Abstract
The role of auxin in controlling leaf expansion remains unclear. Experimental increases to normal auxin levels in expanding leaves have shown conflicting results, with both increases and decreases in leaf growth having been measured. Therefore, the effects of both auxin application and adjustment of endogenous leaf auxin levels on midrib elongation and final leaf size (fresh weight and area) were examined in attached primary monofoliate leaves of the common bean (Phaseolus vulgaris) and in early Arabidopsis rosette leaves. Aqueous auxin application inhibited long-term leaf blade elongation. Bean leaves, initially 40 to 50 mm in length, treated once with α-naphthalene acetic acid (1.0 mm), were, after 6 d, approximately 80% the length and weight of controls. When applied at 1.0 and 0.1 mm, α-naphthalene acetic acid significantly inhibited long-term leaf growth. The weak auxin, β-naphthalene acetic acid, was effective at 1.0 mm; and a weak acid control, benzoic acid, was ineffective. Indole-3-acetic acid (1 μm, 10 μm, 0.1 mm, and 1 mm) required daily application to be effective at any concentration. Application of the auxin transport inhibitor, 1-N-naphthylphthalamic acid (1% [w/w] in lanolin), to petioles also inhibited long-term leaf growth. This treatment also was found to lead to a sustained elevation of leaf free indole-3-acetic acid content relative to untreated control leaves. Auxin-induced inhibition of leaf growth appeared not to be mediated by auxin-induced ethylene synthesis because growth inhibition was not rescued by inhibition of ethylene synthesis. Also, petiole treatment of Arabidopsis with 1-N-naphthylphthalamic acid similarly inhibited leaf growth of both wild-type plants and ethylene-insensitive ein4 mutants.
The primary plant auxin indole-3-acetic acid (IAA) appears to be ubiquitous to higher plants and is likely essential to normal development because auxin-deficient mutants have never been isolated. Synthesis of IAA occurs principally in the most apical tissues of the shoot, the apical meristem and the youngest leaves, although decreased synthesis continues in mature leaves (Sitbon et al., 1991; Ljung et al., 2001). Transported basipetally, auxin has a controlling role in diverse aspects of plant development, including apical dominance (Chatfield et al., 2000), leaf abscission (Morgan, 1984), stem elongation (Sánchez-Bravo et al., 1992), tropisms (Jensen et al., 1998; Rashotte et al., 2000), and root development (Reed et al., 1998).
Although recognized as a critical hormone for stem and root growth, auxin was long thought minimally involved in the growth of leaves, limited to control of vein elongation (Went, 1951). More recently, however, auxin has been shown to be important in the initiation of new leaves in tomato (Lycopersicon esculentum; Reinhardt et al., 2000), in leaf vascular development in Arabidopsis (Sieburth, 1999; Mattsson et al., 2003), and in the cell division phase of leaf expansion in Arabidopsis (Ljung et al., 2001).
The post-cell division/cell enlargement phase of leaf expansion clearly is influenced by plant hormones other than auxin (Goodwin and Erwee, 1983; Digby and Fern, 1985) and is also highly responsive to environmental conditions especially light quality and quantity (Van Volkenburgh, 1999). Attempts to determine the role of auxin in leaf expansion by increasing leaf auxin content above normal, endogenous levels, however, have produced conflicting results. On one hand, increasing auxin levels has been shown to increase growth. For example, excised tobacco (Nicotiana tabacum) leaf strips from expanding tobacco leaves curve epinastically with increased growth when incubated in a range of auxin concentrations (Keller and Van Volkenburgh, 1997, 1998), and one-time aqueous applications of auxins to the expanding leaves of intact common bean plants results in substantial but transient hyponastic curvature because of increased abaxial leaf expansion (Lippincott and Lippincott, 1971; Hayes and Lippincott, 1976, 1981; Hayes, 1977, 1978, 1981). On the other hand, increased auxin levels have been reported to have the opposite effect on leaf development. Transgenic petunia (Petunia hybrida), which overproduces auxin, develops leaves that are epinastic but also smaller and narrower than wild-type (Klee et al., 1987). The Arabidopsis mutants sur1 and sur2, which also overproduce auxin, also have reduced leaf expansion (Boerjan et al., 1995). Most recently, growing Arabidopsis seedlings on agar containing the auxin transport inhibitor 1-N-naphthylphthalamic acid (NPA) was reported to lower leaf auxin and reduce leaf expansion (Ljung et al., 2001). The authors suggested that increased leaf auxin trapped by NPA initiates a feedback inhibition mechanism that inhibits auxin synthesis. The results of these latter studies are complicated by the fact that the elevation of auxin occurs throughout the entire plant, whether as the result of transgenics or by root treatment with NPA (Boerjan et al., 1995; Ljung et al., 2001). Therefore, reduced leaf growth may be the indirect result of other auxin-induced changes to whole-plant development.
The principle objective of the current study was to determine whether increased auxin levels, when primarily localized to individual leaves of intact plants, result in increased or decreased leaf expansion and, ultimately, altered final leaf size. The main experimental system used here was the expanding monofoliate (primary) leaves of the common bean. This system has the advantage of large, easily measured leaves that already have been shown to be auxin sensitive (Lippincott and Lippincott, 1971; Hayes and Lippincott, 1976). Furthermore, the paired arrangement of bean monofoliates allows the use of the opposite untreated leaf to act as a within-plant control.
RESULTS
Growth Effects of Exogenous Auxin on Bean Leaf Blades
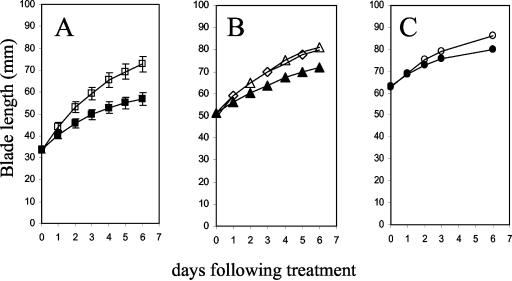
In a first experiment, on d 0, after an initial length measurement, 1 mm α-naphthalene acetic acid (αNAA), buffered to pH 6.0 with 1,3-bis[tris{hydroxymethyl}methylamino]propane (BTP), was applied to one of the pairs of monofoliate leaves of each plant that fell into one of three groups: small leaves (11–12 d postimbibition; Fig. 1A), medium leaves (12–14 d postimbibition; Fig. 1B), and large leaves (15–17 d postimbibition; Fig. 1C). Regardless of initial length, the single aqueous application of 1 mm αNAA inhibited the rate of midrib elongation of the treated leaf when compared with the untreated leaves so that by d 6, the treated blades were significantly shorter than the untreated opposite leaf. Harvested on d 6, the treated leaves had significantly less blade fresh weight than the untreated leaves on the same plants (data not shown), indicating that the shorter midribs of the treated leaves were a reflection of the overall smaller size of these leaves.
Figure 1.
