Abstract
Background:
Rhinitis and obstructive sleep apnea (OSA) often coexist during childhood. To delineate this clinical association, we examined OSA severity and polysomnogram (PSG) features in children with rhinitis and OSA. Given that rapid-eye-movement (REM) sleep is characterized by nasal congestion, we hypothesized that children with rhinitis have more REM-related breathing abnormalities.
Methods:
We conducted a retrospective cross-sectional analysis of 145 children with PSG-diagnosed OSA. Outcomes included PSG parameters and obstructive apnea–hypopnea index (OAHI) during REM and non-REM. Linear multivariable models examined the joint effect of rhinitis and OSA parameters with control for potential confounders.
Results:
Rhinitis was present in 43% of children with OSA (n = 63) but overall OAHI severity was unaffected by the presence of rhinitis. In contrast, OAHI during REM sleep in children with moderate–severe OSA was significantly increased in subjects with rhinitis and OSA (44.1/hr; SE = 6.4) compared with those with OSA alone (28.2/hr; SE = 3.8).
Conclusion:
Rhinitis is highly prevalent in children with OSA. Although OSA is not more severe in children with rhinitis, they do have a distinct OSA phenotype characterized by more REM-related OSA. Further research is needed to delineate the link between REM-sleep and the physiology of the nose during health and disease.
Keywords: Apnea, OSA, pediatric OSA, REM, REM-related OSA, rhinitis, sleep, sleep breathing
Obstructive sleep apnea (OSA) is a common condition that occurs in ∼3–4% of children.1 The pathogenesis of OSA in this age group involves nocturnal obstruction of the nasopharyngeal passages leading to sleep fragmentation and intermittent hypoxemia.2,3 Daytime consequences of this syndrome in children include hyperactivity, excessive daytime sleepiness, and systemic inflammation.3,4 Recent investigations have confirmed that individuals with OSA have marked nasal inflammatory changes,5,6 suggesting a potential link between OSA and rhinitis. This association is also suggested by the clinical observation that patients with allergic rhinitis have poor sleep quality according to actigraphic and Pittsburgh Sleep Quality Index variables.7 Adults with rhinitis have a higher prevalence of OSA,8 and those with rhinitis and OSA have more daytime sleepiness and lower quality of life according to the Epworth sleepiness scale and the Rhinosinusitis Quality of Life Questionnaire.9 The evidence for the association between rhinitis and OSA in children is less strong,10 in part because most pediatric studies use indirect measurements to document sleep-disordered breathing (i.e., snoring), instead of overnight polysomnogram (PSG) to confirm the diagnosis of pediatric OSA.10
The overall aim of this project was to study the connection between clinical rhinitis and OSA examining PSG data of children with both conditions. First, we conducted a retrospective cross-sectional analysis to determine the prevalence of rhinitis in a cohort of 145 children with OSA confirmed by PSG. We hypothesized that rhinitis is a very common condition in children with OSA and that the presence of rhinitis correlates with OSA severity according to standard PSG parameters such as obstructive apnea–hypopnea index (OAHI) and maximal nocturnal oxygen desaturation. Next, we used individual PSG signal processing to evaluate stage-specific breathing abnormalities (i.e., OSA during rapid-eye-movement [REM] sleep stage) as previously described.11 Given that REM sleep is characterized by significant nasal congestion12,13 and that REM sleep modulates the nocturnal phenotypical features of OSA in asthmatic children,11 we postulated that children with rhinitis and OSA would have more REM-related breathing abnormalities relative to those with OSA alone.
