Summary
Background and objectives
Gastric emptying (GE) may be delayed or rapid in diabetes mellitus. We sought to ascertain differences in risk factors or associated features (i.e. diabetic ‘phenotype’) among patients with diabetes who have rapid, slow or normal GE.
Methods
From a database of patients in whom gastrointestinal transit was assessed by scintigraphy, we compared the diabetic phenotype in diabetic patients with rapid, slow and normal GE.
Results
Of 129 patients, 55 (42%) had normal, 46 (36%) had delayed and 28 (22%) patients had rapid GE. In each GE category, there was an approximately equal number of type 1 and type 2 diabetes. In multivariable analyses, significant weight loss (OR, 2·81; 95% CI, 1·09–7·23) and neuropathy (OR, 3·60; 95% CI, 1·007–12·89) were the risk factors for delayed and rapid GE, respectively. Insulin therapy (OR, 0·08; 95% CI, 0·01–0·53) was associated with a lower risk of rapid compared to normal GE. However, other manifestations or characteristics of the diabetes ‘phenotype’ (i.e. type and duration of diabetes, glycosylated haemoglobin levels, and extraintestinal complications) were not useful for discriminating normal from delayed or rapid GE. At a specificity of 60%, clinical features were 73% sensitive for discriminating between normal and delayed GE and 80% sensitive for discriminating normal from rapid GE.
Conclusions
Diabetes is associated with slow and rapid GE. Because the diabetic phenotype is of limited utility for identifying disordered GE, GE should be assessed in patients with diabetes and gastrointestinal symptoms.
Background
It is widely recognized that patients with diabetes mellitus, particularly those with longstanding disease and its associated complications (e.g. the triopathy of retinopathy, nephropathy, and neuropathy, including autonomic neuropathy), may have gastrointestinal (GI) symptoms and abnormal GI transit, particularly delayed gastric emptying (GE).1 In relatively small, tertiary referral studies of patients with predominantly longstanding diabetes, 30–50% of patients with type 1 diabetes have delayed GE,2 while 28% of a cohort of diabetics (57% type 1, 43% type 2) undergoing outpatient evaluation had delayed GE.3 Since the original observations by Kassander, clinicians have relied on the diabetic manifestations or ‘phenotype’ (i.e. duration of diabetes and associated complications), symptoms (e.g. vomiting of undigested food eaten several hours previously and weight loss), and signs (e.g. a gastric succussion splash or features of an autonomic neuropathy) to assess the likelihood of delayed GE in patients with diabetes who are present with gastrointestinal symptoms.4,5 However, previous studies suggest that symptoms are of limited utility for predicting delayed GE in diabetes.3,6–11 For example, in one study abdominal bloating and fullness was the only one of six dyspeptic symptoms associated with delayed GE.11 In fact, delayed GE in diabetes may be asymptomatic, in part because of the afferent dysfunction associated with vagal denervation.12
It is also recognized that diabetes may be associated with rapid GE; this may also cause dyspeptic symptoms.13 In contrast to delayed GE which usually affects solids, rapid emptying of liquids has been mostly described in patients with ‘early’ type 2 diabetes without autonomic dysfunction.14–18 GE is also rapid 1–2 weeks after the onset of hyperglycaemia in diabetic NOD (non-obese diabetic) mice.19 From a clinical perspective, it is unclear whether the diabetic phenotype, a term which will be used to summarize the type, symptoms, signs and other complications of diabetes, can predict the nature of the GE disturbance in diabetes, because most of the previous studies were restricted either to patients with rapid or slow GE. In one study that included both patients with rapid and slow GE, delayed but not rapid GE in diabetes was associated with gastrointestinal symptoms and orthostatic hypotension.20
It is important to identify disordered GE in diabetes because such disturbances may not only cause gastrointestinal symptoms but may also impair glycaemic control. GE affects the delivery of nutrients to the small intestine, which in turn can affect glycaemic control due to a mismatch between the arrival of carbohydrates in the small bowel and insulin levels resulting in larger changes in blood glucose.21 Moreover, rapid GE may contribute to postprandial hypotension in type 2 diabetes.22 Therefore, in this study, our aims were: first, to assess the relative frequencies of normal, delayed, and rapid GE in a database of all patients who had diabetes and GE studies by scintigraphy over a 5-year period; and second to ascertain if other manifestations or characteristics of diabetes (‘phenotype’) were useful for discriminating normal from delayed or rapid GE.
