Abstract
Purpose
64Cu-diacetyl-bis (N 4-methylthiosemicarbazone) (64Cu-ATSM) is a promising theranostic agent that targets hypoxic regions in tumors related to malignant characteristics. Its diagnostic usefulness has been recognized in clinical studies. Internal radiotherapy (IRT) with 64Cu-ATSM is reportedly effective in preclinical studies; however, for clinical applications, improvements to reduce radiation exposure in non-target organs, particularly the liver, are required. We developed a strategy to reduce radiation doses to critical organs while preserving tumor radiation doses by controlled administration of copper chelator penicillamine during 64Cu-ATSM IRT.
Methods
Biodistribution was evaluated in HT-29 tumor-bearing mice injected with 64Cu-ATSM (185 kBq) with or without oral penicillamine administration. The appropriate injection interval between 64Cu-ATSM and penicillamine was determined. Then, the optimal penicillamine administration schedule was selected from single (100, 300, and 500 mg/kg) and fractionated doses (100 mg/kg×3 at 1- or 2-h intervals from 1 h after 64Cu-ATSM injection). PET imaging was performed to confirm the effect of penicillamine with a therapeutic 64Cu-ATSM dose (37 MBq). Dosimetry analysis was performed to estimate human absorbed doses.
Results
Penicillamine reduced 64Cu accumulation in the liver and small intestine. Tumor uptake was not affected by penicillamine administration at 1 h after 64Cu-ATSM injection, when radioactivity was almost cleared from the blood and tumor uptake had plateaued. Of the single doses, 300 mg/kg was most effective. Fractionated administration at 2-h intervals further decreased liver accumulation at later time points. PET indicated that penicillamine acts similarly with the therapeutic 64Cu-ATSM dose. Dosimetry demonstrated that appropriately scheduled penicillamine administration reduced radiation doses to critical organs (liver, ovaries, and red marrow) below tolerance levels. Laxatives reduced radiation doses to the large intestine.
Conclusions
We developed a novel strategy to reduce radiation exposure in critical organs during 64Cu-ATSM IRT, thus promoting its clinical applications. This method could be beneficial for other 64Cu-labeled compounds.
Introduction
64Cu-diacetyl-bis (N 4-methylthiosemicarbazone) (64Cu-ATSM) is a promising theranostic agent that targets hypoxic regions in tumors [1], [2], [3]. Radiolabeled Cu-ATSM was originally developed as a positron emission tomography (PET) imaging agent for hypoxia [2], [4], [5], [6], [7]. Clinical PET studies with Cu-ATSM have been already performed, and the usefulness of this agent has been recognized; for example, Cu-ATSM uptake correlated with therapeutic resistance and metastatic potential in several tumors, including uterine cervical carcinoma and rectal carcinoma [4], [7], [8]. The mechanism of radiolabeled Cu-ATSM accumulation in hypoxic regions of tumor tissues has been well studied. Cu-ATSM has a high membrane permeability and thus rapidly diffuses into cells and is reduced and trapped within cells under highly reduced intracellular conditions such as hypoxia [5], [9], [10], [11], [12]. Cu-ATSM uptake also reportedly reflects the level of the biological reductant NAD(P)H, which is associated with hypoxia or mitochondrial dysfunction, and the activity of NAD(P)H-dependent reductive enzymes, rather than oxygenic conditions directly [10], [13], [14], [15]. From these results, Cu-ATSM is considered a surrogate marker of tumor hypoxia via the detection of an intracellular over-reduced status.
Among the available Cu radioisotopes (60Cu, 61Cu, 62Cu, 64Cu, and 67Cu), 64Cu has several advantages for clinical applications because of its unique properties. First, 64Cu decays via β+ (0.653 MeV, 17.4%), β− (0.574 MeV, 40%), and electron capture (42.6%); thus, γ-ray photons from electron-positron annihilation can be detected by PET, while β− particle and Auger electrons emitted from this nuclide can damage tumor cells [1]. Therefore, 64Cu-ATSM could be used as a theranostic agent, which would enable PET-guided therapy for various tumors [1], [2], [3]. Second, 64Cu can be readily produced with a small on-site cyclotron. Due to its relatively long half-life (t1/2 = 12.7 h) among PET radioisotopes, the 64Cu delivery is also feasible. In fact, 64Cu delivery has already begun in the United States, and a multicenter clinical trial of 64Cu-ATSM has been conducted, although this agent is still limited to diagnostic purposes [8].
The therapeutic effects of 64Cu-ATSM have also been demonstrated in preclinical studies [1], [3], [16], [17]. 64Cu-ATSM has been reported to reduce the clonogenic survival of tumor cells and induce post-mitotic apoptosis in vitro [3]. Lewis et al. have demonstrated the feasibility of 64Cu-ATSM as a radiotherapeutic agent in detail, using tumor-bearing hamsters, and have shown that 64Cu-ATSM treatment increased survival time with no serious toxicity [1]. Furthermore, we recently reported that, in the Colon-26 tumor-bearing mouse model, 64Cu-ATSM preferentially localized in intratumoral regions with high densities of CD133+ cells, which are designated as cancer stem cells or cancer stem cell-like cells. Additionally, 64Cu-ATSM IRT inhibited tumor growth and killed not only CD133− cancer cells, but also CD133+ cells [17], [18]. CD133+ cells are reportedly radiotherapy/chemotherapy resistant and possess a high metastatic potential [19], [20]. These findings indicated that 64Cu-ATSM has great potential not only as a diagnostic tool, but also as a therapeutic option for patients and that 64Cu-ATSM IRT might be a promising therapeutic approach for targeting tumor malignant characteristics such as tumor hypoxia and cancer stem cell-like cells.
However, when considering the clinical applications of 64Cu-ATSM for therapeutic use, the high physiological accumulation of 64Cu in non-target organs is a major barrier. In a clinical PET study with Cu-ATSM, the liver was reported to be the principal dose-limiting organ in humans [21]. In a hamster model, 64Cu accumulation was high in the intestines, as well as in the liver [1]. Hence, the reduction of radiation exposure in these organs, especially the liver, is important to minimize adverse effects and facilitate the clinical application of 64Cu-ATSM IRT.
We focused on the heavy-metal chelator penicillamine to develop an effective method for reducing radiation absorption doses in critical organs. Penicillamine is an active ingredient of drugs used to treat intoxication by heavy metals such as Cu, Hg, Zn, and Pb, and penicillamine treatment enhances the urinary excretion of these metals [22], [23]. Penicillamine has also been approved for the treatment of Wilson’s disease, a genetic copper metabolism disorder. Copper is essential to the physiological functions of a number of enzymes such as tyrosinase, cytochrome oxidase, and superoxide dismutase; however, poorly regulated Cu levels in body fluids can be toxic [24]. Under normal conditions, Cu homeostasis is maintained through enterohepatic circulation, and excess Cu is excreted into the bile and feces. In Wilson’s disease, Cu transport to the bile is disrupted by a mutation in a Cu-transporting P-type ATPase, ATP7B, which leads to an abnormal accumulation of Cu in the liver and subsequently results in hepatic cirrhosis, cardiac dysfunction, pancreatic dysfunction, and neurological abnormalities [25]. The action mechanism of penicillamine in Wilson’s disease has been reported; this agent binds Cu in the small intestine and enhances urinary Cu excretion, resulting in the prevention of Cu enterohepatic circulation and reduced Cu levels in the liver [24]. Additionally, another study of radiolabeled penicillamine showed that this drug, when administered orally (p.o.), is delivered to the liver [26], where it will remove accumulated Cu. Accordingly, we hypothesized that penicillamine could reduce 64Cu accumulation in the liver and small intestine and possibly other organs after 64Cu-ATSM administration. In this study, we developed a method to reduce 64Cu accumulation in non-target organs but not in tumors by appropriately scheduling the administration of penicillamine. The effect of the combined use of laxatives and penicillamine on reduced 64Cu accumulation, particularly in the large intestine, was also tested.
