Abstract
Sucrose (Suc) synthase (SuSy) is believed to function in channeling UDP-Glc from Suc to various β-glucan synthases. We produced transgenic poplars (Populus alba) overexpressing a mutant form (S11E) of mung bean (Vigna radiata) SuSy, which appeared in part in the microsomal membranes of the stems. Expression of SuSy in these membranes enhanced the incorporation of radioactive Suc into cellulose, together with the metabolic recycling of fructose (Fru), when dual-labeled Suc was fed directly into the phloem of the leaf. This overexpression also enhanced the direct incorporation of the glucosyl moiety of Suc into the glucan backbone of xyloglucan and increased recycling of Fru, although the Fru recycling system for cellulose synthesis at the plasma membrane might differ from that for xyloglucan synthesis in the Golgi network. These findings suggest that some of the Suc loaded into the phloem of a poplar leaf is used directly by SuSys associated with xyloglucan and cellulose synthases in the stem. This may be a key function of SuSy because the high-energy bond between the Glc and Fru moieties of Suc is conserved and used for polysaccharide syntheses in this sink tissue.
Suc synthase (SuSy) catalyzes the reversible interconversion of Suc + UDP ⇌UDP-Glc + Fru. This enzyme is involved in cellulose biosynthesis by channeling UDP-Glc to cellulose synthase for secondary wall synthesis in cotton fibers (Amor et al., 1995). The involvement of SuSy also was shown recently to be associated with callose synthase during the development of the root cap and cell plate (Hong et al., 2001; Subbaiah and Sachs, 2001) and with mixed-linkage β(1→3),(1→4)-glucan synthase in the Golgi membrane (Buckeridge et al., 1999). The coupled reaction between mutant (S11E) mung bean (Vigna radiata) SuSy and membrane-bound callose synthase occurred predominantly to channel Glc from Sucderived UDP-Glc into callose (Konishi et al., 2001). The production of UDP-Glc by SuSy is an energy conservation mechanism for ATP in growing cells because the UDP formed from UDP-Glc by β-glucan synthase can be efficiently and rapidly recycled back to UDP-Glc by SuSy. The repression of SuSy by antisense RNA decreased the activity of SuSy, which further resulted in a decrease in the amount of cellulose in carrot (Daucus carota; Tang et al., 1999) and a decrease in fruit setting and Suc unloading capacity of young tomato (Lycopersicon esculentum) fruit (D'Aoust et al., 1999). On the other hand, the expression of the S11E mutant mung bean SuSy in Acetobacter xylinum not only changed Suc metabolism but also enhanced cellulose production by preventing the accumulation of UDP during cellulose biosynthesis (Nakai et al., 1999), likely because UDP is a potent inhibitor of cellulose synthase in A. xylinum (Benziman et al., 1983). It should be possible to produce transgenic (trg) forest trees in which cellulose deposition is enhanced by the overexpression of SuSy. Certainly, SuSy in trunk tissues is related to cambial wood production and heartwood formation (Hauch and Magel, 1998). Thus, the overall aim of the present study is to assess the effect of overexpression of SuSy on the synthesis of cell wall polysaccharides in poplar (Populus alba) stem.
Mutant mung bean SuSy was used in this study because the replacement of Ser-11, the primary phosphorylation site for Ca2+-dependent protein kinases (Zhang and Chollet, 1997; Komina et al., 2002), by Glu-11 significantly decreased the enzyme Km value for Suc by a factor of 7.0 (Nakai et al., 1998). The Kcat to Km ratio, which reflects the overall catalytic efficiency of the S11E mutant enzyme with respect to Suc, was 3.1 times higher than that of the wild-type (wt) enzyme and 2.6 times higher than that of the phosphorylated Ser-11 enzyme. In addition, the S11E mutation resulted in the loss of the primary phosphorylation site in SuSy, whereas the wt enzyme was phosphorylated in vitro at Ser-11 by a Ca2+-dependent protein kinase from soybean (Glycine max) nodules (Nakai et al., 1998). Winter et al. (1997) suggested that the membrane-bound form of SuSy was not phosphorylated because phosphorylation in maize (Zea mays) changed the membrane-bound form to a soluble form. The membrane-bound form of SuSy is phosphorylated in nodules (Komina et al., 2002). The question is whether non-phosphorylatable SuSy is expressed in membranes and associated with β-glucan synthases to form polysaccharides. Expression of SuSy as the plasma membrane-bound form (Carlson and Chourey, 1996) and as the Golgi membrane-bound form (Buckeridge et al., 1999) could enhance the formation of cellulose and xyloglucan, respectively.