METHODS
Subjects
A database of all children that underwent routine overnight PSG at the Penn State Sleep Research and Treatment Center between January 2010 and June 2011 was reviewed. Patients were eligible for the study if a diagnosis of OSA had been made on their initial PSG. Infants, school age children, and young adolescents were included (2–13 years). Patients were excluded if they had respiratory failure (need for supplemental oxygen or positive airway pressure), central hypoventilation syndromes, congenital heart disease, severe developmental delay, cerebral palsy, genetic syndromes, craniofacial abnormalities, or neuromuscular disorders. Patients without complete clinical and PSG data available were also excluded from the study. Rhinitis status was determined based on electronic medical records reviewed in Penn State Children's Hospital and Penn State Sleep Research and Treatment Center. OSA was defined as an OAHI ≥ 1.5 events/hr14 and classified based on OAHI as mild (1.5–5/hr), moderate (5 to <10/hr), or severe (≥10/hr). Only the patient's initial PSG was included in the study. This study was approved by the Institutional Review Board of Penn State College of Medicine.
Sleep and Respiratory Recordings
PSG Protocol.
Standard pediatric overnight PSG was performed on all patients. During 9–10 hours the child's sleep was continuously recorded to a computerized system (Twin PSG software; Grass Technologies, Inc., West Warwick, RI) and scored manually in 30-second epochs according to American Academy of Sleep Medicine (AASM) standardized criteria.14 PSG measurements included electroencephalograms (C4-A1 and O2-A1), right and left electro-oculograms, electrocardiogram, mental–submental electromyogram, leg electromyogram, thoracic and abdominal wall motion (respiratory inductance plethysmography), pulse oximetry (with 2-second averaging time), end-tidal carbon dioxide monitoring (RespSense Capnograph; Grass Technologies. Inc.), combined nasal/oral thermistor, and nasal pressure (model TCT R; Grass-Telefactor, Inc). Objective estimate of snoring during the PSG was obtained with a microphone attached to the neck (model 1250G; Grass Technologies, Inc.). Infrared video monitoring was also routine. Body position was determined by a sensor and confirmed by direct observation throughout the night.
PSG Scoring and Analysis.
Sleep stages and respiratory events were scored according to the AASM pediatric scoring criteria.13 Five AASM sleep stages were identified (wake stage = W, stage 1 = N1, stage 2 = N2, stage 3 = N3, and stage REM = R). For the purpose of this investigation, we used OAHI and respiratory data calculated for W stage (beginning of PSG), non-REM (NREM) stage (represented by N1, N2, and N3), and REM sleep. The OAHI included obstructive apneas, hypopneas, and mixed apneas. The minimum respiratory event duration was ≥2 respiratory cycles. Obstructive apneas were scored if there was an absence of airflow with continued respiratory effort. Obstructive hypopneas were scored if there was a discernible decrease in airflow of ∼50% associated with either a ≥3% pulse oximetry saturation (SaO2) desaturation and/or an arousal. Mixed apneas were scored if there was a discernible decrease in airflow with a period of no respiratory effort and a period of continued respiratory effort associated with either a ≥3% SaO2 desaturation and/or an arousal. The OAHI was calculated separately during REM, NREM sleep, and total sleep time (TST). Pulse oximetry signal was examined separately and carefully cleaned from artifacts. “Maximal percentage of SaO2 desaturation” was calculated with the formula (SaO2 baseline − SaO2 nadir)/SaO2 baseline, and it was individually measured during NREM and REM sleep stages as previously described.11 Additional respiratory analyses were conducted to obtain baseline SaO2 during TST, wake, and NREM and REM sleep.