Effect of 1 mm αNAA on monofoliate midrib length. On d 0, bean plants (each with an approximately equally sized pair of monofoliate leaf blades) were selected in three different stages of development: A, ▪ and □, small leaves 11 to 12 d postimbibition with the monofoliate midribs between 30 and 40 mm long; B, ▴, ▵, and ⋄, medium leaves 12 to 14 d postimbibition with the midribs 40 to 50 mm long; and C, • and ○, large leaves 15 to 17 d postimbibition with the midribs 60 to 70 mm long. After initial midrib measurement, the adaxial surface of one monofoliate leaf on each plant (▪, ▴, and •) was treated with 1 mm αNAA (pH 6.0 with BTP). The opposite leaf was left untreated (□, ▵, and ○). Also shown in B is midrib length of leaf blades for plants of which neither leaf was treated (⋄). Midrib length of both the treated and untreated leaves was measured daily as indicated for 6 d. For clarity, in B, the symbols ▵ and ⋄ are shown at alternating time points only. Errors bars = se in A. In B and C, errors are smaller than symbol size and are not shown. Sample size: n = 12 for ▪, □, ▴, ▵, and ⋄ and n = 20 for • and ○.
Midrib elongation (and d 6 blade fresh weight; data not shown) of the untreated leaves opposite treated leaves was not significantly different from the elongation of the midribs (or d 6 blade fresh weight) of wholly untreated control plants (Fig. 1B), indicating that the effects of auxin were because of growth inhibition of the treated leaves rather than growth stimulation of the untreated leaves.
Inhibition of leaf blade elongation is more responsive to a more active auxin than to a weak auxin (Table I). Applied once at either 0.1 or 1.0 mm, αNAA significantly inhibited leaf blade length, final leaf weight, and final leaf area of the treated leaves compared with the untreated leaf on the same plants harvested after 6 d. The weak auxin β-naphthalene acetic acid (βNAA) also significantly inhibited comparative blade elongation, but only at 1 mm. Application of water alone did not inhibit leaf elongation, final leaf weight, or final leaf area and a weak acid control, benzoic acid, was also ineffective at all tested concentrations.
Table I.
Effect of aqueous applications of auxins on relative leaf blade length, fresh wt, and leaf area after 6 d The adaxial surface of one monofoliate leaf blade of bean plants with a similarly sized pair of monofoliate leaf blades (11 to 12 d postimbibition with both monofoliate midribs between 40 and 50 mm long) was treated either once on d 0 or daily for 6 d beginning on d 0 with aqueous application(s) of solutions (pH 6.0 with BTP) of either αNAA, β -naphthalene acetic acid (βNAA), or benzoic acid, at the indicated concentrations, or of water. Following leaf blade length measurements on d 6, the plants were harvested, and the final leaf blade wts and surface areas of the blades were determined. Data are presented as treated leaf value minus the untreated opposite leaf value on the same plant expressed as mean ± se (n = 8). Significant differences between the midrib length, blade fresh wt, and blade area of treated versus control (untreated) leaves within plants (paired sample Student's t test) are indicated by an asterisk. n.d., Not determined. Initial (d 0) leaf blade fresh wt and area for leaf blades was estimated from destructive sampling of other leaf blades. Leaf blades with 40- to 50-mm-long midribs weighed between 240 and 330 mg and had leaf areas in the range of 14 to 19 cm2. Final (d 6) leaf blade midrib length, blade fresh wt, and blade area of the untreated leaf of plants that received a single water treatment was 81.4 ± 3.5 mm, 735.4 ± 4.6 mg, and 44.6 ± 3.3 cm2, respectively.
| Frequency
|
Treatment Compound
|
Concentration
|
Treated Minus Untreated Leaves
|
||
|---|---|---|---|---|---|
| Difference in Midrib Length | Difference in Fresh Wt | Difference in Area | |||
| μm | mm | mg | cm2 | ||
| Single treatments | αNAA | 1,000 | *—8.0 ± 1.3 | *—94.9 ± 33.8 | *—7.29 ± 2.44 |
| 100 | *—4.7 ± 1.4 | *—84.2 ± 22.4 | *—6.53 ± 1.38 | ||
| 10 | —0.7 ± 0.8 | 11.8 ± 33.2 | 0.11 ± 1.60 | ||
| 1 | 1.0 ± 1.0 | 18.8 ± 18.0 | —0.60 ± 0.88 | ||
| 0.1 | 0.1 ± 0.6 | —29.0 ± 31.0 | —2.01 ± 1.49 | ||
| βNAA | 1,000 | *—4.7 ± 1.4 | —48.40 ± 32.21 | n.d. | |
| 100 | —0.7 ± 1.0 | 34.40 ± 17.07 | n.d. | ||
| 10 | —0.6 ± 2.0 | 23.38 ± 25.95 | n.d. | ||
| Benzoicacid | 1,000 | —1.2 ± 2.2 | 57.80 ± 40.18 | n.d. | |
| 100 | 1.7 ± 1.0 | 20.83 ± 26.08 | n.d. | ||
| 10 | 0.2 ± 0.8 | 34.33 ± 42.44 | n.d. | ||
| IAA | 1,000 | —1.2 ± 1.0 | —26.40 ± 35.27 | n.d. | |
| 100 | —1.3 ± 0.4 | —29.50 ± 37.20 | n.d. | ||
| 10 | —1.2 ± 0.7 | —30.90 ± 37.32 | n.d. | ||
| Water | — | —0.4 ± 1.0 | —28.80 ± 27.24 | 0.88 ± 2.29 | |
| Daily treatments | IAA | 1,000 | *—9.4 ± 1.2 | *—278.30 ± 44.45 | *—11.27 ± 2.24 |
| 100 | *—5.4 ± 1.2 | *—96.10 ± 39.65 | *—4.50 ± 1.73 | ||
| 10 | *—7.0 ± 1.3 | *—157.60 ± 69.03 | *—8.35 ± 2.90 | ||
| 1 | —1.4 ± 0.6 | —37.10 ± 31.49 | n.d. | ||
| Water | — | —1.0 ± 0.8 | —53.38 ± 50.67 | —3.01 ± 1.98 | |
Applied once, IAA was nearly ineffective at inhibiting blade elongation (Table I). Because IAA may be rapidly metabolized and is chemically unstable (Epstein et al., 1980), the effect of daily applications of IAA was also tested (Table I). IAA was found to significantly inhibit leaf blade length, final leaf weight, and final leaf area of the treated leaves compared with the untreated leaf after 6 d of daily application at all concentrations tested (1 μm, 10 μm, 100 μm, and 1 mm).
Hayes and coworkers have characterized extensively a short-term (i.e. maximal within 2–6 h) hyponastic response by attached bean leaves treated with auxin (Lippincott and Lippincott, 1971; Hayes and Lippincott, 1976). The same transient multihour hyponastic response was routinely observed in the leaf blades of bean plants treated with higher concentrations of auxin (i.e. αNAA or IAA) in the experiments described here. Most treated leaves recovered completely from the hyponastic response within 24 h, after which they remained indistinguishable in general appearance from untreated leaves. Daily application, however, of the highest concentration of IAA (1 mm) to bean leaf blades resulted in a gradual development (.i.e. beginning after 2 or 3 d) of epinastic curvature (data not shown).