Methods
This study was approved by the Institutional Review Board at Mayo Clinic. We identified subjects who had diabetes (n = 142) from a database of all patients in whom GE was assessed by scintigraphy between 1 January 2002 and 31 December 2006. From this cohort, we excluded subjects who denied authorization to review their medical records for research (n = 6), subjects in whom GE disturbances were probably attributable to other causes unrelated to diabetes [i.e. post-surgical vagal nerve injury (n = 1)], and subjects who had significant comorbid conditions [i.e. pancreas or combined kidney pancreas transplant (n = 5), acute ischemic colitis (n = 1)], providing 129 subjects for further analysis. These subjects were predominately (78%) women, with a mean (± SEM) age of 50 ± 1 years and a BMI of 29 ± 1 kg/m2. Sixty subjects (46%) had type 1 diabetes.
GE was assessed by scintigraphy using a 300-kcal mixed meal containing 99mTc-sulphur colloid labelled eggs in all patients.23 For our laboratory, normal values for GE are 11–39% at 1 h, 40–76% at 2 h, and 84–98% at 4 h. Delayed emptying was defined as values below the lower limit of normal range at 2 or 4 h, and rapid emptying was defined by values above the upper limit of normal range at 1 or 2 h.
A single physician reviewed and abstracted information from the medical records. The relationship between GE disturbances and several factors [i.e. type and duration of diabetes, insulin therapy and duration, extraintestinal complications of diabetes, weight loss (i.e. more than 4·5 kg)], and regular use of medications with a significant potential for inhibiting motility (i.e. opiates and anticholinergic agents) were assessed. None of the patients were being treated with an exenatide or pramlintide, which also delay GE. Patients were instructed to stop opiates or other agents associated with alteration of GE, 48 h prior to assessing GE. Endoscopic and radiological reports were reviewed to ensure patients did not have any mechanical obstruction; none was found in the 129 patients analysed for this report.
Neurological manifestations of diabetes (i.e. neuropathy or radiculopathy) were defined by abnormal physical examination findings (e.g. diminished or absent knee or ankle reflexes, reduced sensation for light touch, vibration, or pinprick), or by abnormal electromyography (33 patients), or by autonomic (i.e. vagal, sudomotor, or adrenergic) dysfunctions on standardized autonomic function testing were available (23 patients).23 Nephropathy was defined by one of the following criteria: (i) a serum creatinine greater than 123·76 μmol/l in males and 106·08 μmol/l in females, or (ii) proteinuria, as evidenced by a urine protein : osmolarity ratio ≥ 0·2 on a spot urine specimen or urine albumin excretion > 300 mg/day, or (iii) microalbuminuria (i.e. urine albumin excretion between 30 and 300 mg/day).24,25 A retinopathy was defined by features of diabetic retinopathy on examination. Glycaemic control was evaluated by measuring the glycosylated haemoglobin.
Gastrointestinal symptoms were assessed by a physician. During the chart review, symptoms were characterized by the predominant symptom into the following categories: postprandial distress, epigastric pain, gastro-esophageal reflux, vomiting, chronic nausea only, diarrhoea with or without abdominal pain, constipation with or without pain, abdominal bloating, and a miscellaneous group (i.e. rectal pain, chronic abdominal pain, dysphagia, and isolated weight loss). Because numbers in individual groups were small, several symptom categories were combined (i.e. postprandial distress and functional bloating, nausea alone and vomiting, epigastric pain and gastro-esophageal reflux disease) prior to statistical analysis. These clusters conform to those recommended in consensus criteria for functional gastrointestinal symptoms,26 and they in turn are based on factor analyses in large epidemiological studies.27
Statistical analysis
Univariate associations between various factors and GE, characterized as normal, delayed, or rapid was assessed by Fisher’s exact test for categorical variables (e.g. weight loss > 10 lbs over 12 months) and by the Kruskal–Wallis rank test for continuous variables. Poly-chotomous multiple variable logistic regression models were used to identify factors independently associated with rapid or slow GE vs. normal GE. Odds ratios for rapid, and separately, slow GE compared to normal GE are reported. The predicted probabilities from this multiple predictor variable model were used to construct receiver operating characteristic (ROC) curves which illustrate the sensitivity and specificity of clinical features for discriminating normal from delayed, and separately normal from rapid GE.
Results
GE and demographic features
GE was normal in 55 (42%), delayed in 46 (36%), and rapid in 28 (22%) patients. In the delayed GE category, GE was 35% (24–43) [median (IQR)] at 2 h and 75% (58–80) at 4 h. In the rapid GE group, GE was 51% (44–59) at 1 h and 78% (73–85) at 2 h. There were no associations between GE and demographic features (i.e. age categorized as ≤ 50 and > 50 years, gender, and BMI), or the proportion of smokers (Table 1). Similarly, use of opiates [15 (normal), 9 (rapid), and 14 (slow)] and anticholinergic agents [3 (normal) and 2 (slow) GE] was not associated with disordered GE.
Table 1.
