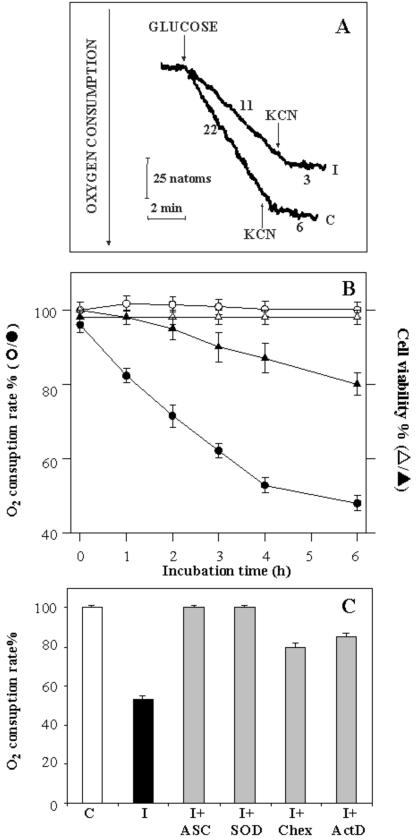
In the light of the results quoted above and given that protein synthesis is needed for plant PCD to occur (Solomon et al., 1999; Clarke et al., 2000), the abilities of two ROS scavengers (ASC and SOD) and of inhibitors of transcription (actinomycin D [Act D]) and translation (cycloheximide [Chex]) to prevent PCD were compared. Both the scavengers and the inhibitors were added to the culture medium 30 min before heat shock, and cell viability was measured in 24-h PCD cells. In the absence of either scavengers or inhibitors, cell survival was about 50% compared with control cells (Fig. 7). About 90% and 80% survival was found for cells pre-incubated with ASC and SOD, respectively. A small but statistically significant (P < 0.01) prevention of cell death (65% cell survival) resulted from addition of either Act D or Chex used at concentrations that totally inhibit protein synthesis both in tobacco (Elbaz et al., 2002) and soybean (Glycine max; Solomon et al., 1999). In experiments not reported in detail, it was confirmed that none of the compounds used had any effect on viability of control cells.
Figure 7.
Effect of antioxidants and inhibitors of transcription and translation on PCD. Cell viability was measured 24 h after heat shock either in the absence (I) or presence of 0.4 mm ASC (I+ASC), 75 units mL-1 SOD (I+SOD), 50 μg mL-1 Chex (I+Chex), or 10 μg mL-1 ActD (I+ActD). Cell viability in control cells (C) was also measured. The percentage cell viability was calculated after counting a population of at least 1,000 cells; values represent mean (±se) of three experiments. The effects of the added compounds were statistically significant (P < 0.01) in all cases.
ASC Pool and cAPX in Heat Shock-Induced PCD
Because steady-state levels of ROS depend on the balance between ROS-producing and -scavenging reactions, we measured levels of both ASC and dehydroascorbate (DHA) and the activity of cAPX; the latter plays a major role in scavenging H2O2 in plants (Asada, 1992; Noctor and Foyer, 1998; Smirnoff, 2000; Shigeoka et al., 2002).
Results of measurements of the ASC and DHA levels are given in Table I. Interestingly, although the total ASC pool (ASC + DHA) remained constant at the control level during the first 6 h after induction of PCD, a 50% increase in concentration of DHA was found already at 0-h PCD. In 24-h PCD cells, the total ASC pool was decreased by 35% with respect to the control, with a further increase (up to 80%) of the percentage of oxidized ASC (DHA). No significant change in either the ASC+DHA pool or in its redox state was found in the control cells over the 24-h period.
Table I.
Time-dependent effect of heat-induced PCD on the ascorbate pool Levels of ASC and DHA were measured in control cells (C) and in cells collected immediately (I0) and 6 (I6) and 24 (I24) h after heat shock. Values represent mean ± se of five experiments. The redox state was calculated as the ratio ASC/(ASC + DHA), using mean values of the concentrations of the individual substances.
| Sample | ASC | DHA | ASC + DHA | Redox State |
|---|---|---|---|---|
| nmoles g-1 fresh wt | ||||
| C | 411 ± 26 | 102 ± 11 | 513 ± 37 | 0,8 |
| I0 | 319a ± 22 | 157a ± 16 | 476 ± 38 | 0.67a |
| I6 | 284a ± 25 | 219a ± 19 | 503 ± 44 | 0.56a |
| I24 | 145a ± 20 | 187a ± 16 | 332a ± 33 | 0.44a |
Indicates the values that are significantly different from control (P < 0.01)
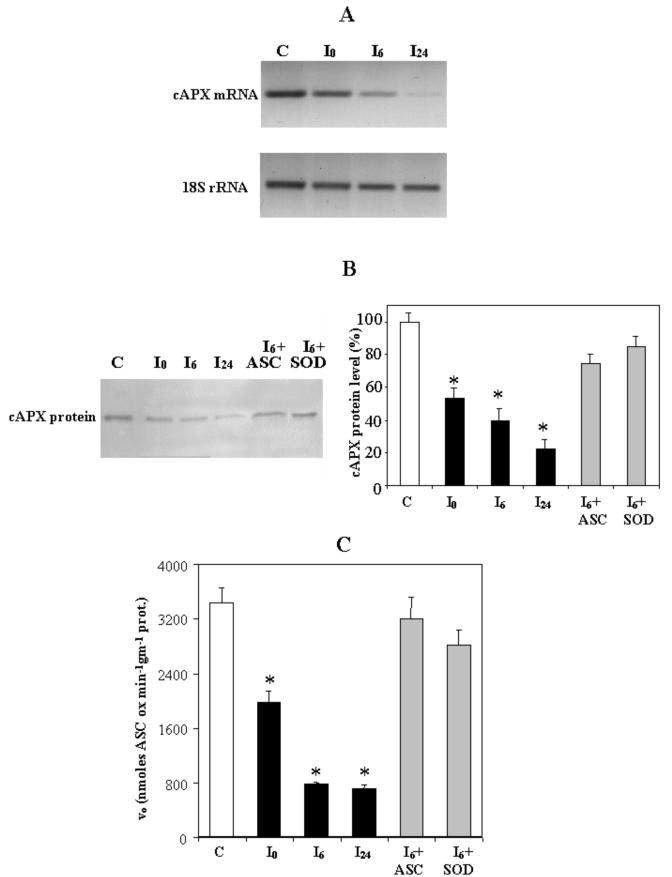
The role of cAPX during PCD was investigated by monitoring the expression of its gene and the amount of cAPX protein present in the cells and by measuring the rate of the enzyme reaction (Fig. 8). Gene expression was analyzed by semiquantitative RT-PCR using a specific primer for cAPX. In 0-h PCD cells, gene expression was found to only have a weak decrease; however, its expression clearly decreased after 6 h and was negligible in 24-h PCD cells (Fig. 8A).