Suc is formed as the major photo-assimilate in the leaf mesophyll of most plant species, loaded by a plasma membrane-bound, phloem-specific Suc transporter from the apoplast into the conducting sieve tubes (Gottwald et al., 2000), translocated via the phloem to the stems, and incorporated into the sink tissues via companion cells either by a Suc transporter or with Suc cleavage by invertase and SuSy. Although the overall mechanism of transport and metabolism of Suc is largely unknown, the sugar is formed in the leaf and is transported to the stem. SuSy, Suc-phosphate synthase, and invertase, which synthesize and degrade Suc, are associated with the phloem unloading zone, in which a high level of SuSy occurs particularly in companion cells (Nolte and Koch, 1993). There are not only a number of ways for the Suc to be involved in the unloading and the loading process but also a number of fates for the Suc to be generated by different reactions. In this study, we employed two phloem loading techniques; one was the reverse flat system (Sovonick et al., 1974), and the other was the direct system. The latter was employed because the asymmetry between 14C and 3H was preserved in Suc. Then, the uptake of Suc was examined to study the process of phloem loading for long-distance transport. The specific question to be addressed is whether Suc loaded into the phloem of a source leaf is used directly for UDP-Glc formation in the sink tissue by conserving the high energy bond between Glc and Fru. Here, we loaded [14C-Glc][3H-Fru]Suc into the phloem of a poplar leaf and traced the movement of the Glc and Fru moieties through the petiole into wall polysaccharides of the stem. This technique allowed us to determine direct channeling of UDP-Glc and the recycling of Fru from the Suc molecule by calculating the relative incorporation of 14C and 3H into various polysaccharides.
RESULTS
trg Expression of Mutant (S11E) SuSy
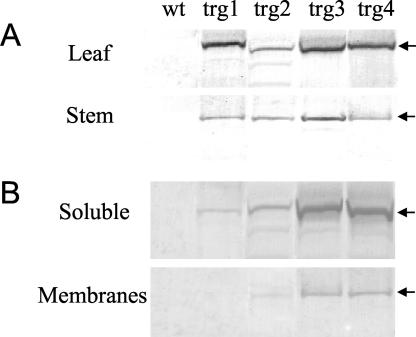
We generated 25 independent trg poplar lines that expressed a mutant mung bean SuSy under the control of a 35S constitutive promoter. Some trg plants grew a slightly better than wt, although the overall morphology of such trg plants was similar to that of the wt. Based on the expression of the transgene (Fig. 1), four typical lines that showed a similar growth pattern (about 30-cm stem length) were selected for further analysis. One-half of the trg plants resembled trg1 and 2 and one-half trg3 and 4 in the western-blot analysis. To assess the expression of the transgene, we used a polyclonal antibody against the recombinant product of the mutant mung bean SuSy gene, which was recognized as a 91-kD band on a western blot. SuSy in the leaves and stem of the trg plants was detected at a position corresponding to the expected size of mung bean SuSy polypeptide: The level of expression was relatively higher in leaves than in the stem (Fig. 1A). Although the level of expression was higher in the S100 fraction, the trg SuSy occurred in both the soluble and microsomal membrane (P100) fractions of the stem; a clearly visible signal was found in the membranes of trg3 and trg4, a much fainter signal in trg2, and no signal in trg1 (Fig. 1B). The activity of SuSy in the microsomal membranes of the stem was higher in trg3 and trg4, by a factor of 10 and 6, respectively, compared with trg1 and wt plants (Table I).
Figure 1.
Western-blot analysis of mutant mung bean SuSy in trg poplar A, Crude extracts from leaf and stem. B, Soluble and microsomal membrane fractions obtained from stem extracts after centrifugation at 100,000g. The arrows indicate 91-kD bands.
Table I.
Levels of SucSy activity in poplar stems se values were calculated from three samples per line.
