Abstract
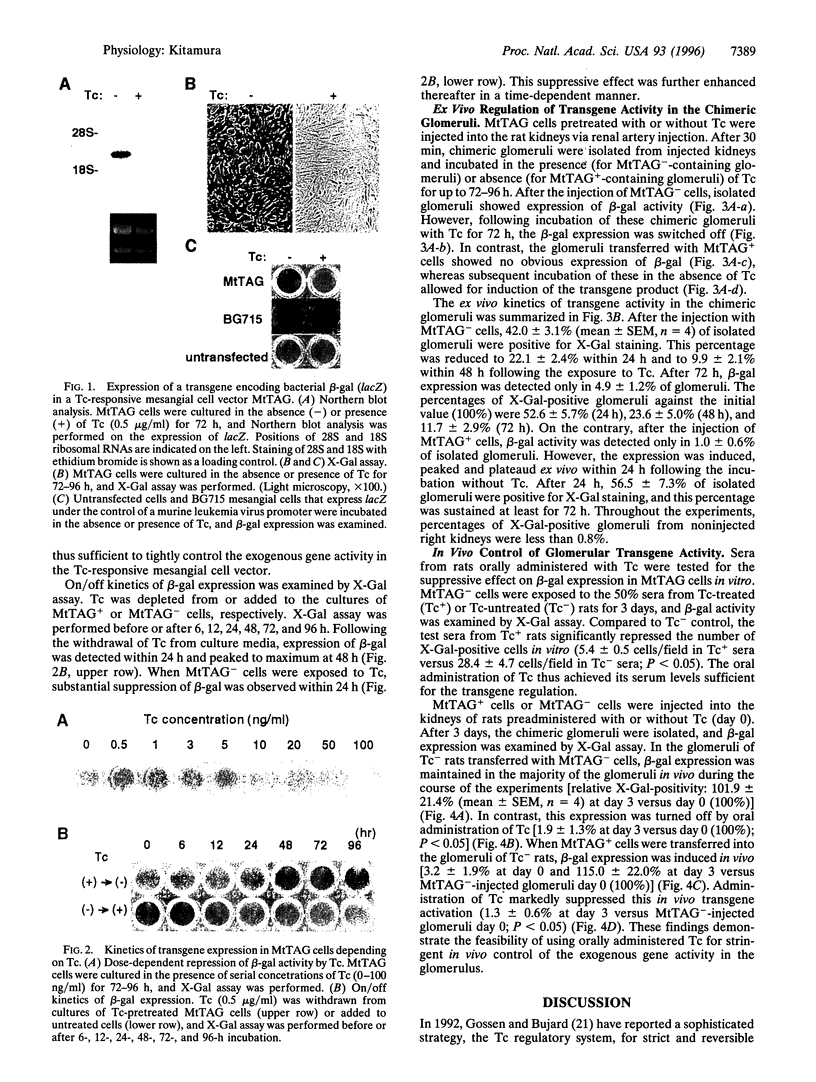
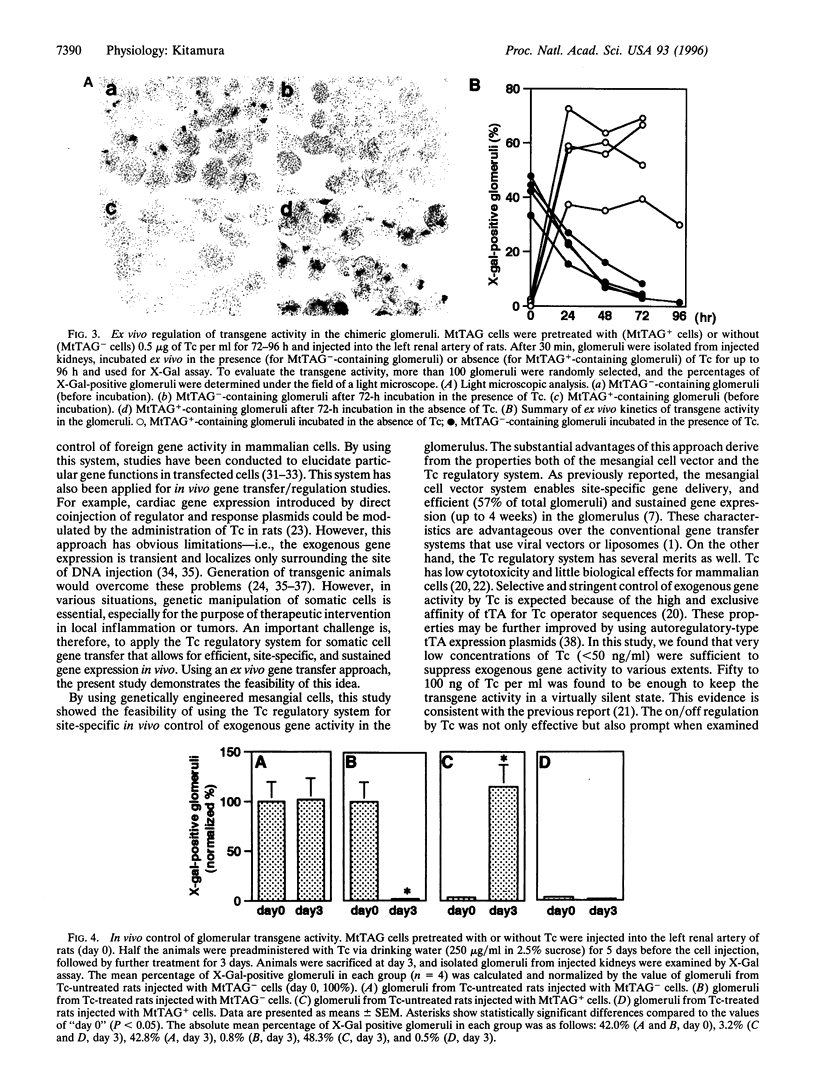
Using genetically engineered glomerular mesangial cells, an in vivo gene transfer approach was developed that specifically targets the renal glomerulus. By combining this system with a tetracycline (Tc)-responsive promoter, the present study aimed to create a reversible on/off system for site-specific in vivo control of exogenous gene activity within the glomerulus. In the Tc regulatory system, a Tc-controlled transactivator (tTA) encoded by a regulator plasmid induces target gene transcription by binding to a tTA-responsive promoter located in a response plasmid. Tc inhibits this tTA-dependent transactivation via its affinity for tTA. In double-transfected cells, therefore, the activity of a transgene can be controlled by Tc. Cultured rat mesangial cells were cotransfected with a regulator plasmid and a response plasmid that introduces a beta-galactosidase gene. In vitro, stable double-transfectant MtTAG cells exhibited no beta-galactosidase activity in the presence of Tc. However, following withdrawal of Tc from culture media, expression of beta-galactosidase was induced within 24 h. When Tc was again added, the expression was rapidly resuppressed. Low concentrations of Tc were sufficient to maintain the silent state of tTA-dependent promoter. MtTAG cells were then transferred into the rat glomeruli via renal artery injection. In the isolated chimeric glomeruli, expression of beta-galactosidase was induced ex vivo in the absence of Tc, whereas it was repressed in its presence. When Tc-pretreated MtTAG cells were transferred into the glomeruli of untreated rats, beta-galactosidase expression was induced in vivo within 3 days. Oral administration of Tc dramatically suppressed this induction. These data demonstrate the feasibility of using mesangial cell vectors combined with the Tc regulatory system for site-specific in vivo control of exogenous gene expression in the glomerulus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baim S. B., Labow M. A., Levine A. J., Shenk T. A chimeric mammalian transactivator based on the lac repressor that is regulated by temperature and isopropyl beta-D-thiogalactopyranoside. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5072–5076. doi: 10.1073/pnas.88.12.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckbinder L., Talbott R., Seizinger B. R., Kley N. Gene regulation by temperature-sensitive p53 mutants: identification of p53 response genes. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10640–10644. doi: 10.1073/pnas.91.22.10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Efrat S., Fusco-DeMane D., Lemberg H., al Emran O., Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3576–3580. doi: 10.1073/pnas.92.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figge J., Wright C., Collins C. J., Roberts T. M., Livingston D. M. Stringent regulation of stably integrated chloramphenicol acetyl transferase genes by E. coli lac repressor in monkey cells. Cell. 1988 Mar 11;52(5):713–722. doi: 10.1016/0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Fishman G. I., Kaplan M. L., Buttrick P. M. Tetracycline-regulated cardiac gene expression in vivo. J Clin Invest. 1994 Apr;93(4):1864–1868. doi: 10.1172/JCI117174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth P. A., St Onge L., Böger H., Gruss P., Gossen M., Kistner A., Bujard H., Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9302–9306. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bonin A. L., Bujard H. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem Sci. 1993 Dec;18(12):471–475. doi: 10.1016/0968-0004(93)90009-c. [DOI] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Anhydrotetracycline, a novel effector for tetracycline controlled gene expression systems in eukaryotic cells. Nucleic Acids Res. 1993 Sep 11;21(18):4411–4412. doi: 10.1093/nar/21.18.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan D. E., Mauceri H. J., Seung L. P., Dunphy E. J., Wayne J. D., Hanna N. N., Toledano A., Hellman S., Kufe D. W., Weichselbaum R. R. Spatial and temporal control of gene therapy using ionizing radiation. Nat Med. 1995 Aug;1(8):786–791. doi: 10.1038/nm0895-786. [DOI] [PubMed] [Google Scholar]
- Hu M. C., Davidson N. The inducible lac operator-repressor system is functional in mammalian cells. Cell. 1987 Feb 27;48(4):555–566. doi: 10.1016/0092-8674(87)90234-0. [DOI] [PubMed] [Google Scholar]
- Isaka Y., Fujiwara Y., Ueda N., Kaneda Y., Kamada T., Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993 Dec;92(6):2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel D. I., Kaufman R. J. Highly inducible expression from vectors containing multiple GRE's in CHO cells overexpressing the glucocorticoid receptor. Nucleic Acids Res. 1989 Jun 26;17(12):4589–4604. doi: 10.1093/nar/17.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. N., Jones P. G., Ibarguen H., Caskey C. T., Craigen W. J. Induction of the Cyp1a-1 dioxin-responsive enhancer in transgenic mice. Nucleic Acids Res. 1991 Dec 11;19(23):6547–6551. doi: 10.1093/nar/19.23.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff S., Köster M., Wirth M., Schaper F., Gossen M., Bujard H., Hauser H. Identification of mammalian cell clones exhibiting highly regulated expression from inducible promoters. Trends Genet. 