Rhinitis Status and other Covariables
Clinical and demographic variables were obtained reviewing electronic medical records. Rhinitis and atopic status were assessed based on preestablished criteria.15,16 The variable “rhinitis” was defined as physician diagnosis of rhinitis based on sneezing, runny or blocked nose without a cold, or the flu in the last 12 months according to International Study of Asthma and Allergies in Childhood methodology used for rhinitis.15,16 Most of the diagnoses were gathered from allergy or ear, nose, and throat visits so we believe that the information was fairly reliable. “Atopy” was defined as one of the following: (1) total IgE of >150 U/mL; (2) at least one positive allergen-specific IgE level (radioallergosorbent test or ImmunoCAP); (3) at least one positive allergen-specific skin test or; (4) eosinophilia, which was considered to be present if eosinophils were >4% of the total white blood cells.14,15 “Asthma” was defined in this pediatric population as the presence of (1) ever being diagnosed with asthma by a physician based on criteria recommended for children 0–4 years and 5–11 years of age in the National Institutes of Health National Asthma Education and Prevention Program guidelines17 and (2) use of asthma therapy and/or presence of asthma symptoms in the past 12 months. Other covariables investigated included age, race, sex, body mass index (BMI), atopy, rhinitis, percent of REM sleep, TST, and TST in supine position. The BMI percentile was used, which was calculated as the percentile of BMI distribution for age and gender, based on Center for Disease Control and Prevention criteria.18
Statistical Analysis
Data were analyzed using the software SAS Version 9.3 or later (SAS Institute, Inc., Cary, NC). Exploratory data analysis on main variables were performed for the entire study population, as well as stratified according to OSA and rhinitis status. OAHI and other respiratory parameters were calculated separately for REM and NREM sleep. The effect of rhinitis and OSA severity was first described by summary statistics (mean/SD) for each combination of levels. For pairwise relationships, two-sample t-test was used to compare the mean value of the continuous outcome measures. Nonparametric testing (i.e., Wilcoxon rank-sum) was also conducted for variables not normally distributed (i.e., OAHI). Chi-square test was used to compare the proportion of positive signals for binary outcomes. Multivariable linear regression model was built to study the joint effect of rhinitis and REM OAHI with control of possible confounders such as gender, age, BMI, atopy, and asthma. Significance was taken at the p < 0.05 level.
RESULTS
Study Population
One hundred forty-five children and young adolescents (2–13 years) were included in this study. Of the 262 subjects identified from the database with OSA, 117 were excluded because of craniofacial abnormalities or dysmorphic genetic syndromes (n = 49), cardiorespiratory conditions (n = 33), neuromuscular disease (n = 32), and incomplete clinical or polysomnographic records (n = 3). The total study population (n = 145) was subdivided into one group with OSA and rhinitis (n = 63) and another group with OSA alone (n = 82). Comparison of demographic, anthropometric, and baseline sleep study variables in these two groups revealed no significant differences (Table 1).
Table 1.
Demographic and polysomnographic profile of subjects
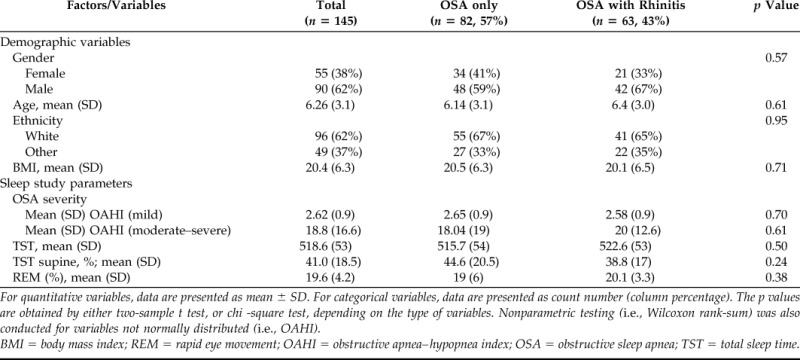
For quantitative variables, data are presented as mean ± SD. For categorical variables, data are presented as count number (column percentage). The p values are obtained by either two-sample t test, or chi -square test, depending on the type of variables. Nonparametric testing (i.e., Wilcoxon rank-sum) was also conducted for variables not normally distributed (i.e., OAHI).
BMI = body mass index; REM = rapid eye movement; OAHI = obstructive apnea–hypopnea index; OSA = obstructive sleep apnea; TST = total sleep time.