Growth Effects of Manipulation of Endogenous Bean Leaf Blade Auxin
If increasing leaf auxin through application inhibits leaf blade expansion, elevation of endogenous auxin within the leaf blade might be expected to have the same effect. IAA produced in leaves moves to the rest of the plant primarily by means of polar auxin transport (Goldsmith, 1977; Estelle, 1998). Thus, application to leaf petioles of NPA, an effective inhibitor of IAA transport (Goldsmith, 1977; Estelle, 1998), would be expected to trap IAA within the leaf and result in elevation of leaf IAA concentration.
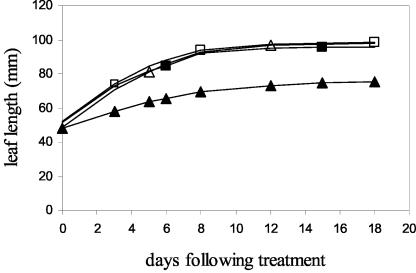
Application of NPA in lanolin to bean leaf petioles inhibited bean leaf elongation in comparison with application of lanolin alone (Fig. 2). On d 0, after an initial length measurement, NPA (1% [w/w] in lanolin) or lanolin alone was applied to the midpoint of the petiole of one of the pairs of monofoliate leaves of plants with similarly sized leaf blades (approximately 40–50 mm in length). By d 3, NPA treatment significantly inhibited leaf blade elongation. Final leaf blade midrib length, final leaf blade fresh weight, and final surface area (i.e. at d 18) also were decreased significantly by NPA treatment (Fig. 2; Table II). Lanolin treatment alone, however, had no effect on leaf blade length, final leaf blade fresh weight, and final surface area because lanolin-treated leaves, control leaves opposite NPA-treated leaves, and control leaves opposite lanolin-treated leaves were never significantly different in length, weight, and area. These results indicate that, as in the case of treatment with auxin, the difference in growth between NPA-treated leaves and the opposite control leaf on the same plant is because of growth inhibition of the treated leaves rather than an induction in growth in the untreated leaves. This conclusion is supported by the results of a separate experiment in which the monofoliate leaf blades opposite NPA-treated leaves were not found to be significantly different in size than those of control, monofoliate leaf blades on untreated plants (data not shown).
Figure 2.
Effect of petiole treatment with NPA on bean leaf expansion. On d 0 (11–12 d postimbibition), bean plants each with an approximately equally sized pair of monofoliate leaf blades (40–50 mm long) were selected. After initial midrib measurement, the middle 5- to 7-mm portion of one petiole of each plant was coated with a band of NPA (1% [w/w] in lanolin; ▴) or lanolin alone (▪). The opposite leaf petiole on each plant was left untreated (▵, opposite NPA; □, opposite lanolin). Midrib length of both the treated and untreated leaves was measured on days shown as indicated for 18 d. For clarity, the symbols ▪, ▵, and □ are shown at alternating time points only. ses are smaller than symbol size and are not shown. Sample size: n = 8.
Table II.
Effect of NPA treatment of the petiole on leaf blade wt and surface area after 18 d After leaf blade length measurement shown in Figure 2, the plants were harvested, and the final wts of the blades were determined. Data for wet wt and surface area of the blade portion of monofoliate leaves are expressed as mean ± se. “Lanolin treated” and “NPA treated” refer to the leaf blades whose petioles were treated with lanolin and NPA (1% [w/w] in lanolin) respectively, whereas “lanolin untreated” and “NPA untreated” refer to the control leaves opposite treated leaves. Initial (d 0) leaf blade fresh wt and area for all leaf blades were estimated to be between 240 and 330 mg and from 14 to 19 cm2.
| Treatment | Final Leaf Blade Wt | Final Leaf Blade Area |
|---|---|---|
| g | cm2 | |
| Lanolin-treated | 1.065 ± 0.055 | 66.26 ± 3.14 |
| Lanolin-untreated | 1.187 ± 0.065 | 68.80 ± 3.57 |
| NPA-treated | 0.774 ± 0.045 | 44.72 ± 2.24 |
| NPA-untreated | 1.186 ± 0.048 | 72.45 ± 2.56 |
An analysis of the free IAA content of NPA-treated and opposite, untreated, leaf blades suggests that the NPA treatment of the petiole did effectively increase the endogenous IAA content of the treated leaf for a sustained period. The free IAA content (Table III), measured after 1, 3, and 6 d of treatment, and expressed as nanograms of IAA per gram leaf blade fresh weight, was higher in the treated leaves on all 3 d. In every plant tested (n = 11), the IAA content of the treated leaf was higher than that of the untreated leaf. In pair-wise analysis (i.e. paired Student's t test), the NPA-treated leaf blades contained significantly more free IAA on d 1 and 6, although a substantial decline in mean detectable free IAA in both treated and untreated blades occurred by d 6. These results support the hypothesis that elevated leaf auxin content inhibits leaf expansion.
Table III.
Effect of NPA treatment of bean petioles on leaf blade auxin content As in Figure 4, plants with monofoliate blades 40 to 50 mm in length had one monofoliate leaf petiole treated with a 5- to 7-mm-wide band of NPA (1% [w/w] in lanolin). Leaf blades (both from treated leaves and from opposite untreated leaves) were harvested from selected plants after 24 h (1 d), 3 d, and 6 d. Free IAA was then extracted and purified by amino ion exchange column chromatography and HPLC and quantified by gas chromatography (GC)-mass spectrometry (MS)-selected ion monitoring as described below. Data are expressed as nanograms of free IAA per gram fresh wt, mean ± se.
| Leaf | After Application | IAA Concentration | No. of Leaf Pairs | Significance (Paired Sample Student's t Test) |
|---|---|---|---|---|
| d | ng g-1 | n | ||
| Treated | 1 | 53.2 ± 17.2 | 4 | 0.025 |
| Untreated | 29.3 ± 14.4 | |||
| Treated | 3 | 52.5 ± 11.6 | 3 | 0.076 |
| Untreated | 14.2 ± 1.4 | |||
| Treated | 6 | 8.9 ± 0.7 | 4 | 0.030 |
| Untreated | 6.3 ± 0.78 |
Effect of Inhibiting Ethylene Synthesis on Auxin Regulation of Bean Leaf Expansion
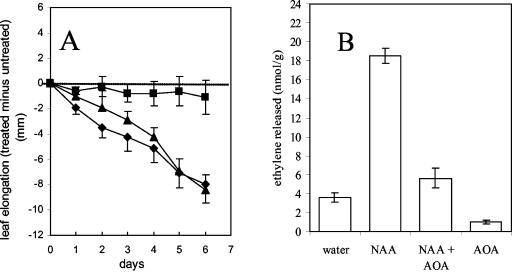
Auxin is well known to induce ethylene production, and many effects of exogenous auxin are, in fact, ethylene responses (Abeles et al., 1992). To address the possibility of a role for ethylene in the auxin-induced leaf growth inhibition observed here, the effect of the ethylene synthesis inhibitor amino-oxyacetic acid (AOA; Abeles et al., 1992) was tested (Fig. 3). When applied daily with 1 mm IAA and 1 mm AOA did not effect auxin-induced inhibition of leaf growth (Fig. 3A). AOA alone (1 mm), applied daily, did not effect leaf growth (Fig. 3A). Over a 24-h period, 1 mm AOA treatment was effective as an inhibitor of ethylene synthesis in detached bean leaf blades (Fig. 3B). As expected, there was substantially more ethylene released by leaves treated with auxin (Fig. 3B), but AOA at 1 mm effectively reduced ethylene production in leaves not treated with auxin and greatly inhibited the production of auxin-induced ethylene.