1995 Jun;11(6):219–220. doi: 10.1016/s0168-9525(00)89053-8. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Burton S., English J., Kawachi H., Fine L. G. Transfer of a mutated gene encoding active transforming growth factor-beta 1 suppresses mitogenesis and IL-1 response in the glomerulus. Kidney Int. 1995 Dec;48(6):1747–1757. doi: 10.1038/ki.1995.473. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Burton S., Yokoo T., Fine L. G. Gene delivery into the renal glomerulus by transfer of genetically engineered, autologous mesangial cells. Exp Nephrol. 1996 Jan-Feb;4(1):56–59. [PubMed] [Google Scholar]
- Kitamura M., Mitarai T., Maruyama N., Nagasawa R., Yoshida H., Sakai O. Mesangial cell behavior in a three-dimensional extracellular matrix. Kidney Int. 1991 Oct;40(4):653–661. doi: 10.1038/ki.1991.257. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Shirasawa T., Maruyama N. Gene transfer of metalloproteinase transin induces aberrant behavior of cultured mesangial cells. Kidney Int. 1994 Jun;45(6):1580–1586. doi: 10.1038/ki.1994.208. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Sütö T., Yokoo T., Shimizu F., Fine L. G. Transforming growth factor-beta 1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells . J Immunol. 1996 Apr 15;156(8):2964–2971. [PubMed] [Google Scholar]
- Kitamura M., Taylor S., Unwin R., Burton S., Shimizu F., Fine L. G. Gene transfer into the rat renal glomerulus via a mesangial cell vector: site-specific delivery, in situ amplification, and sustained expression of an exogenous gene in vivo. J Clin Invest. 1994 Aug;94(2):497–505. doi: 10.1172/JCI117361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M. Transfer of exogenous genes into the kidney. Exp Nephrol. 1994 Nov-Dec;2(6):313–317. [PubMed] [Google Scholar]
- Lin H., Parmacek M. S., Morle G., Bolling S., Leiden J. M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990 Dec;82(6):2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Warren R., Palmiter R. D. The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell. 1982 May;29(1):99–108. doi: 10.1016/0092-8674(82)90094-0. [DOI] [PubMed] [Google Scholar]
- Moullier P., Friedlander G., Calise D., Ronco P., Perricaudet M., Ferry N. Adenoviral-mediated gene transfer to renal tubular cells in vivo. Kidney Int. 1994 Apr;45(4):1220–1225. doi: 10.1038/ki.1994.162. [DOI] [PubMed] [Google Scholar]