Materials and Methods
Ethics Statement
Animal experiments in this study were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Radiological Sciences (Japan). The protocol was approved by the Animal Ethics Committee of the National Institute of Radiological Sciences (Japan; Permit Number: M40-01). All efforts were made to minimize suffering during the animal experiments.
Preparation of 64Cu-ATSM and Penicillamine
64Cu was produced as reported previously [27]. The purification of 64Cu and the preparation of 64Cu-ATSM were performed according to previously reported procedures [18], [27]. The radiochemical purity of the resulting 64Cu-ATSM was greater than 95%, as determined by silica gel thin-layer chromatography (TLC; silica gel 60; Merck, Whitehouse Station, NJ, USA) with ethyl acetate as the mobile phase [28]. Radioactivity levels on the TLC plates were analyzed with a bioimaging analyzer (FLA-7000; Fujifilm, Tokyo, Japan). Penicillamine was obtained from the Tokyo Chemical Industry (Tokyo, Japan).
Penicillamine Challenge to 64Cu-ATSM in Mouse Plasma
The trans-chelation of 64Cu from 64Cu-ATSM to penicillamine was investigated in mouse plasma. For this study, 64Cu-ATSM (370 MBq/mL) and penicillamine (83 mg/mL) solutions were prepared in saline. Freshly prepared mouse plasma was pre-incubated at 37°C for 10 min. The 64Cu-ATSM solution, penicillamine solution or saline, and plasma were mixed together in a volume ratio of 10∶15:100, and the mixture was incubated at 37°C. Aliquots were removed at 0, 5, 10, and 30 min after the start of the incubation and were subsequently loaded onto TLC plates. Radioactivity on the TLC plates was analyzed using a bioimaging analyzer, and the relative ratios of the activity were calculated for the fraction with intact 64Cu-ATSM (Rf = 0.8) and the fraction with free 64Cu and 64Cu-penicillamine at the origin to the total activity.
Cell Line and Animal Model
In these studies, we used the human colon carcinoma cell line HT-29 (HTB-38; American Type Culture Collection, Manassas, VA, USA). The cells were incubated in a humidified atmosphere with 5% CO2 at 37°C. Dulbecco’s modified Eagle’s medium (DMEM 11995-065; Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum and antibiotics, was used for cell culture. Exponentially growing cells were used in the study. The cells were detached from the plates with trypsin.
BALB/c male nude mice (6 weeks old, 20–25 g body weight) were obtained from Japan SLC (Hamamatsu, Japan). Before the experiments, the mice were undisturbed for at least 1 week. HT-29 cells suspended in phosphate-buffered saline (1×107 cells) were subcutaneously injected into the shoulders of the BALB/c nude mice. The biodistribution and PET studies were performed 3 weeks after the tumor cell implantations. HT-29 tumor-bearing mice were fasted for more than 16 h before the administration of 64Cu-ATSM.
In vivo Biodistribution
For the biodistribution study, a tracer dose of 64Cu-ATSM (185 kBq/mouse) was injected into the mice. First, prior to the experiment with penicillamine, the 24-h biodistribution and excretion of 64Cu-ATSM were determined in detail in non-tumor bearing BALB/c nude mice. For the penicillamine study, the biodistribution of 64Cu-ATSM in nude mice with HT-29 tumor treated with penicillamine p.o. (150 µL in volume), as clinically prescribed, was examined and compared with that in mice treated with saline (control). In the first step, the appropriate injection interval between 64Cu-ATSM and penicillamine was determined. Penicillamine (500 mg/kg) was administered at 10 min before, 10 min after, or 1 h after 64Cu-ATSM injection, and the biodistribution in these animals was compared. Next, to determine the adequate penicillamine injection dose, the following treatment conditions for single-dose and fractionated administration were compared. To evaluate single-dose administration, 100, 300, or 500 mg/kg of penicillamine were administered at 1 h after 64Cu-ATSM injection. To evaluate fractionated administration, 3 doses of penicillamine were administered at 100 mg/kg per dose; the doses were given at 1- or 2-h intervals, starting at 1 h after 64Cu-ATSM injection (administration at 1, 2, and 3 h or 1, 3, and 5 h after 64Cu-ATSM injection). For laxative treatment, 3 mL of a Glycerin Enema Solution (Yoshida Pharmaceutical, Tokyo, Japan) was administered rectally, according to the manufacturer’s protocol, at 30 min after the fractionated penicillamine dosage schedule of 1, 3, and 5 h after 64Cu-ATSM injection. Biodistribution was studied in 4 mice per group at 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, and 6 h and 3 mice per group at 16 h and 24 h after 64Cu-ATSM injection. Animals in the 5-min to 6-h sampling groups were housed individually, and the urine and feces were collected with polyethylene-laminated filter paper. Animals in the 16-h and 24-h sampling groups were housed individually in metabolic cages (3600M021; Tecniplast S.p.A, Buguggiate, Italy) to collect urine and feces. The organs of interest (liver, kidney, small intestine, large intestine, muscle, tumor, and remainder of the body) and blood were also collected and weighed. Radioactivity levels were counted with a γ-counter (1480 Automatic gamma counter Wizard 3; PerkinElmer Inc., Waltham, MA, USA). The biodistribution data were calculated and shown as the %ID/g for the organs and blood and the %ID for the urine and feces.
Small-animal PET Imaging with 64Cu-ATSM
The effect of penicillamine on a therapeutic dose of 64Cu-ATSM in HT-29 tumor-bearing mice was examined with PET imaging. In this study, 64Cu-ATSM (37 MBq/mouse) was intravenously injected, followed by a single-dose p.o. administration of 300 mg/kg penicillamine or saline (control) at 1 h after 64Cu-ATSM injection (n = 3/group). At 0.5, 2, 3, 4, 5, 6, 7, 8, and 24 h after the 64Cu-ATSM injection, 5-min emission scans were performed with a small animal PET system (Inveon; Siemens Medical Solutions, Malvern, PA, USA) while the mice remained under 1.5–2% isoflurane anesthesia. Body temperature was maintained with a heat pump during the scans. The images were reconstructed using a 3D maximum a posteriori with Inveon Acquisition Workplace software (Siemens Medical Solutions). Regions of interest (ROIs) were manually drawn over the tumors and livers, and tracer uptake was quantified as the mean standard uptake value (SUVmean) in each ROI measured with ASIPro software (CTI Molecular Imaging/Siemens) as described previously [29], [30].
Dosimetry Analysis
Mean absorbed doses of 64Cu-ATSM (mSv/MBq) in humans were estimated on the basis of the biodistribution data that were obtained as described above from HT-29 tumor-bearing mice. The mean %ID/g values of the mouse liver, kidney, small intestine, large intestine, and remainder of the body were converted into corresponding human values according to the standard body weights for mice (20 g) and humans (73.7 kg) [31]. These values were processed with OLINDA/EXM software [32], which used a dynamic bladder model with a voiding interval of 4.8 h to estimate the organ doses (mSv/MBq).
Statistical Analysis
Data are expressed as the means ± SD. P values were calculated using the two-sided t-test for comparisons between 2 groups or analysis of variance followed by post-hoc Tukey’s test for multiple group comparisons. P values of <0.05 were considered to be statistically significant.