Figure 3.
Effect of daily AOA on leaf elongation through 6 d and on ethylene release after 24 h. A, Effect of the ethylene synthesis inhibitor AOA (1.0 mm) applied daily for 6 d either alone (▪) or with 1 mm IAA (♦) to one of the pairs of monofoliate leaves of bean plants with midribs initially between 40 and 50 mm long. Also shown is the effect of 1 mm IAA alone (▴). The data are expressed as the comparative elongation of the treated leaf (i.e. elongation of the treated leaf minus elongation of the opposite untreated leaf of the same plant; n = 10 plants for each treatment). Final (d 6) midrib lengths for leaf blades that received AOA alone, AOA + IAA, and IAA alone were 75.2 ± 2.1 (control leaf 76.0 ± 2.7 mm), 70.4 ± 2.4 (control leaf 78.6 ± 1.5 mm), and 67.7 ± 1.3 (control leaf 76.2 ± 1.4 mm) mm, respectively. B, Effect of 1 mm αNAA and/or 1 mm AOA treatment on the ethylene released by leaves treated, allowed to dry, then detached and individually enclosed in Erlenmeyer flasks for 24 h. Ethylene production was determined by GC of air samples taken from the flasks (n = 11, 12, 12, and 7 respectively for water control, αNAA, αNAA + AOA, and AOA). Errors bars = se.
The effect of ethylene application on bean leaf blade expansion was also tested in one experiment in which 2-chloroethyphosphonic acid (CEPA; Ethephon), which reacts with water to produce ethylene (Abeles et al., 1992), was applied in solution to leaf blades. Although lesser concentrations (100 and 1 μm) were ineffective, daily treatments with 1 mm CEPA inhibited blade elongation to a degree comparable with the effect of auxin (CEPA-treated blade midribs were 6.4 ± 1.9 mm shorter than the opposite untreated leaf blade midribs after 6 d). However, the 1 mm CEPA-treated leaves did not develop the epinastic appearance of auxin-treated leaves (data not shown). They also had a tendency to abscise before the end of the experiment, something never seen with auxin-treated leaves. In some species, substantially supra-optimal leaf auxin concentrations are known to induce leaf abscission as a result of induction of ethylene, the apparent primary positive regulator of abscission (Morgan, 1984). The absence of leaf abscission among auxin-treated bean leaves argues that the concentration of ethylene produced in leaf blades by daily application of 1 mm CEPA exceeded the leaf ethylene concentration induced by auxin application. Together, the results of experiments using AOA and CEPA imply that long-term inhibition of leaf growth of bean leaves is not the result of auxin-induced ethylene production.
Growth Effects of Auxin on Arabidopsis Wild-Type and ein4 Leaf Blades
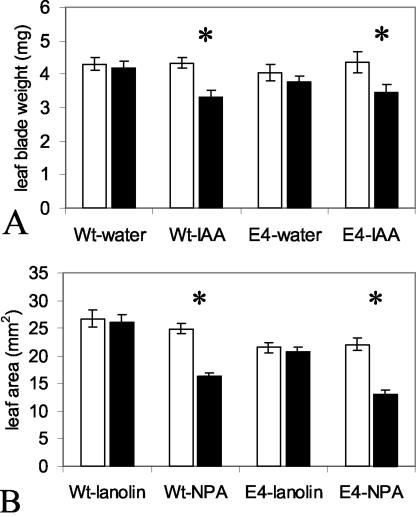
To determine whether elevated auxin has a similar leaf-growth-inhibiting effect in other species and to examine further the potential of ethylene in the phenomenon, Arabidopsis seedlings were used in experiments similar to those described above for beans (Fig. 4). In Arabidopsis, the first two true leaves emerge and expand nearly simultaneously and reach similar final size and shape (Tsukaya et al., 2000). The effect on leaf expansion, after 4 d, of daily applications of 1 mm IAA to young second true leaf blades of Arabidopsis wild-type and ein-4 mutants was examined (Fig. 4A). The harvested fresh weights of the blade portion of both the first (untreated) true leaves (white bars) and the harvested fresh weights of the treated second leaves (black bars) are shown. Daily applications of water alone appeared to have no significant effect on leaf expansion in wild type plants because the first and second leaves grew to a similar final size. Applications of IAA, however, significantly inhibited leaf expansion of the treated leaf relative to the untreated leaf.
Figure 4.
Effect of blade treatment with IAA (A) and of petiole-blade junction treatment with NPA (B) on Arabidopsis wild-type (Wt) and ein4 mutant (E4) leaf expansion. In A, IAA (1 mm) or water was applied daily to the second true leaf of young Arabidopsis seedlings (15–19 d postimbibition) when the first and second true leaves were partially expanded, and the second was 2.5 to 3.5 mm in length. In B, either NPA (1% [w/w] in lanolin) or lanolin alone was applied to petiole-blade point of attachment of the second leaf of seedlings at the same stage of development as in A (i.e. initial blade area and fresh weight were approximately 5–9.5 mm2 and 0.9–1.4 mg). Results shown in A are the mean leaf blade weight of both the first and second true leaves after 4 d of treatment. In B, the mean leaf blade area 4 d after application is shown. White bars, Leaf blade weight or area of the first true leaf; black bars, mean leaf blade weight or area of the second (treated) true leaf. Error bars = se. An asterisk indicates a significant difference between leaves 1 and 2 within plants (paired sample Student's t test). In A, n = 10 for Wt and n = 16 for E4. In B, n = 12 for Wt, n = 20 for E4 water, and n = 21 for E4 IAA.
ein-4 plants are insensitive to ethylene because of a missense mutation in an ethylene receptor (Hua et al., 1998). As with wild-type plants, water did not affect final leaf blade fresh weight (Fig. 4A), but daily IAA application significantly inhibited leaf expansion. Together these results suggest that, as with beans, elevated auxin inhibits leaf blade expansion in Arabidopsis and that this inhibition does not involve ethylene.
Daily application of 1 mm IAA to Arabidopsis (both wild type and ein4) also resulted in considerable epinastic curvature of the leaf blades with a strong tendency to fold across the midvein axis (data not shown). Unlike bean leaf blades, in which auxin-induced epinasty developed gradually over several days, Arabidopsis leaf blades were strongly epinastic within 24 h. The epinasty induced by a single application of 1 mm IAA to Arabidopsis leaf blades was, after 4 d, comparable with that sustained by leaf blades treated daily. Single 1 mm IAA applications, however, did not impact final (i.e. after 4 d) leaf blade fresh weight (data not shown). Unfortunately, the contorted leaf form of leaf blades treated with IAA precluded the measurement of the leaf blade area.