Demographic features and characteristics of diabetes mellitus by gastric emptying categories†
| Characteristic | Normal (N = 55) | Slow (N = 46) | Rapid (N = 28) |
|---|---|---|---|
| Demographics and lifestyle | |||
| Women* | 33 (60%) | 37 (80%) | 16 (57%) |
| Age, mean ± SEM, year | 52·2 ± 2·1 | 47·2 ± 2·3 | 51·7 ± 3·2 |
| BMI, mean ± SEM, kg/m | 29·2 ± 1·2 | 28·7 ± 1·0 | 29·3 ± 1·5 |
| Overweight | 11 (20%) | 14 (30%) | 8 (29%) |
| Obese | 19 (35%) | 16 (35%) | 10 (36%) |
| Current smoker | 14 (25%) | 10 (22%) | 9 (32%) |
| DM-related variables | |||
| Type 1 DM | 23 (42%) | 24 (52%) | 13 (46%) |
| Duration of type 1 DM, year‡ | 19·8 ± 2·5 | 18·4 ± 2·0 | 23·0 ± 4·0 |
| Duration of type 2 DM, year‡ | 12·8 ± 1·4 | 10·4 ± 2·0 | 10·5 ± 2·0 |
| HbA1c level, mean ± SEM, %‡ | 7·2 ± 0·2 | 8·1 ± 0·4 | 7·6 ± 0·6 |
| DM treatment | |||
| No medication | 1 (2%) | 0 (0%) | 2 (7%) |
| Oral hypoglycaemic medications | 10 (18%) | 9 (15%) | 9 (32%) |
| Insulin | 40 (73%) | 37 (80%) | 17 (61%) |
| Insulin + oral agents | 4 (7%) | 0 (0%) | 0 (0%) |
DM, diabetes mellitus; BMI, body mass index; HbA1c, glycosylated haemoglobin.
P = 0·04 for univariate association.
Unless otherwise indicated, data are reported as n (%) of column total.
Values for the duration of DM and Hb1Ac were missing in 5 and 8 patients respectively.
Characteristics of diabetes mellitus
The type and the duration of diabetes were not significantly associated with normal, slow, or rapid GE, and in all groups, the median duration exceeded 10 years (Table 1). Approximately 50% of patients each had type 1 and type 2 diabetes in each GE category. However, type 1 patients had diabetes for approximately 20 years while type 2 diabetics had diabetes for approximately 10 years.
Of 129 patients, 108 (84%) had one more extraintestinal complications of diabetes (i.e. retinopathy, neuropathy, or nephropathy). The presence of any extraintestinal complications and the number of extraintestinal complications in each patient were not significantly associated with rapid or slow compared to normal GE (Table 2). Of the 21 patients who did not have any extraintestinal complications, 10 had normal, 8 had delayed, and 3 had rapid GE. The glycosylated haemoglobin was examined in 10 of these 11 patients with rapid or delayed GE and was normal in six patients with delayed and two patients with rapid emptying. Overall, the glycosylated haemoglobin was abnormal in 95 of 129 (i.e. 74%) patients and was not significantly associated with the GE disturbance.
Table 2.
Extraintestinal complications of diabetes mellitus and associated clinical conditions*
| Feature | Normal, n (%) (N = 55) | Slow, n (%) (N = 46) | Rapid, n (%) (N = 28) |
|---|---|---|---|
| Extraintestinal complications | |||
| None | 10 (18%) | 8 (17%) | 3 (11%) |
| One | 20 (36%) | 16 (35%) | 13 (46%) |
| Two | 16 (29%) | 11 (24%) | 8 (29%) |
| Three | 8 (15%) | 11 (24%) | 3 (11%) |
| Neuropathy or radiculopathy | 29 (53%) | 25 (54%) | 18 (64%) |
| Clinical assessment† | 27 (49%) | 20 (43%) | 16 (57%) |
| Electromyography† | 9 (16%) | 8 (17%) | 6 (21%) |
| Autonomic neuropathy† | 9 (16%) | 4 (9%) | 6 (21%) |
| Retinopathy† | 18 (33%) | 18 (39%) | 8 (29%) |
| Nephropathy† | 30 (55%) | 28 (61%) | 12 (44%) |
| Proteinuria† | 29 (53%) | 24 (52%) | 9 (32%) |
| Renal insufficiency | 16 (29%) | 9 (20%) | 5 (18%) |
| Proteinuria and renal insufficiency | 14 (25%) | 7 (15%) | 3 (11%) |
| Hypertension | 34 (62%) | 29 (63%) | 16 (57%) |
| Coronary artery disease | 11 (20%) | 7 (16%) | 5 (18%) |
| Peripheral vascular disease | 4 (7%) | 4 (9%) | 5 (18%) |
Data are reported as n (%) of column totals.