| Plant
|
SuSy Activity
|
|
|---|---|---|
| S100 fraction | P100 fraction | |
| unit mg-1 protein | ||
| wt | 38 ± 9 | 22 ± 6 |
| trg1 | 67 ± 4 | 23 ± 4 |
| trg2 | 499 ± 148 | 58 ± 8 |
| trg3 | 894 ± 214 | 235 ± 54 |
| trg4 | 821 ± 124 | 131 ± 12 |
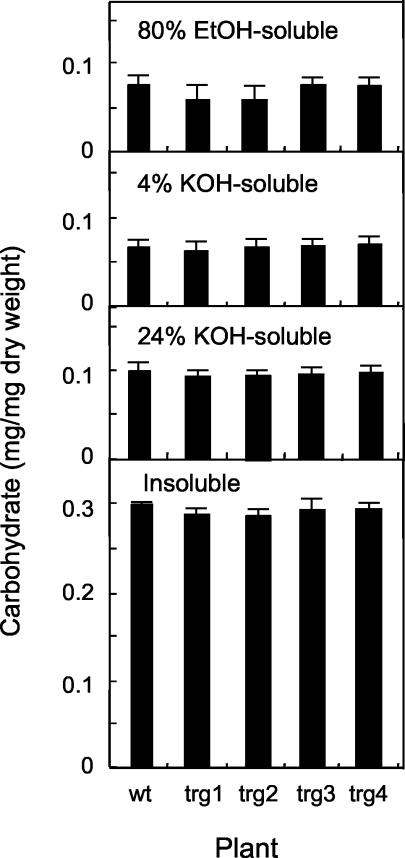
When wt and trg stems were sequentially extracted with 80% (v/v) ethanol, 4% (w/v) KOH, and 24% (w/v) KOH, about 60% of the carbohydrate was recovered in the 24% KOH-insoluble fraction (cellulose). The amounts of cellulose and non-cellulosic polysaccharides per unit dry weight were essentially the same in the four trg lines and wt plants (Fig. 2).
Figure 2.
Amounts of polysaccharides in 3-month-old wt and trg stems. Each sample was extracted sequentially with 80% (v/v) ethanol, 4% (w/v) KOH, and 24% (w/v) KOH, the latter giving rise to both soluble (hemicelluloses) and insoluble (cellulose) fractions. se values were calculated from five samples per line.
Incorporation of Dual-Labeled Suc into the Stem
To examine the effect of overexpressed mutant SuSy on Suc metabolism in the stem, a classical sink tissue, we loaded [14C-Glc][3H-Fru]Suc into the leaf phloem directly as described in “Materials and Methods” and measured the resulting radioactivity incorporated into various wall polysaccharide fractions of the stem. The efficiency of radioactivity transport from the leaf to the stem was 10% to 11% of the total dual-labeled Suc arriving at the stem and 11% to 12% at the root, but less than 2% of the Suc might be converted to [3H-Glc][14C-Fru]Suc in the phloem of the basal petiole. When the reverse flat system was employed for loading the dual-labeled Suc into the leaf phloem (Sovonick et al., 1974), the efficiency of the transport was increased up to 40% of the total radioactivity at the stem. In the phloem of basal petiole, however, the asymmetry between 14C and 3H was not preserved in Suc, in which the same levels of 14C to 3H were obtained in both Glc and Fru moieties.
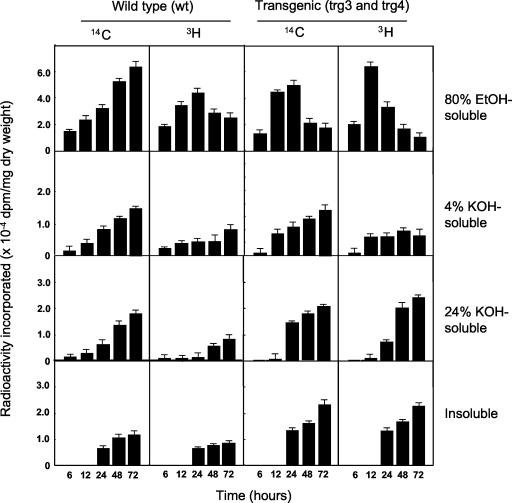
Overexpression of the S11E mutant SuSy in stem microsomal membranes (trg3 and trg4) caused a marked increase at 12 h and decrease after 12 or 24 h in the level of both 14C and 3H (80% [w/v] ethanolsoluble fraction), suggesting a high turnover of free sugars. Both 14C and 3H were gradually incorporated into the 4% (w/v) KOH- and 24% (w/v) KOH-soluble fractions (non-cellulosic polymers) with a distinct lag period in both the wt and trg plants. Radioactivity was initially incorporated into the 24% (w/v) KOH-insoluble fraction (cellulose) after 24 h. This result shows that radioactivity was incorporated into cellulose later than into non-cellulosic polymers, although cellulose is the major component of cell wall polysaccharides in the poplar stem (Fig. 3). In the wt, both 14C and 3H were gradually incorporated into the 4% (w/v) KOH- and 24% (w/v) KOH-soluble fractions, but the 3H moiety was less incorporated than the 14C moiety. Overexpression of mutant SuSy in the microsomal membranes of trg3 and 4 enhanced the incorporation of both 14C and 3H into the 24% (w/v) KOH-soluble and -insoluble fractions compared with wt. Furthermore, the overexpression of SuSy markedly increased the incorporation of 3H into the 24% (w/v) KOH-soluble fraction (hemicelluloses). At 24 h, this overexpression enhanced the incorporation of 14C and 3H into the 24% (w/v) KOH-soluble fraction (hemicelluloses) by factors of 2 and 4, respectively, and that into the 24% (w/v) KOH-insoluble fraction (cellulose) by a factor of 2, compared with wt (Table II). This incorporation of radioactivity was not significantly increased by the modest overexpression of mutant SuSy in trg1 and trg2, although the incorporation of the Suc-derived fructosyl moiety (3H) into the 24% (w/v) KOH-soluble fraction was increased 2- to 3-fold. It is likely that overexpression of the S11E mutant SuSy enhances the subsequent recycling of Fru.