The effect of modulating endogenous auxin was also examined in Arabidopsis (Fig. 4B). NPA (1% [w/w] in lanolin) or lanolin alone was applied to the petiole-blade junction of the partially expanded second true leaves of Arabidopsis. Leaf blade areas of both the untreated first leaf (white bars) and the treated second leaf (black bars) at harvest (4 d after treatment) are shown. ein4 Arabidopsis consistently showed smaller leaf areas than wild type (Fig. 4B). Lanolin-treated second leaf blades expanded to reach a similar size as untreated first leaf blades, suggesting that the lanolin itself did not significantly affect expansion in either wild-type or ein4 plants. Application of NPA in lanolin, however, significantly slowed expansion of the treated leaf relative to the untreated leaf in both wild-type and ein4 plants. Little or no epinasty was evident with NPA- or lanolin-treated leaves (data not shown). These results clearly suggest that modulation of endogenous leaf blade auxin content does impact leaf expansion in Arabidopsis.
DISCUSSION
The results presented here of the effects of NPA treatment of intact plants adds to the growing understanding of the role of NPA on auxin levels and distribution. The treatment of bean leaf petioles with that auxin transport inhibitor slowed bean leaf blade expansion and final size (Fig. 2; Table II). Ljung et al. (2001) also reported the effects of NPA on the growth and free IAA content of Arabidopsis seedlings. They found, in liquid culture experiments, that transferring 10-d-old Arabidopsis seedlings into media containing 40 μm NPA for up to 24 h produced an initial increase in leaf free IAA content (maximal after 16 h) that subsequently declined. In other experiments, 10-d-old seedlings grown on agar were transferred to agar containing 100 μm NPA for up to 5 d. In these experiments, leaf free IAA content was significantly reduced compared with control plants. Plants incubated in NPA for several days also developed smaller, fully expanded leaves. The authors explained these and other results by suggesting that NPA does appear to trap auxin within expanding leaves but that a feedback inhibition mechanism then inhibits IAA synthesis, leading to lowered leaf auxin content and retardation of growth.
Our results (Table III), however, suggest that elevation of leaf blade IAA content through localized application of NPA to petioles does not initiate an effective feedback inhibition in beans because free IAA content remained as high or higher in NPA-treated leaf blades as in the opposite, untreated leaf. The decline in auxin content, evident in both treated and untreated leaves, between d 1 and 6 may simply reflect the normal developmental decline in auxin synthesis and auxin content as leaves reach maturity (Sitbon et al., 1991; Ljung et al., 2001).
In our experiments, application of auxin or NPA to Arabidopsis leaves inhibited leaf growth. We did not measure leaf auxin content in Arabidopsis, and it is possible that both NPA and auxin initiated feedback inhibition in this system as Ljung et al. (2001) have suggested. This would suggest that bean leaf auxin synthesis responds differently to elevation of leaf auxin than does Arabidopsis leaf auxin synthesis. However, in the experiments of Ljung et al. (2001), NPA was applied to the entire plants (in liquid culture) or to the roots (NPA-containing agar culture). Auxin transport has been shown to be essential to root development (Reed et al., 1998). When Arabidopsis seedlings were grown in agar containing just 10 μm NPA, initiation of lateral roots was completely blocked, and primary root elongation was greatly inhibited (Casimiro et al., 2001). Perhaps the decline in leaf auxin content reported by Ljung et al. (2001) was not initiated by an initial rise in auxin within leaves but instead resulted from some root-derived changes.
Daily application of high concentrations of IAA (1 mm) directly to both bean and Arabidopsis leaves should increase leaf auxin content. Although much of the applied auxin probably does not cross the cuticle barrier, reducing the effect of application, repeated applications will elevate auxin levels. This was confirmed by a spot check with single measurement of free IAA. A bean leaf blade treated daily with 1 mm IAA for 6 d (as in Table I) had approximately 1,000-fold higher free IAA level compared with that found in found in NPA treated leaves (data not shown). Daily applications of IAA to bean leaf blades at more reduced concentrations, as low as 10 μm, inhibited leaf expansion (Table II). This confirms that only modest elevations of leaf auxin appear necessary to inhibit growth (Table III).
In general, the effect of exogenous auxin is to induce an increase in growth rate of excised auxinresponsive plant tissues. Auxin is generally less effective when applied to intact plants (Hall et al., 1985). For example, application of high concentrations of auxins to dark-grown intact plants, including to pea (Pisum sativum) epicotyls (Hall et al., 1985), sunflower (Helianthus annuus) and marrow (Cucurbita pepo) hypocotyls (Tamimi and Firn, 1985), and watermelon (Citrullis lanutus) hypocotyls (Carrington and Esnard, 1988), produced a short-term stimulation of growth (3–5 h) followed by insensitivity. Application of auxin to intact, light-grown pea stem internodes, however, resulted in up to 48 h of growth acceleration (Yang et al., 1993), and transgenic Arabidopsis with elevated auxin content also exhibited increased hypocotyl elongation in the light (Romano et al., 1995).
Inhibition of root elongation by exogenous auxin, however, has been observed frequently (Burström, 1950; Åberg, 1957; Morré and Bonner, 1965; Andreae, 1967; Rauser and Horton, 1975). Auxin is known to induce production of ethylene (Abeles et al., 1992), and some have suggested that inhibition of root growth by auxin results from increased ethylene synthesis (Chadwick and Burg, 1967, 1970). This idea, however, is challenged by a study that showed that inhibition of ethylene synthesis does not inhibit auxin-induced root growth inhibition in corn (Zea mays) at higher concentrations (Mulkey et al., 1982) and by another study that shows a poor correlation between the effectiveness of ethylene alone to inhibit root elongation and the quantity of ethylene synthesis actually induced by auxin (Eliasson et al., 1989).
Could elevation of leaf blade auxin inhibit leaf blade expansion through ethylene induction? Ethylene is associated with the development of epinasty (Abeles et al., 1992), and in our experiments, single applications of IAA induced leaf blade epinasty in Arabidopsis, and daily applications of 1 mm IAA produced a similar effect in bean leaf blades. A role for ethylene in auxin-induced leaf blade expansion, however, seems unlikely. First, Romano et al. (1993) reported that the epinastic appearance of the leaves of transgenic Arabidopsis with elevated auxin content was not relieved by either of two ethylene-insensitive mutations. Second, ethylene-induced leaf epinasty is usually only associated with the petiole and not the blade portion of the leaf (Abeles et al., 1992). Third, beans are among the many plant species that have no epinastic response to ethylene (Crocker et al., 1932). Fourth, inhibition of ethylene synthesis did not block the inhibitory effects of auxin on bean leaf expansion (Fig. 3). Fifth, the expansion of leaf blades in the ethylene-insensitive Arabidopsis ein4 mutant was inhibited by daily auxin applications (Fig. 4). Sixth, application of ethylene (in the form of CEPA) to bean leaf blades only produced inhibition of expansion at concentrations that tended to also produce leaf abscission, something not associated with inhibition of leaf blade expansion by auxin.