Figure 8.
Levels of cAPX mRNA and protein in PCD cells and the effects of heat-dependent PCD induction on specific activity of cAPX. Cells were collected immediately (I0), 6 h (I6), and 24 h (I24) after induction of PCD. C, Control cells. A, Total RNA was extracted, and semiquantitative reverse transcriptase (RT)-PCR for cAPX was performed as described in “Materials and Methods.” Levels of 18S rRNA were also determined so that results could be normalized for recovery of RNA from the initial samples. B, Immunoblotting analysis performed using a specific cAPX antibody (see “Materials and Methods”). Protein extracts (15 μg) were loaded in each line. Where indicated, 0.4 mm ASC and 75 units mL-1 SOD were added to the cells before heat shock. After densitometry, levels of cAPX protein were calculated as a percentage of that in control cells. Values represent the mean (±se) of three experiments. C, Specific activity of cAPX. Where indicated, 0.4 mm ASC and 75 units mL-1 SOD were added to the cells before heat shock. Values represent the mean (±se) of five experiments. An asterisk indicates the values that are significantly different from control (P < 0.01).
Levels of cAPX protein were measured using immunoblotting with a specific monoclonal antibody (Fig. 8B). In 0-h PCD cells, the amount of cAPX protein was about 50% of that in control cells and decreased by about 80% in 24-h PCD cells. These decreases were largely prevented by addition of either ASC or SOD.
Because changes in gene expression do not necessarily reflect changes in enzyme catalysis, the activity of cAPX was also measured in both control and PCD cells. A reduction of more than 40% was found in the cAPX activity in 0-h PCD cells (Fig. 8C). At 6 h, the reduction reached 80%, after which the cAPX activity remained constant. On the other hand, when TBY-2 cells were pretreated with either ASC or SOD, no significant decrease in the enzyme activity was found as a result of heat shock (Fig. 8C).
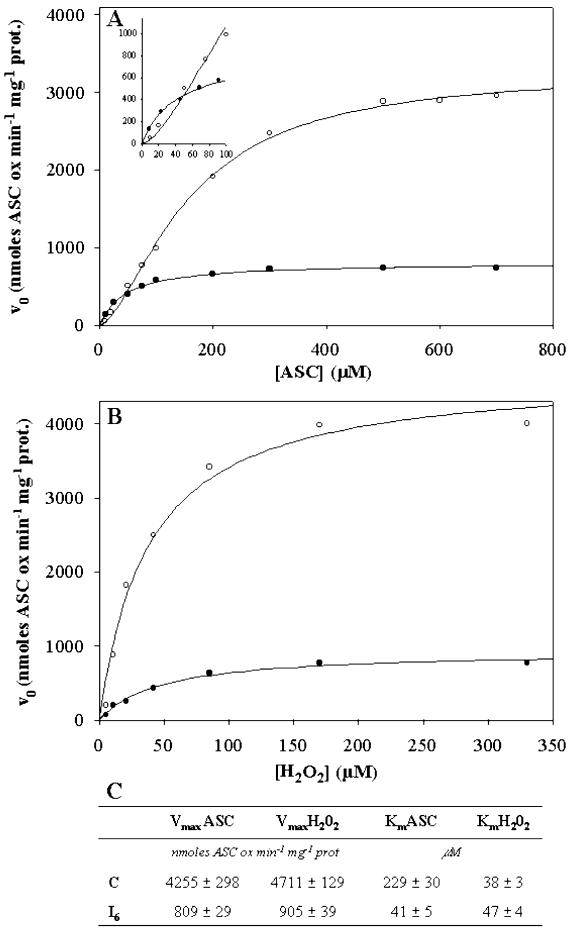
To check whether the kinetics of cAPX were modified as a result of heat shock, the dependence of the reaction rate on ASC (Fig. 9A) or H2O2 (Fig. 9B) concentrations was investigated at a fixed concentration of the second substrate. At a fixed concentration of H2O2 (Fig. 9A), a sigmoidal dependence of rate on ASC concentration was found for control cells showing the occurrence of cooperativity. The Hill coefficient was found to be between 1.4 and 1.6 in four different experiments. On the other hand, in PCD cells, the dependence of rate on ASC concentration was hyperbolic. As expected in light of the results in Figure 8, a very large reduction in the Vmax was found for the enzyme in PCD cells (about 5-fold). Similarly, Km for ASC was reduced 5-fold compared with K0.5 (i.e. the substrate concentration that gives one-half maximum rate). A hyperbolic dependence of rate on H2O2 concentration at a fixed level of ASC was found for the enzyme from both control and heat-shocked cells (Fig. 9B). The enzyme from shocked cells showed a decrease in the Vmax (again about 5-fold) compared with that from control cells, but the Km values were essentially the same.
Figure 9.
Kinetic behavior of cAPX in control and PCD cells. The analysis was performed in extracts from control cells (○) and from cells 6 h after the heat shock (•). A, Concentration of H2O2 was 170 μm, and the concentration of ASC was varied over the range 10 to 700 μm. B, Concentration of ASC was 500 μm, and the concentration of H2O2 was varied over the range 5.5 to 330 μm. C, Vmax and Km values for ASC and H2O2 in control and heat-shocked cells. Values represent the mean (±se) of five experiments.