Values for clinical neurological assessment, electromyography, autonomic function tests, ophthalmological assessment (retinopathy), urinary protein excretion, and serum creatinine were missing in 3, 96, 106, 1, 20, and 4 patients respectively.
Univariate analysis revealed a nonsignificant association (P = 0·11) between insulin use and GE. Among type 2 diabetics, these differences were more pronounced, that is, only 27% of patients with rapid emptying but 66% of patients with normal and 66% with delayed emptying were being treated with insulin.
Gastrointestinal symptoms
Of 129 patients, 124 (96%) had one or more gastrointestinal symptoms (Table 3). In the remaining patients (i.e. the asymptomatic category in Table 3), transit studies were prompted exclusively by suboptimal glycaemic control. Univariate analysis suggested that symptoms were associated (P = 0·076) with GE disturbances (Table 3). Thus, compared to normal GE, patients with delayed GE were more likely to present with epigastric distress. The prevalence of significant weight loss was higher (P = 0·01) with delayed (48%) than with normal (31%) or rapid (14%) GE.
Table 3.
Distribution of predominant gastrointestinal symptoms by gastric emptying category in diabetes mellitus*
| Predominant symptom† | Normal, n (%) (N = 55) | Slow, n (%) (N = 46) | Rapid, n (%) (N = 28) |
|---|---|---|---|
| Postprandial distress1 | 6 (11%) | 12 (26%) | 3 (11%) |
| Epigastric pain or reflux Vomiting2 | 7 (12%) | 2 (4%) | 6 (21%) |
| Less than once/week | 3 (6%) | 5 (11%) | 5 (18%) |
| Once/week or more often | 12 (22%) | 13 (28%) | 5 (18%) |
| Chronic nausea only2 | 2 (4%) | 3 (7%) | 1 (4%) |
| Diarrhoea +/− pain | 11 (20%) | 3 (7%) | 3 (11%) |
| Constipation +/− pain | 3 (6%) | 4 (9%) | 0 (0%) |
| Abdominal bloating1 | 4 (7%) | 2 (4%) | 2 (7%) |
| Asymptomatic | 2 (4%) | 1 (2%) | 2 (7%) |
| Other‡ | 5 (8%) | 1 (2%) | 1 (4%) |
P = 0·076 for univariate association (Fisher’s exact test).
Data are reported as n (%) of column totals;
Numbers (1 and 2) refer to symptoms that were grouped in categories.
Includes dysphagia, isolated weight loss (i.e., without GI symptoms), and chronic abdominal pain.
Multivariable analysis of factors associated with GE disturbances
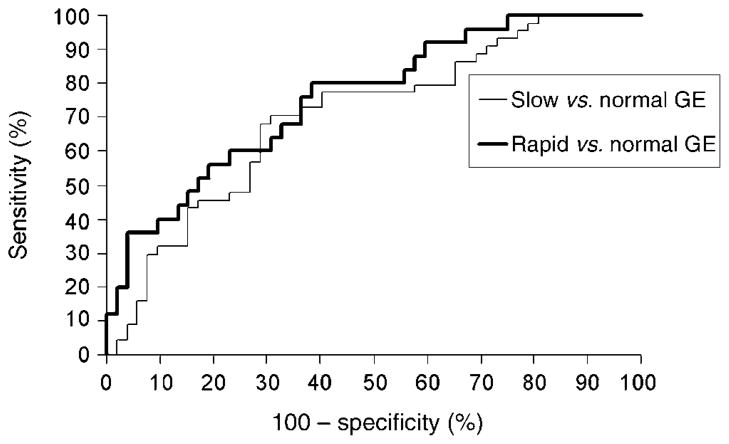
In this analysis (Table 4), significant weight loss and a neuropathy were risk factors for delayed and rapid GE, respectively. In contrast, insulin therapy was associated with a lower risk of rapid compared to normal GE. The logistic regression model incorporating multiple variables resulted in an area under the ROC curve of 0·70 for discriminating delayed from normal emptying and 0·76 for discriminating rapid from normal emptying (Fig. 1). This model suggests that at a specificity of 60%, demographic and clinical features were 73% sensitive for discriminating between normal and delayed GE, and 80% sensitive for discriminating normal from rapid GE.
Table 4.