Results
Penicillamine-induced Depletion of 64Cu from 64Cu-ATSM in Mouse Plasma
First, the ability of penicillamine to deplete 64Cu from 64Cu-ATSM was examined in vitro with mouse plasma (Figure S1). 64Cu-ATSM was stable in the plasma in the absence of penicillamine. The relative ratio of intact 64Cu-ATSM activity to total activity decreased immediately after the addition of penicillamine, whereas the ratio of the activity of the origin fraction with the free 64Cu and 64Cu-penicillamine increased. This indicated that trans-chelation from 64Cu-ATSM to penicillamine occurred in the plasma and that hydrophilic 64Cu-complexes such as 64Cu-penicillamine remained at the origin during normal-phase TLC.
Biodistribution and Excretion of 64Cu-ATSM in Mice
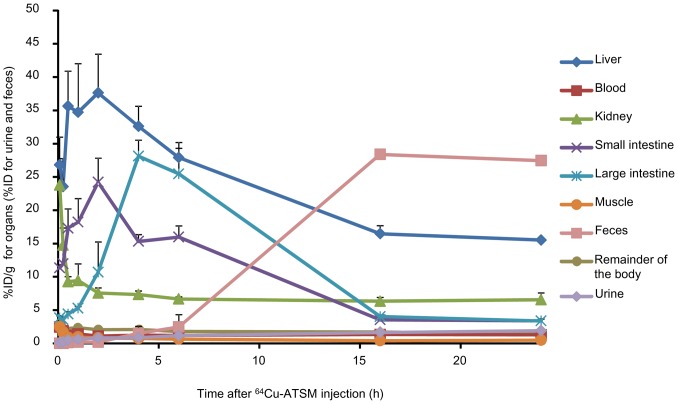
Next, the 24-h biodistribution and excretion of 64Cu-ATSM was examined in tumor-free BALB/c nude mice after i.v. injections of 64Cu-ATSM; this was conducted because there were no sufficient data describing the distribution and excretion of 64Cu-ATSM in mice, particularly at later time points (e.g., 16 h and 24 h) [2], [6], [21], [28]. Time-activity curves for the collected organs and urinary and fecal excretion are shown in Figure 1. Noticeable 64Cu accumulation was observed in the liver, small intestine, and large intestine during the first 6 h after injection. Large amounts of 64Cu were excreted in the feces by 16 hours after the injection, but little urinary excretion was observed in mice.
Figure 1. Biodistribution of 64Cu-ATSM in BALB/c nude mice.
Data were obtained at 564Cu-ATSM injection. Values are expressed as the %ID/g for the organs (liver, kidney, small intestine, large intestine, muscle, and remainder of the body) and blood and as the %ID for the urine and feces. Values are shown as means ± SD; n = 4 for 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, and 6 h and n = 3 for 16 h and 24 h.
In vivo Biodistribution with Penicillamine Administration
The biodistribution of 64Cu-ATSM was compared between various administration schedules in HT-29 tumor-bearing mice that received various p.o. penicillamine doses. First, we examined the biodistribution in animals that were treated with penicillamine (500 mg/kg) at 10 min before, 10 min after, or 1 h after the 64Cu-ATSM injection (Figure 2A). In this experiment, the penicillamine dose was set at 500 mg/kg, as this was the maximum concentration that could be dissolved in the injection volume; also, this dose was shown to be below the LD50 in mice that received a single-dose oral injection (720 mg/kg, Material Safety Data Sheet of penicillamine, CAS#52-67-5). Penicillamine treatments at 10 min before or 10 min after 64Cu-ATSM injection significantly reduced the 64Cu accumulation in the liver (P<0.05); however, tumor uptake was also significantly reduced (P<0.05). In contrast, treatment with penicillamine at 1 h after 64Cu-ATSM injection significantly reduced the liver accumulation (P<0.05), whereas there were no significant decreases in tumor accumulation. The time activity curves for the blood and tumors of HT-29 tumor-bearing mice without penicillamine treatment showed that the radioactivity was mostly cleared from the blood and tumor uptake had plateaued by 1 h (Figure 2B).
Figure 2. The effect of penicillamine administration timing on the biodistribution of 64Cu-ATSM.
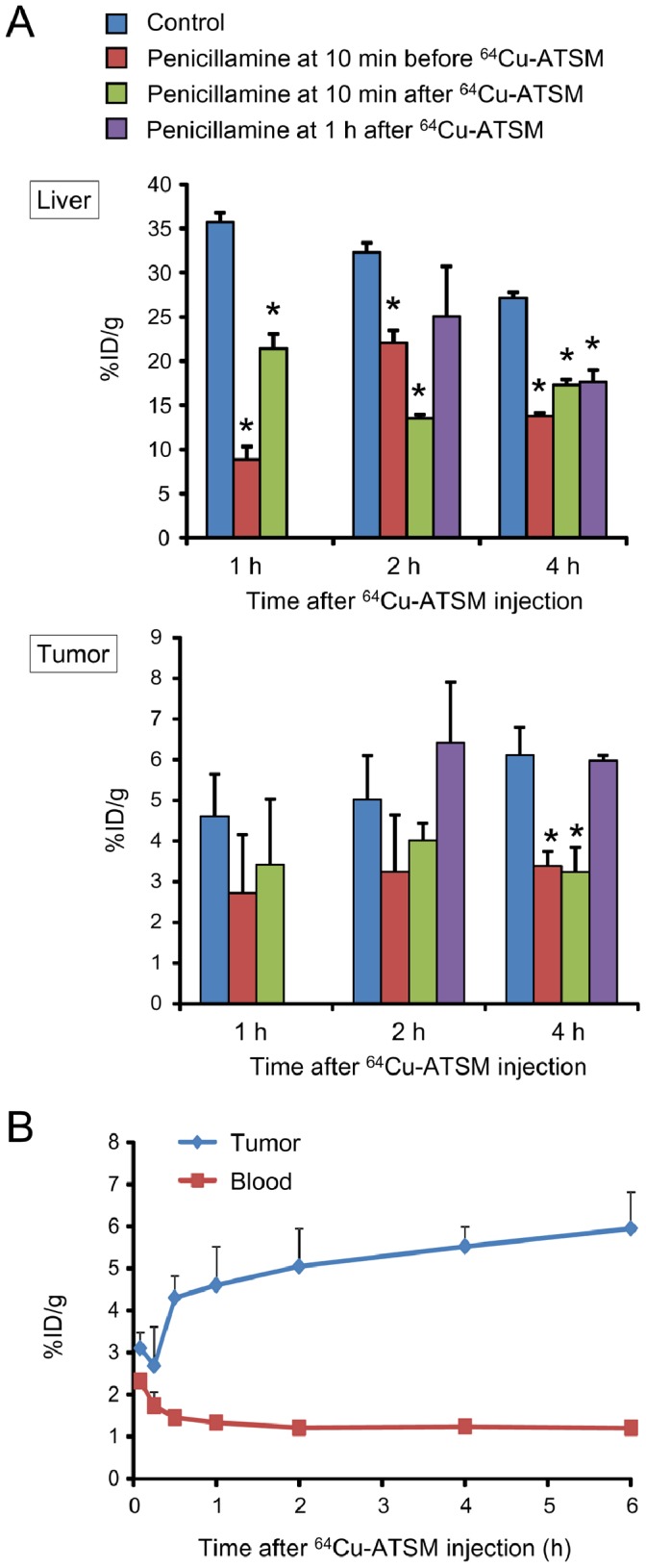
(A) Biodistribution in the liver (upper) and tumor (lower) in HT-29 tumor-bearing mice that were treated p.o. with a single-dose of 500 mg/kg penicillamine at 10 min before or after 64Cu-ATSM injection or at 1 h after 64Cu-ATSM injection. Data were obtained at 1, 2, and 4 h after 64Cu-ATSM injection. (B) Time activity curves for the tumors and blood, based on the biodistribution data from HT-29 tumor-bearing mice (control animal). Values are shown as means ± SD; n = 4 for each time point.