Figure 3.
Incorporation of radioactivity from [14C-Glc][3H-Fru]Suc into the 80% (w/v) ethanol-soluble, 4% (w/v) KOH-soluble, and 24% (w/v) KOH-soluble and -insoluble fractions from poplar stems. se values were calculated from six samples for the wt plant and six samples each of trg3 and trg4 for trg plants.
Table II.
Incorporation of [14C-Glc][3H-Fru]Suc into various cell wall polysaccharide fractions of poplar stems after 24 h
Nos. in the table show picomoles of sugar incorporated from 1 mm [14C-Glc] (250 dpm pmol-1) and [3H-Fru] (250 dpm pmol-1) Suc per milligram dry wt after 24 h of incorporation. se values were calculated from six samples per line.
| Plant
|
4% (w/v) KOH-Soluble Fraction
|
24% (w/v) KOH-Soluble Fraction (Hemicelluloses)
|
Insoluble Fraction (Cellulose)
|
|||
|---|---|---|---|---|---|---|
| 14C | 3H | 14C | 3H | 14C | 3H | |
| pmol sugar incorporated mg dry wt-1 | ||||||
| wt | 73.8 ± 3.7 | 36.4 ± 5.1 | 45.1 ± 4.4 | 11.1 ± 1.5 | 39.2 ± 2.3 | 40.4 ± 4.8 |
| trg1 | 85.7 ± 4.6 | 30.8 ± 3.1 | 36.6 ± 4.5 | 20.7 ± 1.3 | 38.5 ± 3.6 | 39.7 ± 5.5 |
| trg2 | 86.4 ± 9.1 | 37.7 ± 6.2 | 31.7 ± 5.7 | 30.1 ± 4.4 | 41.3 ± 3.3 | 51.2 ± 4.5 |
| trg3 | 84.1 ± 1.0 | 38.8 ± 4.8 | 89.9 ± 3.4 | 47.5 ± 3.8 | 87.8 ± 5.5 | 84.7 ± 4.5 |
| trg4 | 74.1 ± 1.2 | 45.4 ± 4.6 | 82.3 ± 4.2 | 48.1 ± 3.5 | 82.3 ± 4.7 | 81.1 ± 3.0 |
Distribution of Radioactivity into Stem Xyloglucan
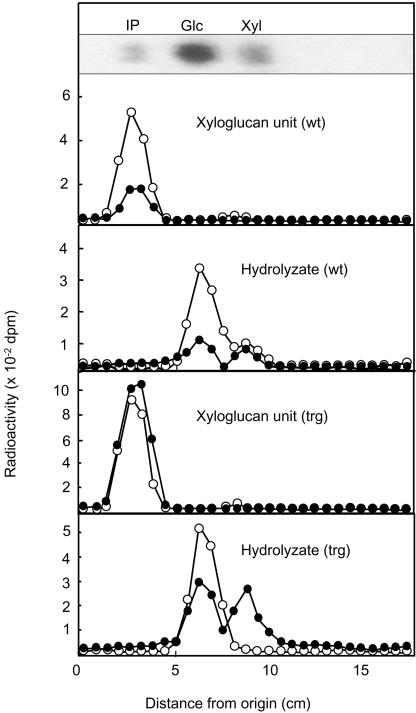
Next, the distribution of radioactivity in the heterogenous polysaccharide xyloglucan was examined in the wt and trg stems. Although xyloglucan consists of a 1,4-β-glucan backbone with xylosyl residues along the backbone, hydrolysis of this polysaccharide by Aspergillus oryzae enzymes yields isoprimeverose (6-O-α-d-xylopyranosyl-d-Glc), in which all of the xylosyl residues are recovered (Hayashi and Matsuda, 1981). Therefore, the stem hemicelluloses in the 24% (w/v) KOH-soluble fraction were digested with such enzymes to produce isoprimeverose, which is the smallest unit of xyloglucan. The radioactivity in isoprimeverose in wt poplar stems was composed of relatively high 14C and low 3H (Fig. 4). When the isoprimeverose was hydrolyzed with acid, 14C was recovered in Glc and Xyl in a molar ratio of 5:1, and 3H was distributed in Glc and Xyl in a molar ratio of 1:1. These results indicate that the [14C]glucosyl moiety of Suc was mainly incorporated into the glucan backbone, whereas the [3H]fructosyl moiety was incorporated equally into both glucosyl and xylosyl residues. Overexpression of mutant SuSy enhanced the incorporation of both 14C and 3H into isoprimeverose, although the level of 3H was somewhat higher than that of 14C. In the acid hydrolyzate of isoprimeverose, most of the 14C was in Glc, with a nearly equal distribution of 3H in Glc and Xyl. Because the total radioactivity in Xyl was not at all the same level as that in the glucosyl residue, some endogenous sugars might serve as the xylosyl residue for xyloglucan synthesis. Nevertheless, overexpression of the S11E mutant SuSy in trg poplars promoted the direct incorporation of Sucderived [14C]Glc into the glucosyl residue by lowering the interconversion of the Glc and Xyl residues.
Figure 4.
Distribution of radioactivity into the isoprimeverose unit of stem xyloglucan. White and black circles, 14C- and 3H-radioactivities, respectively. IP, Isoprimeverose.
DISCUSSION