Multiple variable analysis of risk factors for rapid and delayed gastric emptying among patients with diabetes mellitus
| Variable | Odds ratios (95% CI)
|
|
|---|---|---|
| Rapid vs. normal | Delayed vs. normal | |
| Age (per year) | 0·97 (0·92, 1·02) | 0·98 (0·94, 1·02) |
| BMI (per unit) | 1·01 (0·93, 1·09) | 1·01 (0·95, 1·07) |
| Female sex | 0·91 (0·29, 2·82) | 2·80 (0·98, 7·96) |
| Smoking | 1·91 (0·58, 6·24) | 0·81 (0·28, 2·34) |
| Weight loss | 0·43 (0·11, 1·72) | 2·81 (1·09, 7·23) |
| Type 2 diabetes | 0·35 (0·06, 1·95) | 0·62 (0·19, 2·08) |
| Diabetes duration (per year) | 1·02 (0·97, 1·09) | 0·97 (0·92, 1·02) |
| Insulin therapy | 0·08 (0·01, 0·53) | 0·71 (0·18, 2·91) |
| Oral hypoglycaemic agent | 2·01 (0·24, 16·55) | 1·09 (0·11, 10·70) |
| Neuropathy | 3·60 (1·01, 12·89) | 1·33 (0·51, 3·52) |
| Retinopathy | 0·62 (0·18, 2·21) | 1·50 (0·58, 3·84) |
| Nephropathy | 0·82 (0·26, 2·56) | 1·62 (0·64, 4·14) |
| Coronary artery disease | 1·30 (0·28, 5·92) | 1·42 (0·38, 5·28) |
| Peripheral vascular disease | 4·15 (0·68, 25·19) | 2·04 (0·36, 11·36) |
Fig. 1.
Receiver operating characteristic curves demonstrating utility of clinical features for discriminating between normal and delayed and separately normal and rapid gastric emptying.
Discussion
In this group of 129 unselected, predominantly tertiary referral, patients with diabetes and predominantly upper gastrointestinal symptoms undergoing scintigraphy to measure GE, the latter was normal in 42%, delayed in 36%, and rapid in 22% of patients. In general, patients in this cohort had prolonged diabetes and 84% had one or more extraintestinal complications of diabetes, consistent with previous studies demonstrating that diabetic enteropathy affects patients with longstanding disease.2 Insulin therapy, weight loss, and a neuropathy were useful for predicting abnormal (i.e. slow vs. normal or rapid vs. normal) GE in patients with gastrointestinal symptoms. However, these observations generally confirm a previous study showing that the clinical features (e.g. type and duration of diabetes, presence of autonomic neuropathy, peripheral neuropathy, retinopathy, and vascular disease) were not significantly associated with the severity of gastrointestinal dysmotility assessed by gastro-duodenojejunal manometry in the previous study.28 In the prior study from Mayo Clinic, Kim et al. found a significant association between the number of extraintestinal complications of diabetes and the severity of gastrointestinal dysmotility.
Diabetes mellitus is associated not only with delayed but also with rapid GE.14,16,18 Indeed, one-in-five patients with diabetes and predominantly upper gastrointestinal symptoms in this cohort had rapid GE. While delayed GE has been associated with prolonged type 1 diabetes often complicated by an autonomic neuropathy, rapid GE for liquids has been linked to ‘early’ type 2 diabetes.14–16,18 Autonomic dysfunction is rare when diabetes is diagnosed, usually absent in the first decade of diabetes, and it is typically associated with the duration and severity of peripheral neuropathy.29 These observations suggest that in contrast to delayed emptying,30 rapid GE observed in early diabetes is unlikely to be attributable to autonomic dysfunctions. However, in the present study, a neuropathy, as defined by physical examination, abnormal EMG, or objective autonomic dysfunctions was associated with an increased risk of rapid (vs. normal) GE. Moreover, the distribution of type 1 and type 2 diabetes, the duration of diabetes, and the duration of insulin therapy were not significantly different among patients with normal, rapid, and slow GE of solids. In general, the current study and previous studies suggest that rapid GE is not always associated with ‘early’ type 2 diabetes.17,20 Our data also suggest that even patients with a specific GE disturbance (i.e. rapid or slow) comprise a heterogeneous group in which one or more pathophysiological mechanism(s) may explain the disordered GE observed.
Previous studies have demonstrated that acute hypoglycaemia accelerates GE in healthy subjects and longstanding diabetes mellitus.31,32 This effect of hypoglycaemia is probably not mediated by insulin as euglycaemic hyperinsulinaemia did not affect GE of solids or liquids in patients with uncomplicated type 1 and 2 diabetes.33 Intriguingly, insulin therapy was associated with a lower risk for rapid GE in this study even after correcting for the type and duration of diabetes in the multivariate analysis. Impaired gastric accommodation is associated with and may partly explain rapid GE of liquids in patients with type 2 diabetes who do not have a neuropathy.15 Impaired gastric accommodation was observed in about a quarter of diabetes patients with upper gastrointestinal symptoms.34 As gastric accommodation is a vagally mediated response, it is conceivable that impaired accommodation explains the observed association between a neuropathy and rapid GE of liquids.35,36 We postulate that insulin facilitates gastric accommodation, reducing the risk of rapid GE. Reduced expression of neuronal nitric oxide synthase (nNOS) has been implicated as a cause of impaired gastric accommodation in diabetes mellitus.37 In experimental animals, insulin has neurotrophic effects independent of its ability to restore glycaemia.38 Insulin also restores nNOS expression in genetic and streptozotocin-treated diabetic mice,39 particularly if it is given early (i.e. within 12 weeks after the induction of diabetes).40 However, insulin therapy 12 weeks or later after induction of diabetes does not restore NOS expression in the pyloric sphincter, perhaps because reduced NOS expression is likely attributable to apoptotic cell loss rather than defective axonal transport.40 Perhaps this explains why insulin protected against rapid GE, which may be an early manifestation of diabetes, but not delayed GE, which is more likely a late manifestation of diabetes.