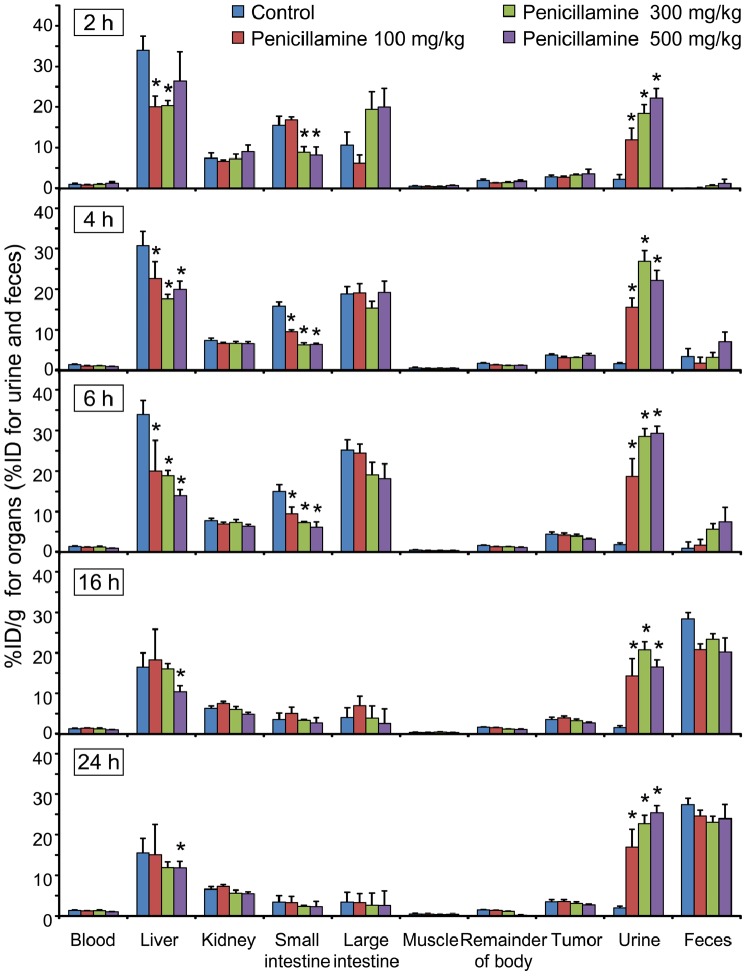
To determine the adequate penicillamine injection dose, various single-dose and fractionated administration conditions were compared in detailed biodistribution studies (Figures 3, 4). For a better understanding, time-activity curves were generated from the biodistribution data for the selected organs with relatively high 64Cu accumulation (i.e., liver, small intestine, and large intestine; Figure S2). Initially, we tested single-dose p.o. injections of 100, 300, and 500 mg/kg of penicillamine in HT-29 tumor-bearing mice at 1 h after the 64Cu-ATSM injections. Chronological changes in biodistribution in the collected organs and urinary and fecal excretion for the 24-h period after 64Cu-ATSM injections with single-dose administrations of penicillamine are shown in Figure 3 (time-activity curves, Figure S2A). These demonstrated that all of the penicillamine treatment groups showed significant decreases in 64Cu accumulation in the liver and small intestine, compared to the control (P<0.05). Figure 3 also indicates statistical significance in comparison to the control at each time point and shows significant decreases in 64Cu accumulation in the liver and small intestine at 4 and 6 h in all treatment groups. In contrast, there were no significant differences in tumor uptake between any of the treatment groups in single-dose administration and the control. The penicillamine treatment significantly accelerated the urinary excretion of 64Cu, but increased 64Cu retention was not observed in the kidneys. Additionally, the 300 mg/kg penicillamine group showed significantly reduced 64Cu accumulation in the liver and small intestine in comparison to that of the 100 mg/kg dose group (P<0.05), while there was no significant difference between the 300 and 500 mg/kg dose groups. Among the doses tested in this study, 300 mg/kg was sufficient for single-dose administration of penicillamine at 1 h after 64Cu-ATSM injection.
Figure 3. Chronological changes in biodistribution and excretion after 64Cu-ATSM injection with single-dose penicillamine injections.
Biodistribution in the organs and urinary and fecal excretions at 2, 4, 6, 16, and 2464Cu-ATSM injection in HT-29 tumor-bearing mice that were treated p.o. with a single-dose of 100, 300, or 500 mg/kg penicillamine at 1 h after 64Cu-ATSM injection, compared with those in animals treated with saline (control). Data are shown as the %ID/g for the organs (liver, kidney, small intestine, large intestine, muscle, remainder of the body, and tumor) and blood and the %ID for the urine and feces. Asterisks indicate statistical significance (*P<0.05) in comparison to the control at each time point. There were no significant differences in the %ID/g of the tumors between any of the treatment groups and the control. Values are shown as means ± SD; n = 4 for 2, 4, and 6 h and n = 3 for 16 and 24 h.
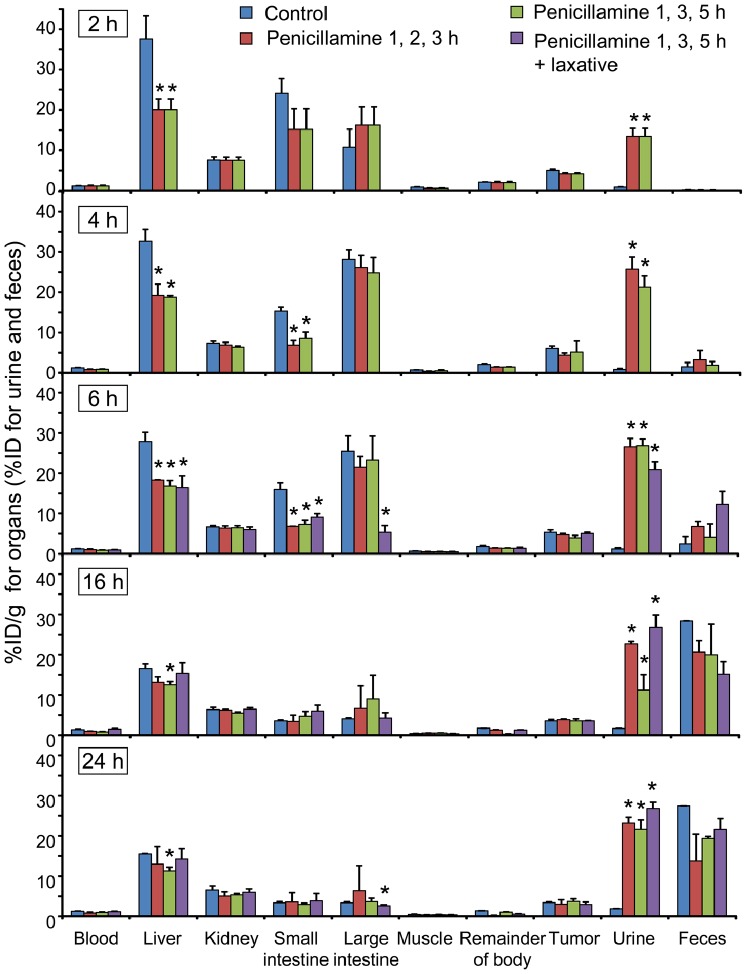
Figure 4. Chronological changes in biodistribution and excretion after 64Cu-ATSM injection with fractionated penicillamine injections.