The leaves of plants either transformed (including those of tobacco; Romano et al., 1993) or mutated to contain higher than normal levels of endogenous auxin are invariably abnormally small and epinastic (Klee et al., 1987; Romano et al., 1993; Boerjan et al., 1995). Incubation of excised tobacco leaf strips in auxin, however, produces increased growth, which is also epinastic resulting from relatively greater auxin-induced growth of the adaxial epidermis than the abaxial epidermis (Keller and Van Volkenburgh, 1997). The auxin response of excised tobacco leaf tissues is complete within 24 h.
A possible explanation for why the response to auxin by excised tobacco leaf tissues appears the opposite of the response of intact transformed or mutated auxin overproducers is suggested by the response of the leaves of intact bean plants to elevated auxin levels. In bean leaves, auxin treatment initially produced a hyponastic curvature (complete within 6 h), apparently because a surge in auxin-induced growth is limited to cells of the abaxial side of the leaf (Hayes and Lippincott, 1976). Within 24 h, however, treated leaves recovered their planar form, and auxin-induced growth inhibition was evident (Fig. 1). Auxin-induced growth inhibition was then sustained for the duration of leaf development (Fig. 2; Tables I and II). Besides a gradually developing epinasty in the leaves treated daily with a high concentration of auxin (1 mm IAA), the form of the leaves remained otherwise unperturbed. Expanding leaf tissues may have two sequential responses to elevated auxin; an initial growth acceleration followed by growth inhibition. Multiphasic auxin responsiveness by hypocotyls sections has been reported (Penny et al., 1974; Vanderhoef et al., 1976). In excised leaf tissues, though, only the growth acceleration response to auxin is evident because the growth of such tissues greatly slows or arrests after about 24 h. In auxin overproducers, only the growth inhibition response is apparent because high auxin levels are continuously present as the tissues transition to cell expansion growth. Because auxin levels normally decline as leaves develop (Wightman, 1977; Sitbon et al., 1991; Ljung et al., 2001), it is also tempting to suggest that in normal leaf expansion in intact plants, auxin may initially act to inhibit cell expansion growth and that the decline in auxin content might be a prerequisite for full leaf expansion.
Early tests of the effects of auxin pastes applied to leaves produced epinasty (Avery, 1935) in a way that suggested that auxin was functioning to stimulate unequal growth of the veins; this, in turn, distorted the shape of the leaves (Went and Thimman, 1937). It is now clear, however, that auxin sensitivity during leaf expansion is not limited to veins because leaf strips excised from interveinal regions of tobacco leaves have a greater epinastic response to auxin than do veinal tissues excised from the same leaves (Keller and Van Volkenburgh, 1997). Auxin-induced epinasty of excised interveinal tobacco leaf tissues is strongly developmentally sensitive, correlating with the transition of tissues from cell division growth to cell expansion growth (Keller and Van Volkenburgh, 1997) and with the initiation of expression of auxin-binding protein 1 (Jones et al., 1998; Chen et al., 2001). The auxin responsiveness of excised tobacco leaf veins appears to occur somewhat earlier than it does in interveinal tissue (C.P. Keller, unpublished data), which probably explains why application of auxin paste to young intact leaves (Avery, 1935; Went and Thimman, 1937) appeared to affect only the veins.
Because leaves of plants like tobacco and Arabidopsis normally develop essentially planar in form, the epinastic response to elevated auxin by the leaf tissues suggests that differential auxin sensitivity might exist across the lamina with adaxial cells being more responsive to higher concentrations of the hormone. The normal distribution of auxin, adaxial to abaxial, within developing leaves may also be unequal and may be disrupted by auxin treatment. Beans might be unusual, compared with tobacco and Arabidopsis, in that the initial auxin sensitivity is reversed, resulting in initial hyponasty.
MATERIALS AND METHODS
Plant Material
Bean (Phaseolus vulgaris L. cv Contender; Southern States Cooperative Inc., Richmond, VA) seeds were first selected for intact condition and uniform weight (0.425–0.525 g), imbibed for 24 h on moist paper towels, and then planted individually in vermiculite in 500-mL pots. Seedlings were grown under greenhouse conditions (ambient light with temperature maintained about 20°C and below 35°C). Plants were watered every 1 to 2 d as needed and treated once weekly with one-quarter-strength Miracle-Gro (Scotts Miracle-Gro Products Inc., Port Washington, NY). Plants to be used for analysis of released ethylene were watered daily with Peters Peat Lite Special 20-10-20 (W.R. Grace, Tukwila, WA), diluted to 100 μm nitrogen, and supplemented with 5 μm iron from iron chelate dispersable powder (Miller Chemical and Fertilizer, Hanover, PA) and magnesium at 10 μm from MgSO4, or watered (once a week) with water alone.
For most experiments, bean plants were selected 12 to 14 d after imbibition when the primary or monofoliate leaves were expanding and the first trifoliate leaf remained part of the apical bud. The plants selected were free of apparent deformities, had approximately equally sized monofoliates, and had monofoliate leaf midribs between 40 and 50 mm in length with the longer at least 45 mm in length. In one experiment, plants with smaller leaves (midribs 30–40 mm with the longer 35–40 mm long; 11–12 d postimbibition) and with larger leaves (midribs 60–70 mm long with the longer 65–70; 15–17 d postimbibition) were also employed.
Arabidopsis seed stocks (Columbia wild type [CS1092] and ein4 mutant [CS8053]) were provided by the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Seeds were sown on moist potting soil, refrigerated for 4 d at 4°C, and grown under greenhouse conditions as described above.
Bean Leaf Growth Assays
Once bean plants had reached the desired stage of development (i.e. monofoliate blade 40–50 mm), midrib length was measured daily using a dial caliper. After an initial d 0 measurement of length, one monofoliate leaf, chosen randomly on each plant, was then treated by application of the appropriate aqueous solution. Solutions were spread uniformly over the adaxial blade surface using cotton-tipped applicators. Each treated leaf was completely covered with solution to the point that excess dripped from the plant. In some experiments, leaves were not treated with aqueous solutions. In these experiments, one monofoliate leaf petiole was treated with a 5- to 7-mm-wide band of lanolin ± 1% (w/w) NPA. Immediately after treatment and daily in most experiments, midrib length was measured using a dial caliper. At the conclusion of each experiment, the blade of each monofoliate was excised and weighed. Leaf area was estimated from digitized images of photocopies of flatted leaves using a OneTouch 8800 scanner and associated Paperport software (Visioneer Inc., Pleasanton, CA) and NIH Image software (National Institutes of Health, Bethesda, MD).
Stock solutions of 1 mm IAA, αNAA, and βNAA were prepared by dissolving in water with sufficient heating (i.e. briefly to approximately 80°C). Once cool, these solutions were brought to pH 6.0 with BTP. Stock solutions of AOA (1 mm) and CEPA (1 mm) were dissolved in water. AOA was also brought to pH 6.0. CEPA was prepared for immediate use only. Lower concentration solutions were prepared by appropriate dilution of stock solutions. NPA (1% [w/w] in lanolin) was prepared by dissolving the compound in molten lanolin. All chemicals (and lanolin) were purchased from Sigma Chemical Co. (St. Louis), except NPA, which was purchased from Chem Service (West Chester, PA).