Rhinitis Is a Common Comorbidity in Children with OSA
Rhinitis was present in 43% (n = 63) of children with OSA, indicating that daytime nasal symptomatology is highly prevalent in this population. This prevalence is significantly higher than what has been reported in the same age group in the general population.15 Interestingly, OSA severity was unaffected by the presence of rhinitis in children with OSA. As illustrated in Table 1, the OAHI, which is a PSG parameter of OSA severity, was not impacted by the diagnosis of rhinitis in the group of children with mild OSA (2.65/hr OSA alone versus 2.58/hr OSA + rhinitis; p = 0.73) or in those with moderate/severe OSA (18/hr OSA alone versus 20/hr OSA + rhinitis; p = 0.65). Moreover, oxygen saturation (SaO2) parameters obtained during wake, and REM and NREM sleep in children with rhinitis and OSA were not significantly different relative to those in children with OSA alone (Table 2). Taken together, these results indicate that almost one-half of the children with OSA have associated rhinitis but that this comorbidity does not impact OSA severity or nocturnal hypoxemia in children with OSA.
Table 2.
Effect of rhinitis on nocturnal oxygenation in pediatric OSA
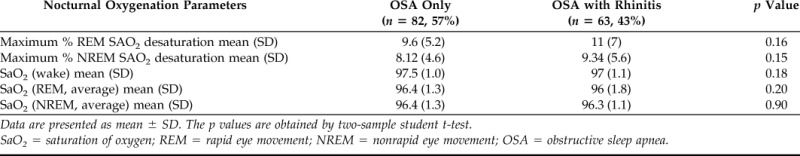
Data are presented as mean ± SD. The p values are obtained by two-sample student t-test.
SaO2 = saturation of oxygen; REM = rapid eye movement; NREM = nonrapid eye movement; OSA = obstructive sleep apnea.
Children with Rhinitis Have More OSA Events during REM Sleep
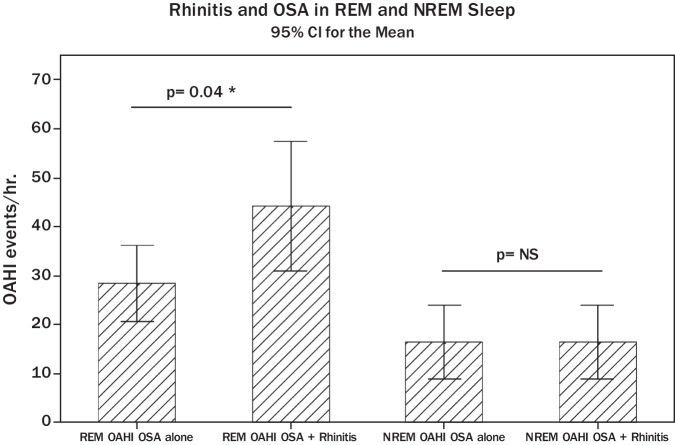
REM sleep stage is characterized by marked nasal congestion.12,13 Accordingly, we next addressed the hypothesis that children with rhinitis have more REM-related OSA. To this end, we compared the OAHI value during REM and NREM in the group of children with rhinitis and OSA with that seen in individuals with OSA alone. Figure 1 A shows that children with rhinitis and moderate–severe OSA had significantly elevated absolute OAHI value during REM sleep (mean REM OAHI = 44.1/hr; SE = 6.4 event/hr) compared with children with OSA alone (mean REM OAHI = 28.2/hr; SE = 3.8 events/hr; p = 0.04). There were no significant differences in the NREM-related OAHI between children with asthma and moderate–severe OSA (mean NREM OAHI = 15.4/hr; SE = 2.5) and individuals with OSA alone (mean NREM OAHI = 16.2/hr; SE = 3.2; p = 0.8). The OAHI mean value during REM or NREM was not significantly different in children with mild OSA with or without rhinitis (data not shown). These results show that children with rhinitis and moderate–severe OSA have a distinct phenotype characterized by OSA clustered during REM sleep compared with those with OSA alone.