Energy Metabolism in PCD Cells
The protection of cells against PCD by antioxidants posed the question as to whether impairment of cell oxidative metabolism, involving mitochondria, occurs together with induction of PCD. Thus, oxygen consumption by the cell suspension arising from addition of glucose (Glc) (10 mm) was measured (Fig. 10). In a typical experiment, in which control and 4-h PCD cells were used, the oxygen content of the cell suspensions incubated in the absence of Glc remained constant, as expected because endogenous respiratory substrates had been oxidized previously (see “Materials and Methods”). When Glc was added, the oxygen content decreased at rates of 22 and 11 natoms min-1 mg-1 cell protein in control and 4-h PCD cells, respectively. In both cases, O2 consumption was largely inhibited by 1 mm cyanide (KCN), a powerful inhibitor of mitochondrial cytochrome c oxidase. Residual oxygen consumption (6 and 3 natoms min-1 mg-1 cell protein in control and 4-h PCD cells, respectively) was observed after addition of KCN, probably because of the cyanide-insensitive alternative oxidase activity (Vanlerberghe et al., 1995). The rate of oxygen consumption measured as a function of the time after heat shock is shown in Figure 10B. In distinction to the control cells, a continuous decrease in this rate was observed over 0 to 6 h of PCD. In heat-shocked cells, viability decreased more slowly than did oxidative capacity, and at 2 h, viability was still about 100%, whereas 30% inhibition of Glc oxidation had already occurred (Fig. 10B). In a parallel experiment, we found no impairment of oxidative metabolism when TBY-2 cells were incubated with the antioxidants ASC and SOD, whereas partial prevention (60%) occurred in the presence of ActD or Chex (Fig. 10C). None of the compounds used had any effect on the rate of Glc oxidation in control cells (not shown).
Figure 10.
Oxidation of Glc in PCD-induced cells. A, Control and 4-h PCD cells (0.2 mg of protein) were incubated at 25°C in 1.5 mL of the medium reported in “Materials and Methods”. At the arrows, Glc (10 mm) and KCN (1 mm) were added. The rate of oxygen uptake is expressed as nanoatoms of oxygen per minute per milligram of cell protein. B, Consumption of O2 was monitored polarographically in 0- to 6-h PCD cells, as reported in Figure 10A. The rate of Glc-dependent oxygen consumption and the viability of both control (white symbols) and PCD (black symbols) cells were measured as a function of time and expressed as a percentage of the control. C, Glc-dependent rate of oxygen consumption was measured 4 h after heat shock in the absence (I) or in the presence of 0.4 mm ASC (I+ASC), 75 units mL-1 SOD (I+SOD), 50 μg mL-1 Chex (I+Chex), or 10 μg mL-1 ActD (I+ActD). The rate of O2 consumption measured in control cells (C) was set at 100. For B and C, values represent the mean (±se) of three experiments.
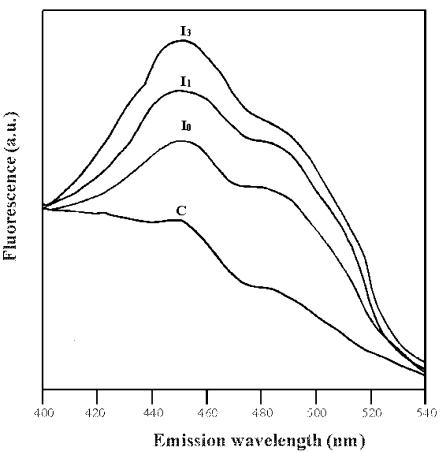
To confirm that impairment of Glc oxidation involved intracellular metabolism, a further experiment was carried out under the same experimental conditions, in which the fluorescence emission spectra of both control and PCD cells were measured using a wavelength pair designed to monitor the intracellular NAD(P)/NAD(P)H ox/redox state as described by Atlante et al., 1999 (Fig. 11). PCD, but not control cells, showed an increase in fluorescence emission close to a wavelength of 456 nm, arising from an increase in intracellular NAD(P)H concentration. The effect increased with time after heat shock. Pretreatment with ASC and SOD was found to prevent the increase in intracellular NAD(P)H fluorescence (data not shown).
Figure 11.
Fluorescence emission spectra of control and PCD cells. Fluorescence emission spectra (λexc=334; emission: 400 < λ < 540) of TBY-2 cells (0.2 mg protein) were measured immediately (I0), 1 h (I1), and 3 h (I3) after the heat shock and compared with the control (C). The experiment was repeated four times with different cell preparations, giving values with 5% to 10% variation. Fluorescence intensity is reported in arbitrary units (a.u.).
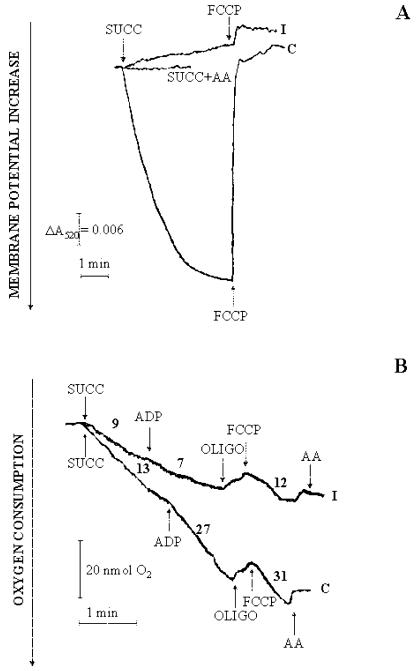
Irrespective of the mechanism by which cytosolic NADH oxidation takes place, i.e. via the malate/oxaloacetate shuttle as in durum wheat (Triticum durum) mitochondria (Pastore et al., 2003) or via other shuttles and/or an external NADH dehydrogenase, the increase of fluorescence in Figure 11 suggests that the mitochondria were somehow impaired. As a consequence, mitochondria were isolated from both control and 4-h PCD cells and assessed with respect to their ability to generate a membrane potential (ΔΨ); this was monitored by changes in absorbance of safranin (Fig. 12A). In control mitochondria, ΔΨ generation after addition of succinate (5 mm) was revealed by the decrease in absorbance of safranin. Generation of ΔΨ was found to be prevented by antimycin and was abolished by the uncoupler carbonyl cyanide p-trifluoromethoxyphenyl-hydrazone (FCCP). In mitochondria from 4-h PCD cells, addition of succinate failed to generate a membrane potential and showed low uncoupling by FCCP (Fig. 12A). In a parallel experiment (Fig. 12B), mitochondria isolated from control and 4-h PCD cells were compared with respect to their capability to oxidize succinate in a manner controlled by ADP. In control mitochondria, measurements of oxygen uptake made in both states 4 and 3 in the presence of 0.5 mm ADP exhibited a control respiratory index (the ratio between the rate of oxygen uptake in the presence or absence of ADP) equal to about 2. Oligomycin inhibited oxygen uptake completely. The uptake was restored by addition of the uncoupler FCCP but with a further strong inhibition arising from addition of antimycin. In contrast, mitochondria isolated from PCD cells showed a decrease in state 4 respiration; more importantly, they were insensitive to addition of ADP but not to FCCP, which caused oxygen uptake at a rate equal to 12 nmol min-1mg-1 protein. Oxygen uptake was inhibited by antimycin (Fig. 12B). The experiments reported in Figure 12 were repeated three times, with similar results in each case.