Determination of Bean Leaf Free IAA Content
Bean plants with equal approximately sized monofoliate blades (i.e. 40–50 mm) were chosen for experimentation. One monofoliate leaf petiole of each plant was treated with a 5- to 7-mm band of NPA (1% [w/w] in lanolin) or with lanolin alone. Individual plants were harvested after 24 h (1 d), after 3 d, and after 6 d. Monofoliate leaf blades (both from treated and from opposite, untreated leaves) were weighed and frozen immediately in liquid N2. Subsequent storage was at -80°C, and transportation to St. Paul for IAA analysis was on dry ice.
Purification and quantification was largely as described by Chen et al. (1988). In brief, entire leaf blades (0.47–1.15 g blade-1) were ground with a mortar and pestle in approximately 4 mL g-1 sample 65% (v/v) isopropanol with 0.2 m imidazole (pH 7) and 40 ng g-1 sample [13C6]IAA as internal standard. After 1 h of incubation, the extract was centrifuged, and 50,000 dpm of 3H-IAA (Amersham, Piscataway, NJ) was added to the supernatant combined as a radioactive tracer. The sample was then diluted 10-fold with double-distilled water, applied to a conditioned amino ion-exchange column (Baker SPE 3 mL, J.T. Baker, Phillipsburg, PA), washed sequentially with hexane, ethyl acetate, acetonitrile, and methanol (3 mL each), and eluted with methanol containing 2% (w/v) acetic acid. The eluate was evaporated to near dryness, and the residue was resuspended in 100 μL of 50% (v/v) methanol. The resuspended residue was then passed through an HPLC column (Ultracarb 30, Phenomenex, Torrance, CA) with a 1% (v/v) acetic acid and 25% (v/v) methanol aqueous running solvent. Radioactive fractions from the HPLC were pooled, reduced to dryness, and resuspended in 100 μL of methanol. The purified free IAA was then methylated by incubation with 1 mL of diazomethane for several minutes. The methylated IAA was subsequently dried under N2 and resuspended in 20 μL of ethyl acetate. Quantification was by GC-MS-selected ion monitoring as previously described by Ribnicky et al. (1996) using a model 6890N GC/5973Network MS (Agilent Technologies, Palo Alto, CA) equipped with a DB-1701 fused silica capillary column (Agilent Technologies). Injector temperature was at 280°C and an initial oven temperature of 70°C ramping 20°C m-1 to 280°C. The monitored ions were mass-to-charge ratio 130 and 136 (quinolinium ions from sample IAA and from the 13C6-labeled internal standard, respectively) and mass-to-charge ratio 189 and 195 for the corresponding molecular ions. Dwell times were 50 ms for each ion.
Ethylene Release Assay
As in the long-term growth assays described above, monofoliate bean leaves initially approximately 45 mm in length were treated with water, 1 mm αNAA, 1 mm AOA, or both 1 mm αNAA and 1 mm AOA. After drying for approximately 15 to 30 min, the blade portion was detached from the plant and placed individually in a 50-mL Erlenmeyer flask containing 1.0 mL of water. The flasks were then closed with serum stoppers. The detached and enclosed leaves were then incubated for 24 h under laboratory conditions, after which gas samples were removed by syringe from the flasks for GC analysis.
Ethylene was detected using a gas chromatograph (model GC-mini 2, Shimadzu, Columbia, MD) equipped with a flame ionization detector and a 6-× 18-inch (15.2 cm × 3.2 mm) stainless steel column packed with Porapac N 80/100 (Alltech, Deerfield, IL). The oven temperature was 60°C, and the detector was 80°C. Gas flow rates were 0.5, 1.0, and 1.5 kg cm-2, respectively, for the air (oxygen), hydrogen, and nitrogen (carrier). Ethylene concentration was estimated from the average of three repeated injections per sample and using peak height standard curves prepared with ethylene standards from Scott Specialty Gases (Plumsteadville, PA). Results shown are data averaged from two separate experimental series each with five to seven samples per treatment.
Arabidopsis Leaf Growth Assay
Arabidopsis plants were selected for study 15 to 19 d after sowing, when the first and second true leaves were partially expanded and of similar size, with the second 2.5 to 3.5 mm in length. The second leaf was identified on each plant by the position of the just-emerging third leaf approximately 140° around the stem axis from the second leaf and close to the insertion of the first leaf. For some plants, the second leaf was treated either with water or 1 mm IAA applied daily to the blade portion of the leaf. For other plants, a 0.8-mm-diameter wire was used to apply a small amount (approximately 1 μL) of lanolin ± NPA (1% w/w) to the second leaf on the adaxial surface at the petiole-blade point of attachment. After 4 d, both first and second leaves were removed from each plant, the fresh weight of the blade portion of each leaf was determined for the auxin- and water-treated plants, and the blade area was determined of the NPA- or lanolin-treated plants from microscope images using a digitizing camera (Video Flex 7600, Ken-a-vision, Kansas City, MO).
Acknowledgments
The Arabidopsis Biological Resource Center is gratefully acknowledged for providing the Arabidopsis wild-type and mutant stocks used here. Credit for inspiring this project goes to a serendipitous botanical inquiry by Dr. Christopher King Beachy.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.032300.
This work was supported by the Minot State University Small Grant Program for Faculty Research (grant to C.P.K.), by the North Dakota Biomedical Research Infrastructure Network (grant to C.P.K.), and by the National Science Foundation (grant no. NSF DBI–0077769 to J.D.C.).