Figure 1.
REM OAHI and NREM OAHI by rhinitis status in children with moderate–severe OSA. Data are presented as mean and 95% confidence interval (CI). REM, rapid eye movement; NREM, nonrapid eye movement; OAHI, obstructive apnea–hypopnea index (p values obtained by two-sample student t-test or nonparametric testing [i.e., Wilcoxon rank-sum] were appropriate).
The Association between Rhinitis and REM-Related OSA Is Independent of Atopy, Gender, Age, BMI, and Ethnicity
REM-related OSA has been linked to younger age, female gender, and obesity.19–21 In addition, allergic status is a covariable associated with increased risk for rhinitis and sleep breathing disorders in children.22 Accordingly, we built a multivariable linear regression model to assess the confounder effect of atopy, obesity, age, gender, and ethnicity in the relationship between rhinitis and REM-related breathing abnormalities (Table 3). After adjusting for covariables we found that the effect of rhinitis in REM-related OSA (REM OAHI) is independent of BMI, age, gender, ethnicity, and atopy (adjusted p = 0.026). The latter results indicate that the link between rhinitis and upper airway obstruction during REM sleep in children with OSA is present regardless of atopic status (i.e., allergic and nonallergic rhinitis) and that it is not significantly influenced by demographic and anthropometric parameters.
Table 3.
Multivariable regression analysis results for the association between rhinitis and REM OAHI adjusted by covariables
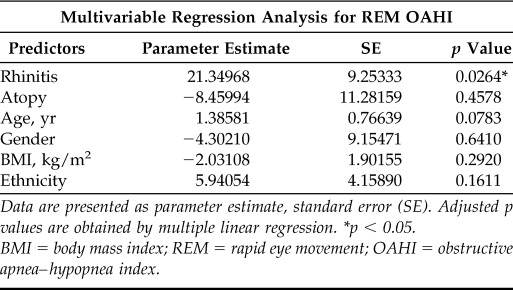
Data are presented as parameter estimate, standard error (SE). Adjusted p values are obtained by multiple linear regression. *p < 0.05.
BMI = body mass index; REM = rapid eye movement; OAHI = obstructive apnea–hypopnea index.
Asthma but Not Rhinitis Is Linked to More REM-Related Hypoxemia in Children with OSA
We have recently indicated that REM sleep modulates the phenotypical expression of OSA in children with asthma.11 Asthma and rhinitis are two airway conditions that often coexist in children. To evaluate the concomitant effect of REM sleep in upper and lower airway disease, we built a multivariable linear regression model with asthma and rhinitis as joint predictors for the maximal percentage of SaO2 desaturation during REM sleep (Table 4). Our data showed that asthma, but not rhinitis, is associated with higher levels of maximal SaO2 desaturation during REM sleep relative to those children with OSA alone (Table 4). These data suggest that children with rhinitis and OSA appear to have more REM-related upper airway obstruction (REM OAHI), and that those with asthma and OSA tend to have more REM-related hypoxemia. The latter data implicate that REM sleep modulates the nocturnal phenotypical expression of airway disorders in children via sleep-related mechanisms that affect upper and lower airway function.
Table 4.
Multivariable regression analysis results for the joint association between asthma, rhinitis, and REM-related hypoxemia
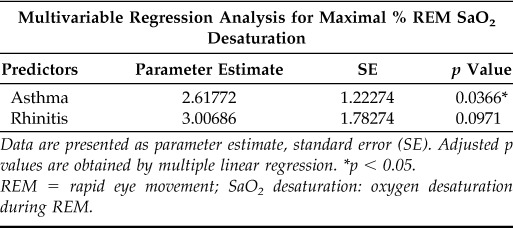
Data are presented as parameter estimate, standard error (SE). Adjusted p values are obtained by multiple linear regression. *p < 0.05.
REM = rapid eye movement; SaO2 desaturation: oxygen desaturation during REM.