Figure 12.
Generation of ΔΨ and oxygen uptake by mitochondria isolated from control and PCD cells arising from externally added succinate. A, Mitochondria isolated from control and 4-h PCD cells (0.5 mg of protein) were incubated at 25°C in 2 mL of the medium reported in “Materials and Methods” with continuous measurement of the safranin O A520. At the arrows, succinate (5 mm) and FCCP (1.25 μm) were added. When present, antimycin (AA; 2 μg) was added 1 min before succinate addition. B, Mitochondria from control and 4-h PCD cells (0.5 mg protein) were incubated at 25°C in 1.5 mL of the respiratory medium reported in “Materials and Methods,” and oxygen uptake was measured polarographically as a function of time. At the arrows, 5 mm succinate, 0.5 mm ADP, 2.5 μm oligomycin (OLIGO), 1.25 μm FCCP, and 2 μg AA were added. The rate of oxygen consumption is expressed as nanomoles of O2 per minute per milligram of mitochondrial protein.
DISCUSSION
Heat Shock Can Induce PCD in TBY-2 Cells
Plant cells respond to a variety of externally added inducers by initiating PCD (McCabe and Leaver, 2000). However, study of the role played by cell organelles in the PCD pathway is complicated by the fact that interactions between the inducer and cell organelles could occur, resulting in effects not related to PCD. We tried to overcome this complication by inducing PCD in TBY-2 cells using heat shock, as already done with other plant cells including those from carrot (Daucus carota), Arabidopsis (McCabe and Leaver, 2000), and cucumber (Cucumis sativus; Balk et al., 1999). Here, we show that TBY-2 cells, when heat shocked at 55°C, undergo PCD as judged by the common criteria of cytoplasm shrinkage, chromatin condensation, and DNA laddering (Figs. 2 and 3). This provides a model for investigation of PCD-related processes and, in particular, of the possible roles played by cellular constituents and organelles in the early phase of PCD, i.e. when the viability of the cells undergoing PCD was almost unaffected.
ROS Production in Heat Shock PCD
We show that PCD cells produce dramatically increased levels of ROS. This phenomenon has been reported already for cells subject both to pathogen attack or to abiotic stress (Lamb and Dixon, 1997; Mittler, 2002; Yao et al., 2002), but the novelty of our results is that ROS generation was induced without the addition of exogenous compounds that could, per se, alter the cellular redox homeostasis. That an increase in ROS is a prerequisite for heat-induced PCD was shown by the fact that the cells survived if the ROS scavengers ASC and SOD were present during heat shock. The results in Figures 4 to 7 imply that both H2O2 and O2-. are required for triggering the process of PCD; removal of either of them alone allows the cells to resist the challenge of heat shock. In the case of SOD, protection was exerted presumably by causing efflux of intracellular superoxide.
At present, the temporal connection between ROS production and the qualitative connection between H2O2 and superoxide must remain a matter of speculation as the ROS potential source is. Even though mitochondria are considered the main intracellular source of ROS, we cannot suggest them as responsible for the ROS production because of heat shock; this point merits further investigation.
Because partial prevention of PCD was also found when inhibitors of transcription and translation were present, we conclude that PCD in TBY-2 cells triggered by ROS is reinforced by modifications in cellular protein biosynthesis (see also Yao et al., 2002). The finding that cell death and the accompanying impairment of Glc oxidation and cAPX expression were completely prevented by antioxidants, but only partially by ActD or Chex, supports the view that the primary events of PCD are triggered by ROS, with biosynthetic events occurring downstream.
It should be noted that in the cascade of events leading to cell death, the cellular level of ROS is critical. A threshold level of ROS is required to activate the signal transduction pathway that results in PCD, but at high doses, the process is subverted and death occurs rapidly by necrosis (Desikan et al., 1998). Consistently, we show that necrotic cell death occurs as a result of heat shock at temperatures higher than 55°C and is accompanied by a much higher production of H2O2 than that in cells undergoing PCD; this is similar to the situation in mammals (Fleury et al., 2002; Atlante et al., 2003a).
Decrease in the ASC Pool and Early Impairment of cAPX in PCD
The level of ROS in a cell derives from a balance between ROS-producing and -scavenging systems. As a consequence, reduced efficiency of ROS scavenging by metabolites and enzymes would be expected to play a major role in induction of PCD. Our results clearly show the importance of ASC in heat-induced PCD. Heat shock resulted in an increase in ASC oxidation, which started immediately after heat-induced PCD and continued over time (Table I). We also show that a change in the activity of cAPX is an early event in the process, with a 50% decrease in activity occurring immediately after induction of PCD. This is consistent with a role for the enzyme in maintaining ROS below threshold levels in non-PCD cells.
The regulation of the activity of cAPX is complex. Although there was a decrease in the amount of mRNA for cAPX in PCD cells (Fig. 8A), which was mirrored by a decrease in amount of cAPX protein present (Fig. 8B), the two phenomena are not necessarily related. The loss of protein was very substantially reduced by the presence of ROS scavengers, which suggests that it may result from oxidative damage and subsequent removal of damaged protein. This is consistent with the reported posttranscriptional suppression of cAPX in hypersensitive PCD, where cAPX gene expression is increased over normal levels (Mittler et al., 1998).