Biodistribution in organs and urinary and fecal excretions at 2, 4, 6, 16, and 2464Cu-ATSM injection in HT-29 tumor-bearing mice that were treated p.o. with fractionated doses of penicillamine (100 mg/kg×3) at 1, 2, and 3 h or 1, 3, and 5 h after 64Cu-ATSM injection, compared with those in animals treated with saline (control). Additional laxative treatments at 5.5 h after 64Cu-ATSM injection along with penicillamine administration (100 mg/kg×3; 1, 3, and 5 h after 64Cu-ATSM injection) are also shown. Data are shown as the %ID/g for the organs (liver, kidney, small intestine, large intestine, muscle, remainder of the body, and tumor) and blood and the %ID for the urine and feces at 2, 4, 6, 16, and 24 h after 64Cu-ATSM injection. Asterisks indicate statistical significance (*P<0.05) in comparison to the control at each time point. There were no significant differences in the %ID/g of the tumors between any of the treatment groups and the control. Values are shown as means ± SD; n = 4 for 2, 4, and 6 h and n = 3 for 16 and 24 h.
Next, we examined the effects of fractionated administration in order to maintain a constant effective penicillamine concentration in the blood for a longer time, as well as to lower the dose of penicillamine given in each injection. Administration regimens that comprised an initial 100 mg/kg dose of penicillamine at 1 h after 64Cu-ATSM injection followed by 2 additional 100 mg/kg doses at either 1-h or 2-h intervals were compared (penicillamine administration at 1, 2, and 3 h or 1, 3, and 5 h after 64Cu-ATSM injection). Chronological changes in biodistribution and excretion for the 24-h period after the 64Cu-ATSM injections while examining the fractionated administration of penicillamine are shown in Figure 4 (time-activity curves, Figure S2B). At earlier time points by 6 h, 3 penicillamine doses of 100 mg/kg per dose reduced 64Cu accumulation in the liver and small intestine to nearly the same extent as a single-dose injection of 300 mg/kg (Figure 4 compared to Figure 3). When penicillamine administration was fractionated at 2-h intervals after 64Cu-ATSM injection, significant decreases in liver accumulation were observed when compared to the control group at later time points (16 and 24 h; P<0.05); however, this was not true for the 300 mg/kg single-dose administration or fractionated administration at 1-h intervals (Figures 3, 4). Mice that received fractionated penicillamine administration did not show significant decreases in tumor uptake when compared to the controls. Overall, the biodistribution study demonstrated that the fractionated administration of penicillamine (100 mg/kg×3) at 1, 3, and 5 h after 64Cu-ATSM injection was the most favorable of the treatment protocols examined in this study.
Effect of Penicillamine on a Therapeutic 64Cu-ATSM Dose
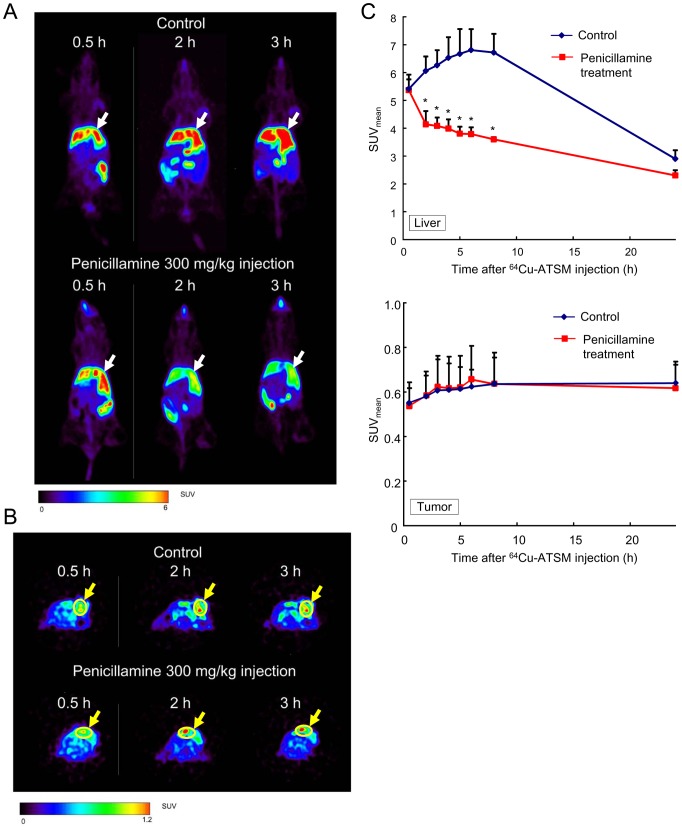
The biodistribution study demonstrated the effect of penicillamine on the tracer dose (185 kBq/mouse) of 64Cu-ATSM. Next, small animal PET with 64Cu-ATSM was performed to examine the effect of penicillamine on a therapeutic dose of 64Cu-ATSM (37 MBq/mouse), which has been reported to induce therapeutic effects in mice [16], [17] and is equivalent to the therapeutic dose required to increase the survival times of tumor-bearing hamsters [1]. In the PET study, a single-dose p.o. injection of 300 mg/kg at 1 h after 64Cu-ATSM injection was employed, rather than fractionated administration, as it allowed us to simply analyze serial changes in 64Cu accumulation after a penicillamine administration. Representative whole-body coronal and transverse PET images and time-activity curves from the image analysis are shown in Figure 5. Similar to the results of the biodistribution study, 64Cu accumulation in the liver was significantly reduced by penicillamine treatment (P<0.05), while tumor uptake was not affected. This indicates that penicillamine functioned similarly with both the therapeutic and tracer doses of 64Cu-ATSM.
Figure 5. Representative PET images.
PET images of HT-29 tumor-bearing mice that were administered 64Cu-ATSM and a single dose of penicillamine (300 mg/kg p.o.) or saline (control) at 1 h after the 64Cu-ATSM injection are shown. (A) Coronal PET images show the liver (white arrows). (B) Transverse PET images show the tumor. HT29 tumors are indicated by yellow arrows and circles. Images were obtained at 0.5, 2, and 3 h after the 64Cu-ATSM injection. (C) Time activity curves from the PET analysis. Time activity curves for the liver (upper) and tumor (lower) are shown for animals treated with penicillamine or saline (control; n = 3/group). Values are shown as means ± SD.
Co-administration of a Laxative with Penicillamine
Among the organs with relatively high 64Cu accumulation after 64Cu-ATSM administration, penicillamine administration reduced 64Cu accumulation in the liver and small intestine, but not in the large intestine. Therefore, we performed an additional experiment to determine whether a laxative could reduce 64Cu accumulation in the large intestine. For this experiment, we used animals that were treated with a fractionated dose of penicillamine (100 mg/kg per dose at 1, 3, and 5 h) after 64Cu-ATSM injection. Prior to the experiment, we confirmed that a glycerin enema significantly increased defecation (P<0.05; Figure S3). Anatomical examinations showed that the feces were almost completely evacuated from the large intestine (data not shown). Biodistribution data from the animals treated with both penicillamine and laxative are shown in the latter 3 panels of Figure 4 (time-activity curves, Figure 2B). At 6 h after the 64Cu-ATSM injection (30 min after laxative injection), 64Cu accumulation in the large intestine was reduced to 20.9% of the control level (P<0.05), and the amount of excreted 64Cu was increased in the fecal matter (Figure 4). Biodistribution in the other organs was almost similar to that in animals treated with only penicillamine (100 mg/kg×3 at 1, 3, and 5 h after 64Cu-ATSM injection), although the significant reductions in liver accumulation at the later time points (16 and 24 h) was not observed in animals treated with penicillamine and laxative (Figure 4). These data indicated that the co-administration of a laxative with penicillamine was effective for quickly reducing 64Cu accumulation in the large intestine, although the prolonged effect of penicillamine was slightly weakened.