DISCUSSION
The most important findings of the current study are that (1) rhinitis is a condition highly prevalent (43%) in children with OSA but it is not associated with more severe OSA and (2) children with rhinitis have more REM-related upper airway obstruction independently of confounding factors. Collectively, our current data provide new evidence supporting the link between rhinitis and OSA in children and present novel insights into the potential role of REM sleep in the pathogenesis of nocturnal nasal congestion in children with OSA and rhinitis.
There is clear evidence supporting the association between OSA and rhinitis in adults. The Wisconsin Sleep Study, a prospective population study of 1032 healthy volunteers, showed a threefold increased incidence of snoring and daytime sleepiness in volunteers with self-reported nocturnal nasal congestion.23 More recently, a recent large retrospective longitudinal cohort study (446,480 electronic medical records) identified that adults with chronic rhinitis have a higher premorbid prevalence of sleep apnea (p < 0.001).9 Another large study (524 subjects mean ± SD; aged 51 ± 12 years) established that nasal symptoms are highly prevalent in subjects with PSG-confirmed diagnosis of OSA: 54% had nasal stuffiness and 33% reported sneezing.24 Interestingly, despite the fact that rhinitis and OSA are very common in children,10 a recent systematic review of existing literature over the last 25 years identified only 18 articles on the association between these two conditions in the pediatric population.10 Only six studies (33%) included cases with OSA confirmed by overnight PSG, the gold standard for this disorder in children.2,3 Moreover, 33% of the articles did not identify a statistically significant association between rhinitis and sleep-breathing disorders in children.10 This lack of evidence contrasts with the prevalent notion that OSA is associated with nasal inflammatory changes.5,6 In support of the link between nasal inflammation and OSA in children, our data identified a high prevalence of rhinitis in children with overnight PSG-confirmed OSA (43%), which is similar to what has been previously described in adult studies.24
The presence of rhinitis did not correlate with OSA severity according to standard PSG parameters (OAHI and OSA-related SaO2 desaturation) in our retrospective pediatric cohort. These findings are in general agreement with prior data reported in adults. Lofaso et al. showed that although increased nasal resistance, quantified by rhinomanometry during wake state, is an independent predictor of OSA in adults, it only accounts for 2.3% of the total AHI variance.25 The dissociation of the diagnosis of OSA and AHI severity (determined by total events occurring in REM + NREM) presented by Lofaso25 may be caused by different levels of nasal congestion occurring in REM sleep versus NREM sleep. Given that REM is only a small portion of the night (around 20%) breathing abnormalities during REM may not necessarily correlate with the overall AHI severity score (which includes NREM that represents 80% of the night). Indeed, other adult studies have failed to establish a relationship between daytime nasal resistance and AHI determined by PSG.26,27 In contrast, the evaluation of daytime nasal dimensions and airflow resistance by acoustic rhinometry and rhinomanometry can discriminate habitual snorers from nonsnoring individuals.28 Collectively, these data suggest that although rhinitis is associated with increased daytime nasal resistance and snoring, nasal inflammation/congestion maybe linked to OSA by additional sleep-related mechanisms. A possible explanation to this phenomenon might be found in the modulatory effect of sleep stages in nocturnal nasal obstruction.12,13 This concept was initially explored by Morris et al.,12 who determined that REM sleep is characterized by marked nasal congestion followed by profound decongestion during NREM sleep, as measured by serial acoustic rhinometry before sleep and during REM and NREM sleep.12 More recently, Kimura et al. reported periodic cycles of nasal vascular engorgement linked to alternate nasal obstruction (so-called “nasal cycles”) clustering primarily around REM sleep and never occurring during NREM slow-wave sleep.13 Based on these observations, we hypothesized that children with rhinitis and OSA have more REM-related