- Passman R. S., Fishman G. I. Regulated expression of foreign genes in vivo after germline transfer. J Clin Invest. 1994 Dec;94(6):2421–2425. doi: 10.1172/JCI117609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescini R., Alouani S., Proudfoot A., Power C., Mermod J. J., DeLamarter J. F., Hooft van Huijsduijnen R. Inducible inhibition of eukaryotic gene expression. Biochem Biophys Res Commun. 1994 Aug 15;202(3):1664–1667. doi: 10.1006/bbrc.1994.2125. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Gossen M., Bujard H., Reed S. I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994 Mar;14(3):1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T. E., Follin A., Jones N. C., Jat P. S. Maintenance of cellular proliferation by adenovirus early region 1A in fibroblasts conditionally immortalized by using simian virus 40 large T antigen requires conserved region 1. Mol Cell Biol. 1990 Dec;10(12):6664–6673. doi: 10.1128/mcb.10.12.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinfest C. W., Jorcyk C. L., Fujiwara S., Papas T. S. A heat-shock-inducible eukaryotic expression vector. Gene. 1988 Nov 15;71(1):207–210. doi: 10.1016/0378-1119(88)90093-5. [DOI] [PubMed] [Google Scholar]
- Shockett P., Difilippantonio M., Hellman N., Schatz D. G. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita N., Higaki J., Morishita R., Kato K., Mikami H., Kaneda Y., Ogihara T. Direct in vivo gene introduction into rat kidney. Biochem Biophys Res Commun. 1992 Jul 15;186(1):129–134. doi: 10.1016/s0006-291x(05)80784-3. [DOI] [PubMed] [Google Scholar]
- Totzke F., Marmé D., Hug H. Inducible expression of human phospholipase C-gamma 2 and its activation by platelet-derived growth factor B-chain homodimer and platelet-derived growth factor A-chain homodimer in transfected NIH 3T3 fibroblasts. Eur J Biochem. 1992 Feb 1;203(3):633–639. doi: 10.1111/j.1432-1033.1992.tb16593.x. [DOI] [PubMed] [Google Scholar]
- Wimmel A., Lucibello F. C., Sewing A., Adolph S., Müller R. Inducible acceleration of G1 progression through tetracycline-regulated expression of human cyclin E. Oncogene. 1994 Mar;9(3):995–997. [PubMed] [Google Scholar]
- Yarranton G. T. Inducible vectors for expression in mammalian cells. Curr Opin Biotechnol. 1992 Oct;3(5):506–511. doi: 10.1016/0958-1669(92)90078-w. [DOI] [PubMed] [Google Scholar]
- Yokoo T., Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol. 1996 Jan;270(1 Pt 2):F123–F130. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- von Harsdorf R., Schott R. J., Shen Y. T., Vatner S. F., Mahdavi V., Nadal-Ginard B. Gene injection into canine myocardium as a useful model for studying gene expression in the heart of large mammals. Circ Res. 1993 Mar;72(3):688–695. doi: 10.1161/01.res.72.3.688. [DOI] [PubMed] [Google Scholar]