Dosimetry Analysis
The mean absorbed doses of 64Cu-ATSM (mSv/MBq) in humans were estimated according to biodistribution data from mice that were treated with saline (control) or penicillamine, either via single-dose (300 mg/kg at 1 h after 64Cu-ATSM injection) or fractionated (100 mg/kg each at 1, 3, and 5 h after 64Cu-ATSM injection with/without laxative) administration (Table 1). The dosimetry data indicate that the liver showed the highest absorbed doses during 64Cu-ATSM IRT, with estimated absorbed doses to the liver of 0.108 mSv/MBq in the absence of penicillamine (control) and 0.082 and 0.069 mSv/MBq (24% and 36% reductions from control levels) with single-dose and fractionated penicillamine administration, respectively. Similarly, reduced absorbed doses were seen in the other organs in the penicillamine-treated groups, except for the urinary bladder wall. The accelerated urinary excretion of the radioactive material in penicillamine-treated groups contributed to increased absorbed doses to the urinary bladder wall. The co-administration of a laxative with penicillamine showed a largely reduced radiation dose to the large intestines (46% from control), although reductions in the doses to other organs were slightly lessened (Table 1).
Table 1. Mean estimated human absorbed doses for 64Cu-ATSM, extrapolated from mouse biodistribution data.
| Estimated absorbed dose (mSv/MBq) | ||||
| Target Organ | Control | Penicillamine300 mg/kg | Penicillamine100 mg/kg 1, 3, 5 h | Penicillamine 100 mg/kg 1, 3, 5 h+laxative |
| Adrenals | 0.014 | 0.011 | 0.008 | 0.009 |
| Brain | 0.008 | 0.006 | 0.004 | 0.005 |
| Breasts | 0.009 | 0.007 | 0.004 | 0.005 |
| Gallbladder wall | 0.020 | 0.015 | 0.012 | 0.013 |
| Lower large intestinal wall | 0.062 | 0.043 | 0.056 | 0.033 |
| Small intestine | 0.053 | 0.037 | 0.039 | 0.044 |
| Stomach wall | 0.012 | 0.009 | 0.006 | 0.007 |
| Upper large intestinal wall | 0.061 | 0.042 | 0.055 | 0.033 |
| Heart wall | 0.011 | 0.009 | 0.006 | 0.007 |
| Kidneys | 0.031 | 0.029 | 0.025 | 0.027 |
| Liver | 0.108 | 0.082 | 0.069 | 0.078 |
| Lungs | 0.011 | 0.008 | 0.006 | 0.007 |
| Muscle | 0.010 | 0.008 | 0.005 | 0.006 |
| Ovaries | 0.014 | 0.011 | 0.009 | 0.009 |
| Pancreas | 0.014 | 0.011 | 0.008 | 0.009 |
| Red marrow | 0.009 | 0.007 | 0.005 | 0.006 |
| Osteogenic cells | 0.018 | 0.015 | 0.009 | 0.011 |
| Skin | 0.008 | 0.006 | 0.004 | 0.005 |
| Spleen | 0.010 | 0.008 | 0.006 | 0.007 |
| Testes | 0.009 | 0.007 | 0.005 | 0.006 |
| Thymus | 0.009 | 0.007 | 0.005 | 0.006 |
| Thyroid | 0.009 | 0.007 | 0.004 | 0.005 |
| Urinary bladder wall | 0.012 | 0.069 | 0.045 | 0.067 |
| Uterus | 0.012 | 0.011 | 0.008 | 0.010 |
| Total body | 0.013 | 0.010 | 0.008 | 0.009 |
Discussion
64Cu-ATSM IRT is a promising cancer treatment that targets over-reduced hypoxic regions of tumors, which are related to malignant characteristics, and is expected to be used in clinical applications [1], [3], [16], [17], [18]. However, high physiological 64Cu accumulation in non-target organs is an issue that affects the clinical application of this agent. In particular, the liver is reported to be the principal dose-limiting organ in human 64Cu-ATSM PET [21]. In this study, we showed for the first time that during 64Cu-ATSM IRT, the appropriately scheduled administration of a copper chelator, penicillamine, could reduce radiation doses to critical organs without decreasing the dose to the tumor. These findings indicate that our new method will provide an additional benefit to 64Cu-ATSM IRT by protecting critical organs from radiation damage while maintaining the anti-tumor therapeutic effects.
In this study, we showed that the appropriately scheduled administration of penicillamine could reduce 64Cu accumulation in both the liver and the small intestine. Penicillamine has been reported to bind Cu in the small intestine, leading to excretion through the urine, which prevents the enterohepatic circulation of Cu and reduces the accumulation of Cu in the liver [24]. Additionally, orally administered penicillamine was reportedly transported to the liver [26], which could explain how penicillamine might clear accumulated Cu in the liver. Upon consideration of the pharmacokinetics and pharmacodynamics of penicillamine, the substantial reductions of 64Cu accumulation in the liver and small intestine were reasonable. Our findings suggest that in 64Cu-ATSM IRT, both of the action mechanisms of penicillamine play important roles in reducing the radiation dose to the liver. Furthermore, this study demonstrated that penicillamine administration increased urinary 64Cu excretion, although 64Cu activity was scarcely retained in the kidney. Therefore, as shown in the present study, penicillamine can transchelate 64Cu from 64Cu-ATSM and possibly also from free or protein-bound 64Cu, and subsequently, 64Cu-penicillamine is quickly excreted from the kidney into urine, which adds the benefit of facilitating the clearance of 64Cu from the body.
Our biodistribution findings indicated that penicillamine accelerated the renal clearance of 64Cu relative to the control. As a result, the estimated absorbed dose to the urinary bladder wall was increased by penicillamine administration (Table 1). We used the dynamic bladder model in the OLINDA/EXM software with a voiding interval of 4.8 h to estimate the absorbed dose. Therefore, it would be beneficial to hydrate patients during an initial period of several hours after 64Cu-ATSM administration to reduce the voiding interval. In such cases, the radiation dose from the urinary bladder to critical organs such as the ovaries could be reduced by more than half in cases with voiding intervals of 2 h or less.
According to the biodistribution data, we showed that the fractionated administration of penicillamine at 2-h intervals (100 mg/kg each at 1, 3, and 5 h after 64Cu-ATSM injection) was the most favorable of the examined treatment conditions. This is because of the ability of the fractionated dose, given at 2-h intervals, to decrease 64Cu accumulation in the liver over a prolonged period. Based on the estimated human absorbed 64Cu-ATSM doses (mSv/MBq) in this study (Table 1), the fractionated administration of penicillamine at 2-h intervals showed a 36% reduction in the absorbed dose to the liver relative to the control. Previous therapeutic studies of 64Cu-ATSM IRT in hamsters showed that a single administration of 370 MBq 64Cu-ATSM effectively increased the experimental survival time of tumor-bearing hamsters without serious toxicity. Based on these results, the authors concluded that the sufficient therapeutic dose of 64Cu-ATSM in humans would be 278 GBq [1]. In this study, to anticipate the effects of 64Cu-ATSM IRT on non-target organs, we attempted to estimate the radiation doses from an assumed 64Cu-ATSM injection dose of 278 GBq/man (Table S1) and to compare these estimates with reported tolerance doses [33], [34]. We found that in the absence of penicillamine, the estimated radiation doses to the liver, ovaries, and red marrow reached or exceeded the tolerance doses (Table S2). Therefore, radiation doses to these organs should be carefully considered as dose-limiting factors in 64Cu-ATSM IRT. This study also showed that the fractionated administration of penicillamine could reduce the radiation doses to these critical organs below the tolerance doses (Table S2). In the small and large intestines, radiation doses in the absence of penicillamine were estimated to be below the tolerance doses with an assumed injection dose of 278 GBq/man 64Cu-ATSM (Table S2). This suggests that the intestines are not critical organs in 64Cu-ATSM IRT, although the 64Cu accumulation levels were relatively high. Nonetheless, supportive penicillamine or penicillamine/laxative treatments would help to reduce unnecessary radiation doses to the small and large intestines, respectively. In clinical settings, laxative use might be recommended only if the patients are expected to experience high radiation doses in the large intestine due to colorectal dysfunction or other disorders.