upper airway obstruction likely because of nasal congestion. Our data indicated that children with OSA and rhinitis have clustering of OSA events around REM sleep, although these events did not lead to more severe REM-related hypoxemia unless asthma was also present (Table 4). Indeed, we have recently described that REM sleep modulates the phenotypical expression of OSA in children with asthma11; however, that investigation did not analyze the joint effect of upper and lower airway disease (rhinitis and asthma) in children with OSA. In contrast, our current study identified that children with rhinitis and OSA tend to have more REM-related upper airway obstruction (REM OAHI) and those with asthma and OSA seem to have more REM-related hypoxemia (Table 4), indicating that REM sleep influences upper and lower airway disorders in children. Additional statistical analysis also established that the link between REM OSA and rhinitis was independent of covariables such as obesity, age, and gender that have been previously described to enhance OSA clustering during REM sleep,19–21 and allergic status, which is an important factor in the pathophysiology of rhinitis and sleep-breathing disorders in children.22
The impact of our current study in the field is that it underscores the interplay between clinical rhinitis, nasal congestion, and OSA in children. This clinical association may have some therapeutic implications. Some treatments for rhinitis such as nasal corticosteroids and leukotriene blockers are already recommended for the treatment of mild OSA in children,29–31 but additional therapies for allergic rhinitis (i.e., immunotherapy) are still to be evaluated in the context of pediatric OSA. Moreover, additional studies are needed to establish the potential beneficial effect of rhinitis therapy in the subgroup of children with REM-related breathing abnormalities. In terms of the limitations of our study, it is worth mentioning that the cross-sectional design prevented any type of cause–effect conclusion between rhinitis and OSA. Additional limitations include those that pertain to any retrospective analysis such as selection bias and misclassification of disease (information bias) resulting from inaccurate documentation in medical records. In our case this is particularly applicable to the retrospective determination of rhinitis and atopy, which may have been underestimated. On the other hand, the strengths of our study are the relatively large sample size and the use of objective data (PSG scoring and individual signal processing) to evaluate OSA variables. It should be noted that although we used multivariable regression analysis to identify associations with REM OAHI, the sample size may have not been large enough to identify risk factors with any certainty.
In summary, we feel that enough evidence is presented to link rhinitis and OSA in children, particularly in the context of upper airway obstruction clustered during REM sleep. Additional research is needed to delineate the biological link between REM sleep and the physiology of the nose during health and disease. New knowledge in this area may allow the discovery of mechanistic pathways that connect nasal inflammation and congestion with sleep biology, which in turn, may result in novel strategies for the treatment of pediatric OSA and nocturnal symptoms of rhinitis in children.
Footnotes
G Nino received funding in part by the National Institutes of Health (NIH) Career Development Award 1K12HL090020/NHLBI, Bethesda, Maryland. The remaining authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: Association with race and prematurity. J Pediatr 142:383–389, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Marcus CL, Loughlin GM. Obstructive sleep apnea in children. Semin Pediatr Neurol 3:23–28, 1996. [DOI] [PubMed] [Google Scholar]
- 3. Gozal D. Sleep, sleep disorders and inflammation in children. Sleep Med 10(suppl 1):S12–S16, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol 44:417–422, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Rubinstein I. Nasal inflammation in patients with obstructive sleep apnea. Laryngoscope 105:175–177, 1995. [DOI] [PubMed] [Google Scholar]