Although symptoms differed among GE categories, these differences were not significant. Compared to normal GE, patients with delayed GE were more likely to complain of postprandial distress and less likely to complain of epigastric pain or gastro-esophageal reflux. However, symptoms were assessed by physician interview rather than by a standard questionnaire. Although the relationship between symptoms and delayed GE is elusive,41 these associations reinforce the utility of discriminating between epigastric pain and postprandial distress as codified in the Rome III criteria.26 The association between significant weight loss and delayed GE may be explained by malnutrition secondary to vomiting. In contrast to previous studies, we did not observe an association between rapid emptying and diarrhoea.42,43
There are several limitations to this study, which was conducted at a tertiary referral centre. This patient population is probably not representative of people with diabetes mellitus in the community. Gastrointestinal symptoms were not assessed by a standardized questionnaire. Transit studies were predominantly prompted by gastrointestinal symptoms, potentially confounding the assessment of the relationship between GE and symptoms. Blood glucose concentrations were not assessed during the GE study. Autonomic functions were only assessed in a minority of patients. Thus, these observations need to be confirmed by prospective assessments utilizing bowel symptom questionnaires in randomly selected subjects with diabetes mellitus from the community. Future studies should also incorporate more sensitive markers for early neuropathy (i.e. nerve conduction tests),44 standardized assessments of autonomic functions, and retinal examination by an ophthalmologist in all patients.
In summary, diabetes mellitus is associated with delayed and rapid gastric emptying. Weight loss and a neuropathy were associated with an increased risk of delayed and rapid gastric emptying, respectively, while insulin use was associated with a lower risk of rapid gastric emptying. From a clinical perspective, these data underscore the importance of assessing GE in diabetic patients, particularly as altered GE can affect the delivery of nutrients to the small intestine and thereby modulate glycaemic control,21 and because newer pharmacological approaches for diabetes (e.g. pramlintide, GLP-1) can delay gastric emptying.45,46 However, because symptoms did not predict abnormal GE in this study, the optimum strategy for selecting patients in whom GE should be assessed is unclear.
Acknowledgments
This study was supported in part by USPHS NIH Grant P01 DK068055, and by the Mayo CTSA grant MO1-RR00585 from the National Institutes of Health in support of the Physiology Laboratory and Patient Care Cores. An abstract of this work was published in Neurogastroenterology and Motility 19 : 423, 2007.
References
- 1.Park MI, Camilleri M. Gastroparesis: clinical update. American Journal of Gastroenterology. 2006;101:1129–1139. doi: 10.1111/j.1572-0241.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz M, O’Donovan D, Jones KL, et al. Gastric emptying in diabetes: clinical significance and treatment. Diabetic Medicine. 2002;19:177–194. doi: 10.1046/j.1464-5491.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 3.Samsom M, Vermeijden JR, Smout AJ, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116–3122. doi: 10.2337/diacare.26.11.3116. [DOI] [PubMed] [Google Scholar]
- 4.Kassander P. Asymptomatic gastric retention in diabetics: gastroparesis diabeticorum. Annals of Internal Medicine. 1958;48:797–812. doi: 10.7326/0003-4819-48-4-797. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M. Clinical practice. Diabetic gastroparesis. New England Journal of Medicine. 2007;356:820–829. doi: 10.1056/NEJMcp062614. [Erratum appears in New England Journal of Medicine, 357, 427.] [DOI] [PubMed] [Google Scholar]
- 6.Keshavarzian A, Iber FL, Vaeth J. Gastric emptying in patients with insulin-requiring diabetes mellitus. American Journal of Gastroenterology. 1987;82:29–35. [PubMed] [Google Scholar]
- 7.Horowitz M, Maddox AF, Wishart JM, et al. Relationships between oesophageal transit and solid and liquid gastric emptying in diabetes mellitus. European Journal of Nuclear Medicine. 1991;18:229–234. doi: 10.1007/BF00186645. [DOI] [PubMed] [Google Scholar]