Furthermore, in this study, we found that penicillamine treatment at 1 h after 64Cu-ATSM injection, when the blood radioactivity level was already low and tumor uptake had plateaued, could reduce liver uptake without decreasing tumor uptake. On the other hand, penicillamine treatment at 10 min before or after 64Cu-ATSM injection reduced tumor uptake. This is likely because penicillamine depleted 64Cu from the circulating 64Cu-ATSM. These data indicate that the initial penicillamine dose should be administered after the blood radioactivity level has been adequately reduced and the tumor uptake has plateaued. In a clinical study with radiolabeled Cu-ATSM, the blood radioactivity level reportedly decreased and the tumor uptake achieved steady-state at approximately 20 min after injection [35]. Together, these data suggest that in humans, the adequate timing for penicillamine administration after 64Cu-ATSM injection might be earlier than in mice. PET guidance would be helpful not only for determining the therapeutic 64Cu-ATSM dose, but also for optimizing the penicillamine dose and administration schedule to improve patient outcomes in response to 64Cu-ATSM IRT.
Our study showed that the single-dose administration of 300 mg/kg penicillamine or the fractionated administration of 100 mg/kg×3 doses was appropriate for reducing radiation exposure to critical organs during 64Cu-ATSM IRT. Animal tests with penicillamine have also demonstrated that the LD50 in mice for single-dose administration is 720 mg/kg (Material Safety Data Sheet of penicillamine, CAS#52-67-5) and that doses less than 400 mg/kg/day did not induce any adverse effects [36]. These findings suggest that the penicillamine dose used in this study was sufficiently low, compared with the toxic levels, and could be safely applied to humans, although the human doses of penicillamine in combination with 64Cu-ATSM IRT must be carefully investigated in future clinical studies.
In recent years, due to the recognition of the usefulness of 64Cu and its improved availability, drug development and human translational studies with 64Cu-labeled compounds have been accelerated. For example, 64Cu-chelator conjugates such as 64Cu-TETA-octreotide and 64Cu-DOTA-trastuzumab have been used in clinical studies [37], [38]. It is noted that 64Cu-chelator conjugates often cause 64Cu accumulation in the liver due to the hepatic clearance and transchelation of 64Cu [39], [40], [41]. In the present study, we developed a novel strategy to remove accumulated 64Cu in the liver after 64Cu-ATSM injection. This method might be also applied to remove transchelated 64Cu in the liver subsequent to other 64Cu-labeled compounds. Further studies would be necessary to expand the applicability of this method in the future.
Conclusions
This study revealed that appropriately scheduled penicillamine administration could reduce radiation to critical organs during 64Cu-ATSM IRT without reducing radiation to the tumor. This method therefore promotes the clinical application of 64Cu-ATSM IRT.
Supporting Information
Ability of penicillamine to remove 64Cu from 64Cu-ATSM. Incubation of 64Cu-ATSM in plasma with or without penicillamine. The y-axis shows the relative ratios of the fraction with intact 64Cu-ATSM and of the fraction at the origin with free 64Cu and 64Cu-penicillamine. Values are means ± SD, n = 3.
(TIF)
Time-activity curves for liver, small intestine, and large intestine. Time-activity curves were generated using biodistribution data in Figure 3 and Figure 4. (A) Single-dose administration of penicillamine. (B) Fractionated-dose administration of penicillamine and co-administration of a laxative with penicillamine. Statistical significance at each time point is shown in Figure 3 and Figure 4.
(TIF)
Quantification of defecation after glycerin enema in mice. Values indicate the weight of collected fecal matter during a 30-min period after glycerin treatment or from untreated control mice. *P<0.05. Values are means ± SD, n = 3.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank Mr. Hisashi Suzuki for providing the radiopharmaceuticals.
Funding Statement
This work was supported by the Japan Advanced Molecular Imaging Program from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lewis J, Laforest R, Buettner T, Song S, Fujibayashi Y, et al. (2001) Copper-64-diacetyl-bis(N 4-methylthiosemicarbazone): An agent for radiotherapy. Proc Natl Acad Sci U S A 98: 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ (1999) Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med 40: 177–183. [PubMed] [Google Scholar]
- 3. Obata A, Kasamatsu S, Lewis JS, Furukawa T, Takamatsu S, et al. (2005) Basic characterization of 64Cu-ATSM as a radiotherapy agent. Nucl Med Biol 32: 21–28. [DOI] [PubMed] [Google Scholar]
- 4. Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, et al. (2008) Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N 4-methylthiosemicarbazone). J Nucl Med 49: 201–205. [DOI] [PubMed] [Google Scholar]
- 5. Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, et al. (1997) Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med 38: 1155–1160. [PubMed] [Google Scholar]
- 6. Lewis JS, Sharp TL, Laforest R, Fujibayashi Y, Welch MJ (2001) Tumor uptake of copper-diacetyl-bis(N 4-methylthiosemicarbazone): effect of changes in tissue oxygenation. J Nucl Med 42: 655–661. [PubMed] [Google Scholar]
- 7. Dietz DW, Dehdashti F, Grigsby PW, Malyapa RS, Myerson RJ, et al. (2008) Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rectum 51: 1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewis JS, Laforest R, Dehdashti F, Grigsby PW, Welch MJ, et al. (2008) An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J Nucl Med 49: 1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dearling JL, Lewis JS, Mullen GE, Welch MJ, Blower PJ (2002) Copper bis(thiosemicarbazone) complexes as hypoxia imaging agents: structure-activity relationships. J Biol Inorg Chem 7: 249–259. [DOI] [PubMed] [Google Scholar]
- 10. Obata A, Yoshimi E, Waki A, Lewis JS, Oyama N, et al. (2001) Retention mechanism of hypoxia selective nuclear imaging/radiotherapeutic agent Cu-diacetyl-bis(N 4-methylthiosemicarbazone) (Cu-ATSM) in tumor cells. Ann Nucl Med 15: 499–504. [DOI] [PubMed] [Google Scholar]
- 11. Oh M, Tanaka T, Kobayashi M, Furukawa T, Mori T, et al. (2009) Radio-copper-labeled Cu-ATSM: an indicator of quiescent but clonogenic cells under mild hypoxia in a Lewis lung carcinoma model. Nucl Med Biol 36: 419–426. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka T, Furukawa T, Fujieda S, Kasamatsu S, Yonekura Y, et al. (2006) Double-tracer autoradiography with Cu-ATSM/FDG and immunohistochemical interpretation in four different mouse implanted tumor models. Nucl Med Biol 33: 743–750. [DOI] [PubMed] [Google Scholar]