Expression of mutant mung bean SuSy in microsomal membranes varied among trg lines of poplar. Its level of expression was always higher in the S100 soluble fraction than in the corresponding P100 membrane fraction and relatively higher in the leaf than in the stem. Thus, constitutive expression was low not only in the stem but also low in the microsomal membrane fraction of this organ. Nevertheless, the mutant SuSy expressed in some of the poplar stems was located in both the soluble and membrane fractions of the cells (trg3 and trg4 in Fig. 1B). This result shows that the localization of SuSy is not absolutely dependent on the phosphorylation state of Ser-11, although phosphorylation of SuSy in maize varied between the membrane-bound form and soluble forms (Winter et al., 1997). Our results are in general agreement with previous findings of Komina et al. (2002) that phosphorylation and dephosphorylation have no marked effect on the solubilization of membrane-bound SuSy. Some unknown control mechanism may be required for the expression of SuSy in the microsomal membranes because different isoforms of SuSy were shown to channel carbon from Suc into different metabolic fates in pea cells (Barratt et al., 2001).
This study provided evidence that at least some Suc was directly used for UDP-Glc and glucan formation without interconversion during transport from the phloem of the poplar leaf to the stem cells. By overexpression of an S11E mutant, SuSy, the Glc moiety of Suc was predominantly incorporated into the glucan backbone of xyloglucan in the stem. This may be a key function of SuSy because the high-energy bond between the Glc and Fru in Suc is preserved and used for polysaccharide syntheses in this sink tissue. It is possible that xyloglucan synthase forms a putative synthase complex, including SuSy, for utilizing this energy for the transfer of the glucosyl residue. Thus, xyloglucan 4-β-glucosyltransferase likely has a catalytic site and mechanism similar to those in the glucosyltransferases of cellulose, callose, and mixed-linkage β(1→3),(1→4)-glucan (Carpita and Vergara, 1998). The direct transfer of the glucosyl residue from Suc via UDP-Glc to xyloglucan and to cellulose may be a key step for their synthesis in poplar stems. The overexpression of SuSy could possibly enhance β-glucan synthesis, together with Fru recycling, if its expression occurs only in the membranes of this sink tissue.
Therefore, enhancement of cellulose and xyloglucan syntheses was derived not only from the direct transfer of the glucosyl moiety of Suc via UDP-Glc but also from the recycling of the Fru moiety by overexpression of SuSy. Enhanced Fru recycling was probably caused by the enhancement of UDP-Glc and Fru production by the increased activity of SuSy. It appears that Fru formed from Suc cleavage is directly and rapidly converted into UDP-Glc via the hexose-phosphate pool (i.e. Fru-6-P ⇌ Glc-6-P ⇌ Glc-1-P). Fru buildup may be prevented during UDP-Glc channeling, not only because a high concentration of Fru inhibits the activity of SuSy (Doehlert, 1987), but also because Fru buildup in this sink tissue may be coupled with a negative signaling related to photosynthesis (Quereix et al., 2001). Nevertheless, the Fru recycling system for cellulose synthesis at the plasma membrane may be different from that for xyloglucan synthesis in the Golgi network, possibly because the UDP-[3H]Glc formed in the Golgi could be interconverted to UDP-3H-sugars for the synthesis of heterogenous polysaccharides, whereas the Glc nucleotide formed at the plasma membrane is predominantly destined for the synthesis of cellulose. Although Suc-phosphate synthase for Suc biosynthesis also might be involved in the recycling of Fru in the stem (Haigler et al., 2001), an increased activity of SuSy in the stem resulted in the enhancement of Fru recycling for the synthesis of cellulose and xyloglucan.