In addition to the effect on amount of enzyme present, we also show that its kinetic properties change in response to PCD (Fig. 9). The enzyme from control cells showed a sigmoidal dependence on ASC concentration, as has been reported already for cAPX purified from several sources (Lad et al., 2002, and refs. therein). In contrast, the enzyme from PCD cells showed a hyperbolic dependence on ASC concentration. This strongly suggests that the very large decrease in the Vmax for the reaction in PCD cells arises not only from a decrease in the amount of enzyme protein but also from modifications involving substrate-enzyme interaction. It is known that cAPX from other sources possesses two ASC-binding sites (Lad et al., 2002); therefore, the loss of cooperativity in the enzyme from PCD cells suggests that the modification results in loss of one of these sites.
As far as H2O2 is concerned, cAPX from both control and PCD cells showed saturation characteristics, with the latter having decreased Vmax but an unchanged Km; that is, there is a noncompetitive type of inhibition. In this case, we conclude that the processes resulting in modification of cAPX in PCD cells do not involve the H2O2 binding site interaction but cause damage to other parts of the enzyme protein. It should be noted that inactivation of purified cAPX because of H2O2 has been reported already, but this was under conditions of complete ASC depletion (Hiner et al., 2000).
Impairment of Energy Metabolism in PCD
We investigated certain aspects of cellular energy metabolism that might be assumed to play a key role in PCD given that the involvement of mitochondria in oxidative stress (Jones, 2000; Tiwari et al., 2002), cytochrome c release (Balk et al., 1999), and PCD in the hypersensitive response (Lam et al., 2001) has been proposed already. We show here that Glc oxidation is impaired during PCD in a manner completely prevented by ROS scavengers (Fig. 10). It is important to note that oxidation of Glc was already impaired under conditions where cell viability was unaffected; that is, the impairment of energy metabolism is a process occurring in the early phase of PCD, when the cells are still viable. In this regard, plants resemble mammalian cells (Atlante et al., 1996; 1998). Recently, cryptogein has been shown to inhibit Glc transport in tobacco cells (Bourque et al., 2002); whether this occurs in our cells remains to be established. That notwithstanding, the increase in concentration of cellular NAD(P)H in PCD cells (Fig. 11) shows both that the cells can take up Glc and that mitochondrial NAD(P)H oxidation is impaired in PCD. Consistently, we found that mitochondria isolated from 4-h PCD cells were unable to generate a membrane potential or to increase oxygen consumption in state 3 respiration (Fig. 12). Because mitochondrial coupling appeared to be completely impaired when cell viability was almost unaffected, we conclude that mitochondrial damage occurs in the early phase of PCD.
The picture emerging from this work is, then, as follows. After heat shock, as a result of the immediate production of ROS, the death program starts and evokes biosynthetic processes, imbalance in ASC-dependent H2O2 scavenging, and impairment of oxidative mitochondrial metabolism as early events. In addition to genetic control, changes in enzyme kinetics are suggested to contribute to regulation of PCD, thus integrating the regulatory mechanisms acting at transcriptional and posttranscriptional levels.
MATERIALS AND METHODS
Cell Culture, Growth Conditions, and Heat Treatments
The suspension of tobacco (Nicotiana tabacum L. cv Bright-Yellow 2) cells was routinely propagated and cultured at 27°C according to Nagata et al. (1992). In this work, a stationary culture was diluted 4:100 (v/v) and cultured for 4 d. Heat shock at 55°C was induced by transferring the cell suspension into a water bath at 55°C with a heating time of either 5 or 10 min depending on the cell suspension volume (20 or 100 mL, respectively). After heat shock, the cells were returned to 27°C. Where indicated, ASC (0.4 mm), SOD (75 units mL-1), Chex (50 μg mL-1), or Act D (10 μg mL-1) were added to the culture medium 30 min before heat shock. At the indicated times, aliquots of cells were collected by filtration on Whatman 3MM paper (Whatman, Clifton, NJ) for analysis. Heat treatments at the other temperatures (37°C, 45°C, 60°C, and 65°C) were carried out under the same experimental conditions. Assay of cell protein was done according to Bradford (1976).
Cell and Nuclear Morphology
Cell viability was measured using trypan blue staining as described by de Pinto et al. (1999), and cell morphology was investigated by using a phase contrast light microscope.
For the analysis of nuclear morphology, TBY-2 cells were stained with 4,6-diamidino-2-phenylindole (DAPI) as in de Pinto et al. (2002) and visualized using a fluorescence microscope (DMLS, Leica, Wetzlar, Germany) with an excitation filter of 340 to 380 nm and a barrier filter of 400 nm.
DNA Fragmentation Analysis
At time intervals over a 72-h period after the heat shock, cells were collected from cell suspension and homogenized in liquid nitrogen. DNA was extracted by the cetyl-trimethyl-ammonium bromide method (Murray and Thompson, 1980). DNA samples were digested with 100 μg mL-1 DNAse-free RNAse for 1 h at 37°C, and DNA fragments were separated by electrophoresis on a 1.8% (w/v) agarose gel, followed by visualization by staining with ethidium bromide.
Measurement of H2O2
Intracellular H2O2 production was measured using DHR123 (Sigma-Aldrich Italia, Milan) as a probe (Royall and Ischiropoulos, 1993). The cells were stained for 5 min with 20 μm DHR123 and then viewed under a fluorescence microscope (DMLS, Leica) with an excitation filter of 450 to 490 nm and a barrier filter of 510 nm.
H2O2 in the extracellular phase was measured according to Bellincampi et al. (2000). In brief, 1 mL of cell cultures was harvested by centrifugation (10,000g, 20 s, 25°C), and H2O2 concentration was measured in the supernatant. An aliquot of supernatant (500 μL) was added to 500 μL of assay reagent (500 μm ferrous ammonium sulfate, 50 mm H2SO4, 200 μm xylenol orange, and 200 mm sorbitol). After 45 min of incubation the peroxide-mediated oxidation of Fe+ to Fe3+ was determined by measuring the A560 of the Fe3+-xylenol orange complex.