- 6. Koutsourelakis I, Vagiakis E, Perraki E, et al. Nasal inflammation in sleep apnoea patients using CPAP and effect of heated humidification. Eur Respir J 37:587–594, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Yuksel H, Sogut A, Yilmaz H, et al. Sleep actigraphy evidence of improved sleep after treatment of allergic rhinitis. Ann Allergy Asthma Immunol 103:290–294, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol 131:1350–1360, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park CE, Shin SY, Lee KH, et al. The effect of allergic rhinitis on the degree of stress, fatigue and quality of life in OSA patients. Eur Arch Otorhinolaryngol 269:2061–2064, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Lin SY, Melvin TA, Boss EF, Ishman SL. The association between allergic rhinitis and sleep-disordered breathing in children: A systematic review. Int Forum Allergy Rhinol 3:504–509, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Gutierrez MJ, Zhu J, Rodriguez-Martinez CE, et al. Nocturnal phenotypical features of obstructive sleep apnea (OSA) in asthmatic children. Pediatr Pulmonol 48:592–600, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Morris LG, Burschtin O, Setlur J, et al. REM-associated nasal obstruction: A study with acoustic rhinometry during sleep. Otolaryngol Head Neck Surg 139:619–623, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Kimura A, Chiba S, Capasso R, et al. Phase of nasal cycle during sleep tends to be associated with sleep stage. Laryngoscope 123:2050–2055, 2013. [DOI] [PubMed] [Google Scholar]
- 14. Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 15. Pe[tildenaranda A, Aristizabal G, Garcìa E, et al. Rhinoconjunctivitis prevalence and associated factors in school children aged 6–7 and 13–14 years old in Bogota, Colombia. Int J Pediatr Otorhinolaryngol 76:530–535, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 162:1403–1406, 2000. [DOI] [PubMed] [Google Scholar]
- 17. Gupta RS, Weiss KB. The 2007 National Asthma Education and Prevention Program asthma guidelines: Accelerating their implementation and facilitating their impact on children with asthma. Pediatrics 123:S193–S198, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Ogden CL. Defining overweight in children using growth charts. Md Med 5:19–21, 2004. [PubMed] [Google Scholar]
- 19. Mokhlesi B, Punjabi NM. “REM-related” obstructive sleep apnea: An epiphenomenon or a clinically important entity? Sleep 35:5–7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement related sleep-disordered breathing: Influence of age and gender. Chest 134:1156–1161, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haba-Rubio J, Janssens JP, Rochat T, Sforza E. Rapid eye movement-related disordered breathing: Clinical and polysomnographic features. Chest 128:3350–3357, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Ishman SL, Smith DF, Benke JR, et al. The prevalence of sleepiness and the risk of sleep-disordered breathing in children with positive allergy test. Int Forum Allergy Rhinol 2:139–143, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med 161:1514–1519, 2001. [DOI] [PubMed] [Google Scholar]
- 24. Kreivi HR, Virkkula P, Lehto JT, Brander PE. Upper airway symptoms in primary snoring and in sleep apnea. Acta Otolaryngol 132:510–518, 2012. [DOI] [PubMed] [Google Scholar]
- 25. Lofaso F, Coste A, d'Ortho MP, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J 16:639–643, 2000. [DOI] [PubMed] [Google Scholar]
- 26. Miljeteig H, Hoffstein V, Cole P. The effect of unilateral and bilateral nasal obstruction on snoring and sleep apnea. Laryngoscope 102:1150–1152, 1992. [DOI] [PubMed] [Google Scholar]
- 27. Miljeteig H, Savard P, Mateika S, et al. Snoring and nasal resistance during sleep. Laryngoscope 103:918–923, 1993. [DOI] [PubMed] [Google Scholar]
- 28. Yahyavi S, Parsa FM, Fereshtehnejad SM, Najimi N. Objective measurement of nasal airway dimensions and resistance using acoustic rhinometry and rhinomanometry in habitual snorers compared with non-snorers. Eur Arch Otorhinolaryngol 265:1483–1487, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Dayyat E, Serpero LD, Kheirandish-Gozal L, et al. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest 135:1142–1149, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics 122:e149–e155, 2008. [DOI] [PubMed] [Google Scholar]
- 31. Kheirandish-Gozal L, Kim J, Goldbart AD, Gozal D. Novel pharmacological approaches for treatment of obstructive sleep apnea in children. Expert Opin Investig Drugs 22:71–85, 2013. [DOI] [PubMed] [Google Scholar]