- 13. Bowen SR, van der Kogel AJ, Nordsmark M, Bentzen SM, Jeraj R (2011) Characterization of positron emission tomography hypoxia tracer uptake and tissue oxygenation via electrochemical modeling. Nucl Med Biol 38: 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holland JP, Giansiracusa JH, Bell SG, Wong LL, Dilworth JR (2009) In vitro kinetic studies on the mechanism of oxygen-dependent cellular uptake of copper radiopharmaceuticals. Phys Med Biol 54: 2103–2119. [DOI] [PubMed] [Google Scholar]
- 15. Yoshii Y, Yoneda M, Ikawa M, Furukawa T, Kiyono Y, et al. (2012) Radiolabeled Cu-ATSM as a novel indicator of overreduced intracellular state due to mitochondrial dysfunction: studies with mitochondrial DNA-less ρ 0 cells and cybrids carrying MELAS mitochondrial DNA mutation. Nucl Med Biol 39: 177–185. [DOI] [PubMed] [Google Scholar]
- 16. Aft RL, Lewis JS, Zhang F, Kim J, Welch MJ (2003) Enhancing targeted radiotherapy by copper(II)diacetyl- bis(N 4-methylthiosemicarbazone) using 2-deoxy-D-glucose. Cancer Res 63: 5496–5504. [PubMed] [Google Scholar]
- 17. Yoshii Y, Furukawa T, Kiyono Y, Watanabe R, Mori T, et al. (2011) Internal radiotherapy with copper-64-diacetyl-bis (N 4-methylthiosemicarbazone) reduces CD133+ highly tumorigenic cells and metastatic ability of mouse colon carcinoma. Nucl Med Biol 38: 151–157. [DOI] [PubMed] [Google Scholar]
- 18. Yoshii Y, Furukawa T, Kiyono Y, Watanabe R, Waki A, et al. (2010) Copper-64-diacetyl-bis (N 4-methylthiosemicarbazone) accumulates in rich regions of CD133+ highly tumorigenic cells in mouse colon carcinoma. Nucl Med Biol 37: 395–404. [DOI] [PubMed] [Google Scholar]
- 19. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, et al. (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760. [DOI] [PubMed] [Google Scholar]
- 20. Liu G, Yuan X, Zeng Z, Tunici P, Ng H, et al. (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laforest R, Dehdashti F, Lewis JS, Schwarz SW (2005) Dosimetry of 60/61/62/64Cu-ATSM: a hypoxia imaging agent for PET. Eur J Nucl Med Mol Imaging 32: 764–770. [DOI] [PubMed] [Google Scholar]
- 22. Shimada H, Fukudome S, Kiyozumi M, Funakoshi T, Adachi T, et al. (1993) Further study of effects of chelating agents on excretion of inorganic mercury in rats. Toxicology 77: 157–169. [DOI] [PubMed] [Google Scholar]
- 23. Walshe JM (1964) Endogenous copper clearance in Wilson’s disease: a study of the mode of action of penicillamine. Clin Sci 26: 461–469. [PubMed] [Google Scholar]
- 24.Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, et al.. (1999) Basic neurochemistry: molecular, cellular and medical aspects. 6th ed. Philadelphia: Lippincott-Raven.
- 25. Walker LR, Rattigan M, Canterino J (2011) A case of isolated elevated copper levels during pregnancy. J Pregnancy 2011: 385767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nozu T, Tanaka I, Nanpo T, Otsuki T, Yokoshima T (1977) Metabolic fate of 14C-penicillamine (I) Absorption distribution and excretion of 14C-D-penicillamine in rats. Ouyouyakuri 14: 265–276. [Google Scholar]
- 27. Obata A, Kasamatsu S, McCarthy DW, Welch MJ, Saji H, et al. (2003) Production of therapeutic quantities of 64Cu using a 12 MeV cyclotron. Nucl Med Biol 30: 535–539. [DOI] [PubMed] [Google Scholar]
- 28. Jalilian AR, Rostampour N, Rowshanfarzad P, Shafaii K, Kamali-Dehghan M, et al. (2009) Preclinical studies of [61Cu]ATSM as a PET radiopharmaceutical for fibrosarcoma imaging. Acta Pharm 59: 45–55. [DOI] [PubMed] [Google Scholar]
- 29. Tsuji AB, Kato K, Sugyo A, Okada M, Sudo H, et al. (2012) Comparison of 2-amino-[3–11C]isobutyric acid and 2-deoxy-2-[18F]fluoro-D-glucose in nude mice with xenografted tumors and acute inflammation. Nucl Med Commun 33: 1058–1064. [DOI] [PubMed] [Google Scholar]
- 30. Tsuji AB, Morita M, Li XK, Sogawa C, Sudo H, et al. (2009) 18F-FDG PET for semiquantitative evaluation of acute allograft rejection and immunosuppressive therapy efficacy in rat models of liver transplantation. J Nucl Med 50: 827–830. [DOI] [PubMed] [Google Scholar]
- 31. Kirschner AS, Ice RD, Beierwaltes WH (1975) Author’s Reply: Radiation dosimetry of 131-I-19-iodocholesterol: The pitfalls of using tissue concentration data. J Nucl Med 16: 248–249. [PubMed] [Google Scholar]
- 32. Stabin MG, Sparks RB, Crowe E (2005) OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46: 1023–1027. [PubMed] [Google Scholar]
- 33.Fauci A, Braunwald E, Isselbacher K, Wilson J, Martin J, et al.. (1998) Harrison’s Principles of Internal Medicine, 14th ed. New York: Mc Graw-Hill. 525–526.
- 34. ICRP 2007 (2007) The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37: 2–4. [DOI] [PubMed] [Google Scholar]
- 35. Wong TZ, Lacy JL, Petry NA, Hawk TC, Sporn TA, et al. (2008) PET of hypoxia and perfusion with 62Cu-ATSM and 62Cu-PTSM using a 62Zn/62Cu generator. AJR Am J Roentgenol 190: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khalil-Manesh F, Price RG (1983) Effect of D-penicillamine on glomerular basement membrane, urinary N-acetyl-beta-D-glucosaminidase and protein excretion in rats. Toxicology 26: 325–334. [DOI] [PubMed] [Google Scholar]
- 37. Tamura K, Kurihara H, Yonemori K, Takahashi K, Wada Y, et al. (2012) 64Cu-DOTA-trastuzumab-PET imaging in patients with HER2-positive breast cancer. J Clin Oncol 30 suppl:10519 [Google Scholar]
- 38. Anderson CJ, Dehdashti F, Cutler PD, Schwarz SW, Laforest R, et al. (2001) 64Cu-TETA-octreotide as a PET imaging agent for patients with neuroendocrine tumors. J Nucl Med 42: 213–221. [PubMed] [Google Scholar]
- 39. Wadas TJ, Wong EH, Weisman GR, Anderson CJ (2007) Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr Pharm Des 13: 3–16. [DOI] [PubMed] [Google Scholar]
- 40. Liu Z, Li ZB, Cao Q, Liu S, Wang F, et al. (2009) Small-animal PET of tumors with 64Cu-labeled RGD-bombesin heterodimer. J Nucl Med 50: 1168–1177. [DOI] [PubMed] [Google Scholar]
- 41. Paudyal P, Paudyal B, Hanaoka H, Oriuchi N, Iida Y, et al. (2010) Imaging and biodistribution of Her2/neu expression in non-small cell lung cancer xenografts with Cu-labeled trastuzumab PET. Cancer Sci 101: 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ability of penicillamine to remove 64Cu from 64Cu-ATSM. Incubation of 64Cu-ATSM in plasma with or without penicillamine. The y-axis shows the relative ratios of the fraction with intact 64Cu-ATSM and of the fraction at the origin with free 64Cu and 64Cu-penicillamine. Values are means ± SD, n = 3.
(TIF)
Time-activity curves for liver, small intestine, and large intestine. Time-activity curves were generated using biodistribution data in Figure 3 and Figure 4. (A) Single-dose administration of penicillamine. (B) Fractionated-dose administration of penicillamine and co-administration of a laxative with penicillamine. Statistical significance at each time point is shown in Figure 3 and Figure 4.
(TIF)
Quantification of defecation after glycerin enema in mice. Values indicate the weight of collected fecal matter during a 30-min period after glycerin treatment or from untreated control mice. *P<0.05. Values are means ± SD, n = 3.
(TIF)
(DOCX)
(DOCX)