No clear difference in gross phenotype was observed between the trg and wt poplar lines, and the deposition of polysaccharides was not increased in the trg plants compared with wt. This is probably because the level of expression was higher in leaves than in the stem, and photo-assimilated Suc was arrested by the overexpressed SuSy in leaves. In addition, there may be some regulatory mechanism that controls the transport of free sugars and sugar derivatives from source to sink tissues in plants.
MATERIALS AND METHODS
Construction of SuSy Fusion DNAs
The cDNA for mung bean (Vigna radiata) SuSy (VrSuSy; accession no. D10266) was amplified from pM-SS-5 as a template by PCR using a forward primer containing a BamHI site (5′-GAGGATCCGCCACCATGGCTACCGATCGTTTGACCCGTGTTCACGAACTCCGTGAGAGGC-3′) to mutagenize Ser-11 to Glu-11 and a reverse primer containing an SalI site (5′-TCTCGGTCGACAAGCCGGTTCCTCCATTTCTTCATCC-3′; Nakai et al., 1998). The BamHI-SalI fragment for this S11E mutant SuSy was subcloned into pBluescript II (SK-). The fragment was excised with BamHI and SalI and inserted into the BamHI-SalI site of the binary vector pBE2113-GUS, under control of the cauliflower mosaic virus 35S promoter and E12-Ω enhancer sequences (Mitsuhara et al., 1996).
Plant Transformation and Growth Conditions
The plasmid constructs were electroporated into Agrobacterium tumefaciens LBA4404, and leaves of aseptically flask-grown poplar (Populus alba) were inoculated with the bacteria by the tobacco leaf disc method (Ebinuma et al., 1997). Plantlets were grown in a growth chamber at 27°C under a photoperiod of 18 h.
Preparation of an Antibody against Mutant Mung Bean SuSy
Recombinant mutant (S11E) mung bean SuSy was expressed in Escherichia coli cells harboring the pET-32 Xa/LIC expression vector fused with the full-length cDNA for S11E SuSy. The recombinant protein was purified using a His-bind resin, digested with protease Factor Xa, and injected with Freund's adjuvant into a rabbit. The resulting antiserum was precipitated with ammonium sulfate at 20% to 50% (w/v) saturation.
Preparation of Soluble and Membrane Forms of Stem SuSy
Poplar stems were ground in liquid nitrogen and homogenized with 100 mm Tris/HCl (pH 7.0) containing 2 mm dithiothreitol and 1% (w/v) polyvinylpolypyrrolidone. The homogenate was centrifuged at 1,000g at 4°C for 10 min, and the supernatant fraction was centrifuged further at 100,000g at 4°C for 40 min. The S100 fluid was used as the soluble fraction, and the P100 precipitate was used for the crude microsomal membrane fraction.
Assay of SuSy
SuSy activity was measured according to the method described by Uggla et al. (2001). The enzyme preparation was incubated at 30°C for 30 min with 250 mm Suc and 6.25 mm UDP, in 250 mm HEPES/KOH (pH 7.0) and a total volume of 50 μL, and the reaction stopped at 100°C for 1 min. Then, UDP-Glc dehydrogenase (0.005 units) in 200 μL of 300 mm Gly/NaOH buffer (pH 8.6), containing 14 mm NAD, was added to the reaction mixture and further incubated at 25°C for 30 min. One unit of SuSy activity is defined as the amount of enzyme that generates 1 nmol UDP-Glc min-1.
Western-Blot Analysis
Proteins were subjected to electrophoresis by 10% (w/v) SDS-PAGE, electrotransferred to a Hybond-C Extra membrane (Amersham, Piscataway, NJ), and probed with primary antibody against mutant mung bean SuSy and then with secondary antibody using a TOYOBO-ABC high-HRP Immunostaining Kit (Toyobo, Tokyo). Protein was quantified by the method of Bradford using a protein assay kit (Pierce, Rockford, IL) and bovine serum albumin as standard.