Measurement of O2-·
The detection of O2-. in the extracellular phase was performed by using the nitroblue tetrazolium method (Murphy et al., 1998). In brief, 1 mL of cell cultures was harvested by centrifugation (10,000g, 20 s, 25°C), and O2-. concentration was measured in the supernatant by monitoring the reduction of nitroblue tetrazolium (100 μm) at 530 nm. SOD (100 units) was added in a duplicate cuvette to access the specificity of measured changes of absorbance on O2-. concentration. The amount of O2-. was then calculated using ε550 nm = 12.8 mm-1 cm-1 (Murphy et al., 1998).
Total RNA Extraction and Semiquantitative RT-PCR
Total RNA was isolated from TBY-2 cells using the RNeasy plant minikit (QUIAGEN S.p.A., Milan) according to the supplier's recommendation. Residual DNA was removed from the RNA samples using a DNA-free kit (Ambion, Inc., Austin, TX). Synthesis of cDNA was performed from 2 μg of total RNA with 10 μm random primers (Amersham Biosciences Europe GMBH, Milan), utilizing an Omniscript Reverse Transcriptase kit (QUIAGEN S.p.A.) according to the supplier's recommendation. PCR reactions were performed with specific primers for cAPX (cAPX, D85912, 5′-CACTGTAAGCGAGGAGTACC-3′ and 3′-TGAGCCTCAGCATAGTCAGC-5′) and 18S rRNA (18S, AJ236016, 5′-CATGATAACTCGACGGATCG-3′ and 3′-GAAGGCCAACGTAATAGGAC-5′). 18S rRNA was used as an internal control to normalize each sample for variations in the amount of initial RNA. The PCR mix contained 2 μL of the template, 0.2 mm dNTPs, 0.5 mm primers (forward and reverse), 1.5 units of Taq polymerase (Amersham Biosciences Europe GMBH), and 1× PCR buffer (supplied from Amersham with Taq polymerase) in a final volume of 50 μL. PCR was carried out in a programmable Primus Thermal Cycler (MWG-AG Biotech, Ebersberg, Germany) at an annealing temperature of 55°C. For semiquantitative RT-PCR, the cycle number in the linear range was empirically determined. The products of PCR amplification produced a single band at the predicted sizes of 699 and 594 bp for cAPX and 18S, respectively. These were analyzed on 1.5% (w/v) agarose gel containing 0.5 μg mL-1 ethidium bromide.
Immunoblot Analysis
Cells were ground in liquid nitrogen and homogenized at 4°C in extraction buffer (50 mm Tris-HCl [pH 7.5], 0.05% [w/v] Cys, and 0.1% [w/v] bovine serum albumin). The homogenate was centrifuged at 20,000g for 15 min, and the supernatant was electrophoresed on 12.5% (w/v) SDS-polyacrylamide gel under reduction conditions. Immunoblotting was carried out using anti-cAPX monoclonal antibody (AP6 from Saji et al., 1990) and with goat anti-mouse IgG conjugated with alkaline phosphatase (Promega, Madison, WI) as previously described by de Pinto et al. (2002). Densitometry values for immunoreactive bands were quantified using a GS-700 Imaging Densitometer (Bio-Rad Laboratories, Hercules, CA); multiple expressions of the same immunoblots in a linear range were performed. cAPX protein levels were calculated as a percentage of the control taken as 100 in arbitrary units. The variability of protein levels in the control cells, measured by comparing the percentage of protein level from different control samples on the same filter, was always less than 5%.
Activity and Kinetics of cAPX
Cells were homogenized and centrifuged as reported by de Pinto et al. (2002). The supernatant was used for analysis of cAPX activity. The absence of ASC in the extraction buffer made it possible to determine the cytosolic isoenzyme alone (Shigeoka et al., 2002). The reaction catalyzed by cAPX (l-ASC; H2O2 oxidoreductase; EC 1.11.1.11), was determined by following the H2O2-dependent oxidation of ASC as decrease in A290 in a reaction mixture containing 0.1 m phosphate buffer (pH 6.4), 500 μm ASC, 170 μm H2O2, and 50 μg of protein. The extinction coefficient (ε) was found to be 2.8 mm-1 cm-1. The rate of the reaction, measured as a tangent to the initial part of the progress curve, was corrected for spontaneous ASC oxidation measured in the absence of H2O2 and for nonenzymatic ASC oxidation by H2O2 and expressed in nanomoles ASC oxidized per minute per milligram of protein. The values for Vmax and Km were obtained by either Michaelis-Menten or Hill analysis using Sigma Plot software (SPSS Inc., Chicago).
ASC Assay
Cells (0.5–1 g) were homogenized in two volumes of cold 5% (w/v) meta-phosphoric acid at 4°C in a porcelain mortar. The homogenate was centrifuged at 20,000g for 15 min at 4°C, and the supernatant was collected for analysis of ASC. ASC content and redox state were measured as described by de Pinto et al. (1999).
Measurement of Oxygen Uptake by TBY-2 Cells
One milliliter of either control or PCD cell suspension was taken at the indicated times and centrifuged at 360g for 5 min at 25°C; the resulting pellet was washed and suspended in 1 mL of plant salt mixture (PSM) (4.3 g L-1 Murashige and Skoog plant salt mix [ICN Biomedicals Inc., Aurora, OH], and 0.4 mm KH2PO4 [pH 5.8]). The cells (0.2 mg of protein) were then incubated at 25°C in 1.5 mL of 0.5 m Tris-acetate (pH 6.5), and oxygen uptake was started by adding 10 mm Glc to the cell suspension. Oxygen consumption was measured by means of a Gilson 5/6 oxygraph (Gilson Medical Electronics Inc., Middletown, WI) using a Clark electrode according to Atlante et al. (1996). The rate of oxygen consumption was obtained as a tangent to the initial part of the progress curve and expressed as nanoatoms of oxygen per minute per milligram of protein.