References
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego
- Åberg B (1957) Auxin relations in roots. Annu Rev Plant Physiol 8: 153-180 [Google Scholar]
- Andreae WA (1967) Uptake and metabolism of indoleacetic acid, naphtha-leneacetic acid, and 2,4-dichlorophenoxyacetic acid by pea root segments in relation to growth inhibition during and after auxin application. Can J Bot 45: 737-753 [Google Scholar]
- Avery GS Jr (1935) Differential distribution of a phytohormone in the developing leaf of Nicotiana, and its relation to polarized growth. Bull Torrey Bot Club 62: 313-330 [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Onckelen HV, Montagu MV, Inze D (1995) superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burström H (1950) Studies on growth and metabolism of roots: IV. Positive and negative auxin effects on cell elongation. Physiol Plant 3: 277-292 [Google Scholar]
- Carrington CMS, Esnard J (1988) The elongation response of watermelon hypocotyls to indole-3-acetic acid: a comparative study of excised segments and intact plants. J Exp Bot 39: 441-450 [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick AV, Burg SP (1967) An explanation for the inhibition of root growth caused by indole-3-acetic acid. Plant Physiol 42: 415-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick AV, Burg SP (1970) Regulation of root growth by auxin-ethylene interaction. Plant Physiol 45: 192-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield SP, Stirnberg P, Forde BG, Leyser O (2000) The hormonal regulation of axillary bud growth in Arabidopsis. Plant J 24: 159-169 [DOI] [PubMed] [Google Scholar]
- Chen J-G, Shimomura S, Sitbon F, Sandberg G, Jones AM (2001) The role of auxin-binding protein 1 in the expansion of tobacco leaf cells. Plant J 28: 607-617 [DOI] [PubMed] [Google Scholar]
- Chen K-H, Miller AN, Patterson GW, Cohen JD (1988) A simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiol 86: 822-825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker W, Zimmerman PW, Hitchcock AE (1932) Ethylene-induced epinasty of leaves and the relation of gravity to it. Contrib Boyce Thompson Inst 4: 177-218 [Google Scholar]
- Digby J, Firn RD (1985) Growth substances and leaf growth. In NR Baker, WJ Davies, CK Ong, eds, Control of Leaf Growth. Cambridge University Press, UK, pp 57-76
- Eliasson L, Bertell G, Bolander E (1989) Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol 91: 310-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Cohen JD, Bandurski RS (1980) Concentration and metabolic turnover of indoles in germinating kernels of Zea mays L. Plant Physiol 65: 415-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle M (1998) Polar auxin transport: new support for an old model. Plant Cell 10: 1775-1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith MHM (1977) The polar transport of auxin. Annu Rev Plant Physiol 28: 439-478 [Google Scholar]
- Goodwin PB, Erwee MG (1983) Hormonal influences on leaf growth. In JE Dale, FL Milthorpe, eds, The Growth and Functioning of Leaves. Cambridge University, UK, pp 207-232
- Hall JL, Brummell DA, Gillespie J (1985) Does auxin stimulate the elongation of intact stems? New Phytol 100: 341-345 [Google Scholar]
- Hayes AB (1977) Developmental aspects of leaf blade hyponasty. Bot Gaz 138: 52-55 [Google Scholar]
- Hayes AB (1978) Auxin-cytokinin effects in leaf blade hyponasty. Bot Gaz 139: 385-389 [Google Scholar]
- Hayes AB (1981) The interaction of auxin and ethylene in the maintenance of leaf blade form in Phaseolus vulgaris L. var. Pinto. Am J Bot 68: 733-740 [Google Scholar]
- Hayes AM, Lippincott JA (1976) Growth and gravitational response in the development of leaf blade hyponasty. Am J Bot 63: 383-387 [Google Scholar]
- Hayes AM, Lippincott JA (1981) The timing of and effect of temperature on auxin-induced hyponastic curvature of the bean primary leaf blade. Am J Bot 68: 305-311 [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QC, Bleecker AB, Ecker JR, Meyerowitz E (1998) EIN4 and ERS2 are members of the putative ethylene gene family in Arabidopsis. Plant Cell 10: 1321-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M (1998) Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol 116: 455-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Im K-H, Savka MA, Wu M-J, DeWitt G, Shillito R, Binns AN (1998) Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 282: 1114-1117 [DOI] [PubMed] [Google Scholar]
- Keller CP, Van Volkenburgh E (1997) Auxin-induced epinasty of tobacco leaf tissues. A non-ethylene mediated response. Plant Physiol 113: 603-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CP, Van Volkenburgh E (1998) Evidence that auxin-induced growth of tobacco leaf tissues does not involve cell wall acidification. Plant Physiol 118: 557-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffman NL (1987) The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev 1: 86-96 [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28: 465-474 [DOI] [PubMed] [Google Scholar]
- Lippincott BB, Lippincott JA (1971) Auxin-induced hyponasty of the leaf blade of Phaseolus vulgaris. Am J Bot 58: 817-826 [Google Scholar]
- Mattsson J, Ckurshumova W, Berleth T (2003) Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol 131: 1327-1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PW (1984) Is ethylene the natural regulator of abscission? In Y Fuchs, E Chalutz, eds, Ethylene: Biochemical, Physiological and Applied Aspects, Advances in Agricultural Biotechnology. Vol 9. Martinus Nijhoff, The Hague, The Netherlands, pp 231-240 [Google Scholar]
- Morré DJ, Bonner J (1965) A mechanical analysis of root growth. Physiol Plant 18: 635-639 [Google Scholar]
- Mulkey TJ, Kuzmanoff KM, Evans ML (1982) Promotion of growth and shift in the auxin dose/response relationship in maize roots treated with the ethylene biosynthesis inhibitors aminoethoxyvinylglycine and cobalt. Plant Sci Lett 25: 43-48 [Google Scholar]
- Penny D, Penny P, Marshall DC (1974) High resolution measurement of plant growth. Can J Bot 52: 959-969 [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118: 1369-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribnicky DM, Ilic N, Cohen JD, Cooke TJ (1996) The effects of exogenous auxins on endogenous indole-3-acetic acid metabolism: the implications for carrot somatic embryogenesis. Plant Physiol 112: 549-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE, Horton RF (1975) Rapid effects of indoleacetic acid and ethylene on the growth of intact pea roots. Plant Physiol 55: 443-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano CP, Cooper ML, Klee HJ (1993) Uncoupling auxin and ethylene effects in transgenic tobacco and Arabidopsis plants. Plant Cell 5: 181-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano CP, Robson PRH, Smith H, Estelle M, Klee H (1995) Transgene-mediated auxin overproduction in Arabidopsis: hypocotyls elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol 27: 1071-1083 [DOI] [PubMed] [Google Scholar]
- Sánchez-Bravo J, Ortuno AM, Botía JM, Acosta M, Sabater F (1992) The decrease in auxin polar transport down the lupin hypocotyl could produce the indole-3-acetic acid distribution response responsible for the elongation growth pattern. Plant Physiol 99: 108-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE (1999) Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol 121: 1179-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon F, Sundberg B, Olsson O, Sandberg G (1991) Free and conjugated indoleacetic acid (IAA) contents in transgenic tobacco plants expressing the iaaM and iaaH IAA biosynthesis genes from Agrobacterium tumefaciens. Plant Physiol 95: 480-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamimi S, Firn RD (1985) The basipetal auxin transport system and the control of cell elongation in hypocotyls. J Exp Bot 36: 955-962 [Google Scholar]
- Tsukaya T, Shoda K, Kim G-T, Uchimiya H (2000) Heteroblasty in Arabidopsis thaliana (L.) Heynh. Planta 210: 536-542 [DOI] [PubMed] [Google Scholar]
- Vanderhoef LN, Stahl CA, Tse-Yuan SL (1976) Two elongation responses to auxin respond differently to protein synthesis inhibition. Plant Physiol 58: 402-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Volkenburgh E (1999) Leaf expansion: an integrating plant behavior. Plant Cell Environ 22: 1463-1473 [Google Scholar]
- Went FW (1951) The development of stems and leaves. In F Skoog, ed, Plant Growth Substances. University of Wisconsin, Madison, pp 287-298
- Went FW, Thimman KV (1937) Phytohormones. MacMillan, New York
- Wightman F (1977) Gas chromatographic identification and quantitative estimation of natural auxins in developing plant organs. In PE Pilet, ed, Plant Growth Regulation. Springer-Verlag, Berlin, pp 77-90
- Yang T, Law DM, Davies PJ (1993) Magnitude and kinetics of stem elongation induced by exogenous indole-3-acetic acid in intact light-grown pea seedlings. Plant Physiol 102: 717-724 [DOI] [PMC free article] [PubMed] [Google Scholar]