Uptake of Dual-Labeled Suc into Cell Wall Polysaccharides
[14C-Glc]Suc (300 Ci mol-1) and [3H-Fru]Suc (10 Ci mmol-1; American Radiolabeled Chemicals, St. Louis) were purified two times by paper chromatography using 1-propanol:ethyl acetate:water (3:2:1 [v/v]) as solvent and eluting the Suc with water. Then, a 1 mm Suc solution (4 μL) containing [14C-Glc]Suc (1,000,000 dpm), [3H-Fru]Suc (1,000,000 dpm), 2 mm EDTA, and 50 mm MES/KOH (pH 5.5) was prepared.
Two-month-old trg poplar plants (about 30-cm stem length) were decapitated above the fifth or sixth node and defoliated, leaving only one uppermost leaf and about a 5-cm stem section. They were then planted in soil in a growth chamber for 2 weeks. The apical one-third of the leaf was excised, and 4 μL of 1 mm [14C-Glc][3H-Fru]Suc was immediately fed into the central main vein in the cut surface. The seedlings were cultured at 27°C in 14 h of light and 10 h of darkness. The radioactivity of each wall polysaccharide fraction (see below) was determined with a liquid scintillation counter. In the case of the reverse flat system, we loaded 4 μL of 1 mm [14C-Glc][3H-Fru]Suc in a capillary tube pushed over the severed vein of whole leaf.
To analyze the asymmetry level of dual-labeled Suc, both 14C to 3H in the Glc and Fru moieties of Suc were determined in Suc occurring in the basal petiole. The main vein of petiole was excised, and the end of the vein was spotted onto Whatman 3MM filter paper (Whatman, Clifton, NJ). The sample spotted on the paper was subjected directly to paper chromatography described above. Then, the area corresponding to Suc on the paper strip was excised, eluted with water, and subjected to acid hydrolysis with 0.1 m trifluoroacetic acid at 100°C for 1 h. The hydrolyzate was dried to remove trifluoroacetic acid and again subjected to the paper chromatography. The areas corresponding to Fru and Glc on the paper strip were excised, and their radioactivities were determined with a liquid scintillation counter.
Fractionation of Wall Polysaccharides
Stems were ground in liquid nitrogen, and the resulting powder was freeze dried. The sample was extracted sequentially three times each, with 80% (w/v) ethanol, 4% (w/v) KOH containing 0.1% NaBH4, and 24% (w/v) KOH containing 0.1% NaBH4. The final insoluble wall residue (cellulose fraction) was washed twice with water and solubilized with ice-cold 72% (w/v) sulfuric acid. Total sugar in each fraction was determined by the phenol-sulfuric acid method (Dubois et al., 1956).
Analysis of Xyloglucan
The 24% (w/v) KOH-soluble hemicellulose fraction (see above) was neutralized with acetic acid and dialyzed against water. The sample was then digested with 1 mg of Aspergillus oryzae enzyme preparation at 40°C for 12 h (Hayashi and Matsuda, 1981). The digest was passed through a column (0.5 × 1 cm) of Dowex 50W (H+ type) resin, and the flow-through was subjected to paper chromatography using 1-propanol:ethyl acetate:water (3:2:1 [v/v]) as solvent. The areas corresponding to isoprimeverose on the paper strip were excised and eluted with water. This oligosaccharide was hydrolyzed in 0.1 n trifluoroacetic acid at 100°C for 1 h, and the hydrolyzate was dried to remove trifluoroacetic acid. The hydrolyzate was subjected again to paper chromatography.
Acknowledgments
Raymond Chollet critically read this manuscript. We thank Hiroaki Hayashi, Candace H. Haigler, and Donald Geiger for valuable discussion.
We are grateful to Keiko Yamada for helpful comments on the transformation of poplar via Agrobacterium.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.033167.