Fluorescence Emission Spectra of TBY-2 Cells
Cell suspension (1 mL) was taken at the indicated times, centrifuged (360g, 5 min, 25°C), and the resulting pellets were washed and suspended in 1 mL of PSM. The cells (0.2 mg of protein) were then incubated at 25°C in 1.5 mL of PSM, and fluorescence emission spectra (excitation wavelength 334 nm, emission range 400–540 nm) were recorded using an LS50B Luminescence Spectrophotometer (Perkin-Elmer Applied Biosystems, Foster City, CA).
Isolation of Mitochondria from TBY-2 Cells and Measurement of Their Oxygen Uptake
Mitochondria were isolated by protoplast fractionation and lysis, followed by differential centrifugation essentially as described by de Pinto et al. (2000).
Oxygen uptake measurements were carried out at 25°C using a Gilson 5/6 oxygraph with a Clark electrode. Mitochondria (0.5 mg protein) were added to 1.5 mL of the respiration medium containing 210 mm mannitol, 70 mm sucrose (Suc), 20 mm TRIS-HCl, 5 mm potassium phosphate (pH 7.4), 3 mm MgCl2, and 5 mg mL-1 bovine serum albumin in the presence of 2 μg of rotenone. Succinate (5 mm) was used as a respiratory substrate, and either ADP (0.5 mm) or FCCP (1.25 μm) were added to induce state 3 respiration.
Assay of the Safranin Response
This was done at 25°C, essentially as in Pastore et al. (1999), by measuring absorbance changes at 520 nm using a Perkin-Elmer Applied Biosystems Lambda-5 spectrophotometer. Measurements were carried out at 25°C with 2 mL of standard medium consisting of 300 mm Suc, 10 mm KCl, 1 mm MgCl2, and 20 mm HEPES-Tris (pH 7.2) containing 10 μm safranin O and 1 mg of protein.
Statistics
The data are reported as the mean ± se for the indicated experiments. The statistical significance of differences between groups was determined by one-way ANOVA followed by a Student-Newman-Keuls test. Statistical differences between mean values of control and treated cells were determined with the Student's t test. All experiments were repeated at least three times.
Acknowledgments
The authors wish to thank Prof. Shawn Doonan for his critical reading and Mr. Riccardo S. Merafina for technical assistance. The authors thank Dr. Akihiro Kubo (Environmental Biology Division National Institute for Environmental studies, Onogawa, Japan) for kindly supplying ASC peroxidase antibody.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.035956.
This work was supported by the Italian Ministry of Instruction, University, and Research (MIVR) and by the Programmi di Ricerca di Rilevante Interesse Nazionale (“Bioenergetica: Aspetti Genetici, Biochimici e Fisiologici” to S.P. and “Specie Reattive Dell'Ossigeno e Sistemi Antiossidanti Nella Morte Cellulare Programmata e Nell'Interazione Pianta-Patogeno” to L.D.G.).
References
- Asada K (1992) Ascorbate peroxidase: a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 85: 235-241 [Google Scholar]
- Atlante A, Bobba A, Calissano P, Passarella S, Marra E (2003a) The apoptosis/necrosis transition in cerebellar granule cells depends on the mutual relationship of the antioxidant and the proteolytic systems which regulate ROS production and cytochrome c release en route to death. J Neurochem 84: 960-971 [DOI] [PubMed] [Google Scholar]
- Atlante A, de Bari L, Bobba A, Marra E, Calissano P, Passarella S (2003b) Cytochrome c, released from cerebellar granule cells undergoing apoptosis or excytotoxic death, can generate protonmotive force and drive ATP synthesis in isolated mitochondria. J Neurochem 86: 591-604 [DOI] [PubMed] [Google Scholar]
- Atlante A, Gagliardi S, Marra E, Calissano P (1998) Neuronal apoptosis in rats is accompanied by rapid impairment of cellular respiration and is prevented by scavengers of reactive oxygen species. Neurosci Lett 245: 127-130 [DOI] [PubMed] [Google Scholar]
- Atlante A, Gagliardi S, Marra E, Calissano P, Passarella S (1999) Glutamate neurotoxicity in rat cerebellar granule cells involves cytochrome c release from mitochondria and mitochondrial shuttle impairment. J Neurochem 73: 237-246 [DOI] [PubMed] [Google Scholar]
- Atlante A, Gagliardi S, Minervini GM, Marra E, Passarella S, Calissano P (1996) Rapid uncoupling of oxidative phosporylation accompanies glutamate toxicity in rat cerebellar granule cells. Neuroreport 7: 2519-2523 [DOI] [PubMed] [Google Scholar]
- Balk J, Leaver CJ, McCabe PF (1999) Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Lett 463: 151-154 [DOI] [PubMed] [Google Scholar]
- Beers EP, McDowell JM (2001) Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr Opin Plant Biol 4: 561-567 [DOI] [PubMed] [Google Scholar]
- Bellincampi D, Dipierro N, Salvi G, Cervone F, De Lorenzo G (2000) Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxine-regulated rolB gene expression in tobacco leaf explants. Plant Physiol 122: 1379-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque S, Lemoine R, Sequeira-Legrand A, Fayolle L, Delrot S, Pugin A (2002) The elicitor cryptogein blocks glucose transport in tobacco cells. Plant Physiol 130: 2177-2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254 [DOI] [PubMed] [Google Scholar]
- Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ (2000) NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J 24: 667-677 [DOI] [PubMed] [Google Scholar]
- Dat JF, Pellinen R, Van De Cotte B, Langebartels C, Kangasjarvi J, Inzé D, Van Breusegem F (2003) Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J 33: 621-632 [DOI] [PubMed] [Google Scholar]
- De Gara L (2003) Ascorbate metabolisms and plant growth: from germination to cell death. In Asard H, Smirnoff J, May N, eds, Vitamin C: Its Function and Biochemistry in Animals and Plants. Bios Scientific Publisher Ltd., Oxford, pp 83-95
- De Gara L, de Pinto MC, Tommasi F (2003) The antioxidant systems vis à vis reactive oxygen species during plant-pathogen interaction. Plant Physiol Biochem 41: 863-870 [Google Scholar]
- de Pinto MC, Francis D, De Gara L (1999) The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209: 90-97 [DOI] [PubMed] [Google Scholar]
- de Pinto MC, Tommasi F, De Gara L (2000) Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tabacco Bright Yellow 2. Plant Physiol Biochem 38: 541-550 [Google Scholar]