- 8.Iber FL, Parveen S, Vandrunen M, et al. Relation of symptoms to impaired stomach, small bowel, and colon motility in long-standing diabetes. Digestive Diseases and Sciences. 1993;38:45–50. doi: 10.1007/BF01296772. [DOI] [PubMed] [Google Scholar]
- 9.Jones KL, Horowitz M, Carney BI, et al. Gastric emptying in early noninsulin-dependent diabetes mellitus. Journal of Nuclear Medicine. 1996;37:1643–1648. [PubMed] [Google Scholar]
- 10.Ziegler D, Schadewaldt P, Pour Mirza A, et al. [13C]octanoic acid breath test for non-invasive assessment of gastric emptying in diabetic patients: validation and relationship to gastric symptoms and cardiovascular autonomic function. Diabetologia. 1996;39:823–830. doi: 10.1007/s001250050516. [DOI] [PubMed] [Google Scholar]
- 11.Jones KL, Russo A, Stevens JE, et al. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–1269. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 12.Rathmann W, Enck P, Frieling T, et al. Visceral afferent neuropathy in diabetic gastroparesis. Diabetes Care. 1991;14:1086–1089. doi: 10.2337/diacare.14.11.1086. [DOI] [PubMed] [Google Scholar]
- 13.Delgado-Aros S, Camilleri M, Cremonini F, et al. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia [see comment] Gastroenterology. 2004;127:1685–1694. doi: 10.1053/j.gastro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Phillips WT, Schwartz JG, McMahan CA. Rapid gastric emptying of an oral glucose solution in type 2 diabetic patients. Journal of Nuclear Medicine. 1992;33:1496–1500. [PubMed] [Google Scholar]
- 15.Frank JW, Saslow SB, Camilleri M, et al. Mechanism of accelerated gastric emptying of liquids and hyperglycemia in patients with type II diabetes mellitus. Gastroenterology. 1995;109:755–765. doi: 10.1016/0016-5085(95)90382-8. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz JG, Green GM, Guan D, et al. Rapid gastric emptying of a solid pancake meal in type II diabetic patients [see comment] Diabetes Care. 1996;19:468–471. doi: 10.2337/diacare.19.5.468. [DOI] [PubMed] [Google Scholar]
- 17.Weytjens C, Keymeulen B, Van Haleweyn C, et al. Rapid gastric emptying of a liquid meal in long-term Type 2 diabetes mellitus. Diabetic Medicine. 1998;15:1022–1027. doi: 10.1002/(SICI)1096-9136(1998120)15:12<1022::AID-DIA720>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Bertin E, Schneider N, Abdelli N, et al. Gastric emptying is accelerated in obese type 2 diabetic patients without autonomic neuropathy. Diabetes and Metabolism. 2001;27:357–364. [PubMed] [Google Scholar]
- 19.Choi KM, Zhu J, Stoltz GJ, et al. Determination of gastric emptying in non-obese diabetic mice. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2007;293:G1039–G1045. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 20.Nowak TV, Johnson CP, Kalbfleisch JH, et al. Highly variable gastric emptying in patients with insulin dependent diabetes mellitus [see comment] Gut. 1995;37:23–29. doi: 10.1136/gut.37.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rayner CK, Samsom M, Jones KL, et al. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 22.Jones KL, Tonkin A, Horowitz M, et al. Rate of gastric emptying is a determinant of postprandial hypotension in non-insulin-dependent diabetes mellitus. Clinical Science. 1998;94:65–70. doi: 10.1042/cs0940065. [DOI] [PubMed] [Google Scholar]
- 23.Camilleri M, Zinsmeister AR, Greydanus MP, et al. Towards a less costly but accurate test of gastric emptying and small bowel transit. Digestive Diseases and Sciences. 1991;36:609–615. doi: 10.1007/BF01297027. [DOI] [PubMed] [Google Scholar]
- 24.Zelmanovitz T, Gross JL, Oliveira JR, et al. The receiver operating characteristics curve in the evaluation of a random urine specimen as a screening test for diabetic nephropathy [see comment] Diabetes Care. 1997;20:516–519. doi: 10.2337/diacare.20.4.516. [DOI] [PubMed] [Google Scholar]
- 25.Wilson DM, Anderson RL. Protein-osmolality ratio for the quantitative assessment of proteinuria from a random urinalysis sample. American Journal of Clinical Pathology. 1993;100:419–424. doi: 10.1093/ajcp/100.4.419. [DOI] [PubMed] [Google Scholar]
- 26.Tack J, Talley NJ, Camilleri M, et al. Functional gastro-duodenal disorders [erratum appears in Gastroenterology, 131, 336] Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M, Dubois D, Coulie B, et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clinical Gastroenterology and Hepatology. 2005;3:543–552. doi: 10.1016/s1542-3565(05)00153-9. [DOI] [PubMed] [Google Scholar]