References
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 92: 9353-9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt DHP, Barber L, Kruger NJ, Smith AM, Wang TL, Martin C (2001) Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol 127: 655-664 [PMC free article] [PubMed] [Google Scholar]
- Benziman M, Delmer DP, Aloni Y (1983) Unique regulatory properties of the UDP-glucose:1,4-β-d-glucan synthase of Acetobacter xylinum. J Appl Polym Sci 37: 131-143 [PubMed] [Google Scholar]
- Buckeridge MS, Vergara CE, Carpita NC (1999) The mechanism of synthesis of a mixed-linkage (1→3),(1→4)β-d-glucan in maize: evidence for multiple sites of glucosyl transfer in the synthase complex. Plant Physiol 120: 1105-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SJ, Chourey P (1996) Evidence for plasma membrane-associated forms of sucrose synthase in maize. Mol Gen Genet 252: 303-310 [DOI] [PubMed] [Google Scholar]
- Carpita NC, Vergara CE (1998) A recipe for cellulose. Science 279: 672-673 [DOI] [PubMed] [Google Scholar]
- D'Aoust MA, Yelle S, Nguyen-Quoc B (1999) Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. Plant Cell 11: 2407-2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert DC (1987) Substrate inhibition of maize endosperm sucrose synthase by fructose and its interaction with glucose inhibition. Plant Sci 52: 153-157 [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350-356 [Google Scholar]
- Ebinuma H, Sugita K, Matsunaga E, Yamakado M (1997) Selection of marker-free transgenic plants using the isopentenyl transferase gene. Proc Natl Acad Sci USA 94: 2117-2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97: 13979-13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin K, Delmer DP (2001) Carbon partitioning to cellulose synthesis. Plant Mol Biol 47: 29-51 [PubMed] [Google Scholar]
- Hauch S, Magel E (1998) Extractable activities and protein content of sucrose-phosphate synthase, sucrose synthase and neutral invertase in trunk tissues of Robinia pseudoacacia L. are related to cambial wood production and heartwood formation. Planta 207: 266-274 [Google Scholar]
- Hayashi T, Matsuda K (1981) Biosynthesis of xyloglucan in suspension-cultured soybean cells: occurrence and some properties of xyloglucan 4-β-d-glucosyltransferase and 6-α-d-xylosyltransferase. J Biol Chem 256: 11117-11122 [PubMed] [Google Scholar]
- Hong Z, Zhang Z, Olson JM, Verma DPS (2001) A novel UDP-glucose transferase is part of the callose synthase complex and interacts with phragmoplastin at the forming cell plate. Plant Cell 13: 769-779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komina O, Zhou Y, Sarath G, Chollet R (2002) In vivo and in vitro phosphorylation of membrane and soluble forms of soybean nodule sucrose synthase. Plant Physiol 129: 1664-1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T, Nakai T, Sakai F, Hayashi T (2001) Formation of callose from sucrose in cotton fiber microsomal membranes. J Wood Sci 47: 331-335 [Google Scholar]
- Mitsuhara I, Ugaki M, Hirochika H, Ohshima M, Murakami T, Gotoh Y, Katayose Y, Nakamura S, Honkura R, Nishimiya S et al. (1996) Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol 37: 49-59 [DOI] [PubMed] [Google Scholar]
- Nakai T, Konishi T, Zhang X-Q, Chollet R, Tonouchi N, Tsuchida T, Yoshinaga F, Mori H, Sakai F, Hayashi T (1998) An increase in apparent affinity for sucrose of mung bean sucrose synthase is caused by in vitro phosphorylation or directed mutagenesis of Ser11. Plant Cell Physiol 39: 1337-1341 [DOI] [PubMed] [Google Scholar]
- Nakai T, Tonouchi N, Konishi T, Kojima Y, Tsuchida T, Yoshinaga F, Sakai F, Hayashi T (1999) Enhancement of cellulose production by expression of sucrose synthase in Acetobacter xylinum. Proc Natl Acad Sci USA 96: 14-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte KD, Koch KE (1993) Companion-cell specific localization of sucrose synthase in zones of phloem loading and unloading. Plant Physiol 101: 899-905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quereix A, Dewar RC, Gaudillere JP, Dayau S, Valancogne C (2001) Sink feedback regulation of photosynthesis in vines: measurements and a model. J Exp Bot 52: 2313-2322 [DOI] [PubMed] [Google Scholar]
- Sovonick SA, Geiger DR, Fellows RJ (1974) Evidence for active phloem loading in the minor veins of sugar beet. Plant Physiol 54: 886-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Sachs MM (2001) Altered patterns of sucrose synthase phosphorylation and localization precede callose induction and root tip death in anoxic maize seedlings. Plant Physiol 125: 585-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G-Q, Luscher M, Sturm A (1999) Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell 11: 177-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Magel E, Moritz T, Sundberg B (2001) Function and dynamic of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Physiol 125: 2029-2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC (1997) Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett 420: 151-155 [DOI] [PubMed] [Google Scholar]
- Zhang X-Q, Chollet R (1997) Seryl-phosphorylation of soybean noudle sucrose synthase (nodulin-100) by a Ca2+-dependent protein kinase. FEBS Lett 410: 126-30 [DOI] [PubMed] [Google Scholar]