- de Pinto MC, Tommasi F, De Gara L (2002) Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco Bright-Yellow 2 cells. Plant Physiol 130: 698-708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Reynolds A, Hancock JT, Neill SJ (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J 330: 115-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cagno R, Guidi L, De Gara L, Soldatini GF (2001) Combined cadmium and ozone treatments affect photosynthesis and ascorbate-dependent defences in sunflower. New Phytol 151: 627-636 [DOI] [PubMed] [Google Scholar]
- Elbaz M, Avni A, Weil M (2002) Constitutive caspase-like machinery executes programmed cell death in plant cells. Cell Death Differ 9: 726-733 [DOI] [PubMed] [Google Scholar]
- Fath A, Bethke P, Beligni V, Jones R (2002) Active oxygen and cell death in cereal aleurone cells. J Exp Bot 53: 1273-1282 [PubMed] [Google Scholar]
- Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84: 131-141 [DOI] [PubMed] [Google Scholar]
- Fukuda H (2000) Programmed cell death of tracheary elements as a paradigm in plants. Plant Mol Biol 44: 245-253 [DOI] [PubMed] [Google Scholar]
- Hiner ANP, Rodriguez-Lopez JN, Arnao MB, Raven EL, Garcia-Canovas F, Acosta M (2000) Kinetic study of the inactivation of ascorbate peroxidase by hydrogen peroxide. Biochem J 348: 321-328 [PMC free article] [PubMed] [Google Scholar]
- Jones A (2000) Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trends Plant Sci 5: 225-230 [DOI] [PubMed] [Google Scholar]
- Kim M, Ahn JW, Jin UH, Chai D, Poek KH, Pai HS (2003) Activation of the programmed cell death pathway by inhibition of proteasome function in plant. J Biol Chem 278: 19406-19415 [DOI] [PubMed] [Google Scholar]
- Koukalovà B, Kovarik A, Fajkus J, Siroky J (1997) Chromatin fragmentation associated with apoptotic changes in tobacco cells exposed to cold stress. FEBS Lett 414: 289-292 [DOI] [PubMed] [Google Scholar]
- Lad L, Mewies M, Raven EL (2002) Substrate binding and catalytic mechanism in ascorbate peroxidase: evidence for two ascorbate binding sites. Biochemistry 41: 13774-13781 [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411: 848-853 [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251-275 [DOI] [PubMed] [Google Scholar]
- McCabe PF, Leaver CJ (2000) Programmed cell death in cell cultures. Plant Mol Biol 44: 359-368 [DOI] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405-410 [DOI] [PubMed] [Google Scholar]
- Mittler R, Feng X, Cohen M (1998) Post-transcriptional suppression of cytosolic ascorbate peroxidase expression during phatogen-induced programmed cell death in tobacco. Plant Cell 10: 461-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TM, Vu H, Nguyen T (1998) The superoxide synthases of rose cells: comparison of assays. Plant Physiol 117: 1301-1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8: 4321-4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol 132: 1-30 [Google Scholar]
- Noctor G, Foyer CH (1998)Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol 49: 249-279 [DOI] [PubMed] [Google Scholar]
- Pastore D, Stoppelli MC, Di Fonzo N, Passarella S (1999) The existence of the K(+) channel in plant mitochondria. J Biol Chem 274: 26683-26690 [DOI] [PubMed] [Google Scholar]
- Pastore D, Fratianni A, Di Pede S, Passarella S (2000) Effects of fatty acids, nucleotides and reactive oxygen species on durum wheat mitochondria. FEBS Lett 470: 88-92 [DOI] [PubMed] [Google Scholar]
- Pastore D, Di Pede S, Passarella S (2003) Isolated durum wheat and potato cell mitochondria oxidize externally added NADH mostly via the malate/oxaloacetate shuttle, with a rate that depends on the carrier mediated transport. Plant Physiol 133: 2029-2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Davis KR (2001) The physiology of ozone induced cell death. Planta 213: 682-690 [DOI] [PubMed] [Google Scholar]
- Royall JA, Ischiropoulos H (1993) Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys 302: 348-355 [DOI] [PubMed] [Google Scholar]
- Saji H, Tanaka K, Kondo N (1990) Monoclonal antibodies to spinach ascorbate peroxidase and immunochemical detection of the enzyme in eight different plant species. Plant Sci 69: 1-9 [Google Scholar]
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53: 1305-1319 [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multifacetted molecule. Curr Opin Plant Biol 3: 229-235 [PubMed] [Google Scholar]
- Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A (1999) The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11: 431-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhao Y, Hong X, Zhai Z (1999) Cytochrome c release and caspase activation during menadione-induced apoptosis in plants. FEBS Lett 462: 317-321 [DOI] [PubMed] [Google Scholar]
- Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128: 1271-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Day DA, Wiskich JT, Vanlerberghe AE, McIntosh L (1995) Alternative oxidase activity in tobacco leaf mitochondria (dependence on tricarboxylic acid cycle-mediated redox regulation and pyruvate activation). Plant Physiol 109: 353-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranova E, Inze D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53: 1227-1236 [PubMed] [Google Scholar]
- Weaver LM, Gan S, Quirino B, Amasino MR (1998) A comparison of the expression patterns of several senescence associated genes in response to stress and hormone treatment. Plant Mol Biol 37: 455-469 [DOI] [PubMed] [Google Scholar]
- Wu HM, Cheun AY (2000) Programmed cell death in plant reproduction. Plant Mol Biol 44: 267-281 [DOI] [PubMed] [Google Scholar]
- Yao N, Tada Y, Sakamoto M, Nakayashiki H, Park P, Tosa Y, Mayama S (2002) Mitochondrial oxidative burst involved in apoptotic response in oats. Plant J 30: 567-579 [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR (2000) Programmed cell death during endosperm development. Plant Mol Biol 44: 283-301 [DOI] [PubMed] [Google Scholar]
- Yu XH, Perdue TD, Heimer YM, Jones AM (2002) Mitochondrial involvement in tracheary element programmed cell death. Cell Death Differ 9: 189-198 [DOI] [PubMed] [Google Scholar]