- 28.Kim CH, Kennedy FP, Camilleri M, et al. The Relationship Between Clinical Factors and Gastrointestinal Dysmotility in Diabetes Mellitus. Journal of Gastrointestinal Motility. 1991;3:268–272. [Google Scholar]
- 29.Sinnreich M, Taylor BV, Dyck PJ. Diabetic neuropathies. Classification, clinical features, and pathophysiological basis. Neurologist. 2005;11:63–79. doi: 10.1097/01.nrl.0000156314.24508.ed. [DOI] [PubMed] [Google Scholar]
- 30.Bharucha AE, Camilleri M, Low PA, et al. Autonomic dysfunction in gastrointestinal motility disorders. Gut. 1993;34:397–401. doi: 10.1136/gut.34.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schvarcz E, Palmer M, Aman J, et al. Atropine inhibits the increase in gastric emptying during hypoglycemia in humans. Diabetes Care. 1995;18:1463–1467. doi: 10.2337/diacare.18.11.1463. [DOI] [PubMed] [Google Scholar]
- 32.Russo A, Stevens JE, Chen R, et al. Insulin-induced hypoglycemia accelerates gastric emptying of solids and liquids in long-standing type 1 diabetes. Journal of Clinical Endocrinology and Metabolism. 2005;90:4489–4495. doi: 10.1210/jc.2005-0513. [DOI] [PubMed] [Google Scholar]
- 33.Kong MF, King P, Macdonald IA, et al. Euglycaemic hyperinsulinaemia does not affect gastric emptying in type I and type II diabetes mellitus. Diabetologia. 1999;42:365–372. doi: 10.1007/s001250051164. [DOI] [PubMed] [Google Scholar]
- 34.Bredenoord AJ, Chial HJ, Camilleri M, et al. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clinical Gastroenterology and Hepatology. 2003;1:264–272. [PubMed] [Google Scholar]
- 35.Desai KM, Zembowicz A, Sessa WC, et al. Nitroxergic nerves mediate vagally induced relaxation in the isolated stomach of the guinea pig. Proceedings of the National Academy of Sciences of USA. 1991;88:11490–11494. doi: 10.1073/pnas.88.24.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahnberg T, Abrahamsson H, Jansson G, et al. Gastric relaxatory response to feeding before and after vagotomy. Scandinavian Journal of Gastroenterology. 1977;12:225–228. doi: 10.1203/00006450-199404000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Vittal H, Farrugia G, Gomez G, et al. Mechanisms of disease: the pathological basis of gastroparesis – a review of experimental and clinical studies. Nature Clinical Practice Gastroenterology and Hepatology. 2007;4:336–346. doi: 10.1038/ncpgasthep0838. [DOI] [PubMed] [Google Scholar]
- 38.Hoybergs YM, Meert TF. The effect of low-dose insulin on mechanical sensitivity and allodynia in type I diabetes neuropathy. Neuroscience Letters. 2007;417:149–154. doi: 10.1016/j.neulet.2007.02.087. [DOI] [PubMed] [Google Scholar]
- 39.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. Journal of Clinical Investigation. 2000;106:373–384. doi: 10.1172/JCI8273. [Erratum appears in Journal of Clinical Investigation, 106, 803.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–2362. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 41.Camilleri M. Does delayed gastric emptying really cause symptoms in functional dyspepsia? [see comment] Gut. 2006;55:909–910. doi: 10.1136/gut.2005.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charles F, Phillips SF, Camilleri M, et al. Rapid gastric emptying in patients with functional diarrhea. Mayo Clinic Proceedings. 1997;72:323–328. doi: 10.4065/72.4.323. [DOI] [PubMed] [Google Scholar]
- 43.Lawal A, Barboi A, Krasnow A, et al. Rapid gastric emptying is more common than gastroparesis in patients with autonomic dysfunction. American Journal of Gastroenterology. 2007;102:618–623. doi: 10.1111/j.1572-0241.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 44.Dyck PJ, O’Brien PC, Litchy WJ, et al. Monotonicity of nerve tests in diabetes: subclinical nerve dysfunction precedes diagnosis of polyneuropathy. Diabetes Care. 2005;28:2192–2200. doi: 10.2337/diacare.28.9.2192. [DOI] [PubMed] [Google Scholar]
- 45.Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulino-tropic effects in healthy humans. American Journal of Physiology. 1997;273 (5 Part 1):E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 46.Samsom M, Szarka LA, Camilleri M, et al. Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2000;278:G946–G951. doi: 10.1152/ajpgi.2000.278.6.G946. [DOI] [PubMed] [Google Scholar]