Abstract
Dopamine depletion resulting from degeneration of nigrostriatal dopaminergic neurons is the primary neurochemical basis of the motor symptoms of Parkinson’s disease (PD). While dopaminergic replacement strategies are effective in ameliorating these symptoms early in the disease process, more advanced stages of PD are associated with the development of treatment-related motor complications and dopamine-resistant symptoms. Other neurotransmitter and neuromodulator systems are expressed in the basal ganglia and contribute to the extrapyramidal refinement of motor function. Furthermore, neuropathological studies suggest that they are also affected by the neurodegenerative process. These non-dopaminergic systems provide potential targets for treatment of motor fluctuations, levodopa-induced dyskinesias, and difficulty with gait and balance. This review summarizes recent advances in the clinical development of novel pharmacological approaches for treatment of PD motor symptoms. Although the non-dopaminergic pipeline has been slow to yield new drugs, further development will likely result in improved treatments for PD symptoms that are induced by or resistant to dopamine replacement.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0239-9) contains supplementary material, which is available to authorized users.
Keywords: Parkinson’s disease, Non-dopaminergic, Dyskinesias, Motor fluctuations, Glutamate, Adenosine
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is characterized clinically by the classical motor symptoms of bradykinesia, rigidity, and resting tremor. These symptoms are primarily caused by the selective loss of dopaminergic neurons in the substantia nigra pars compacta, which results in decreased levels of dopamine in the striatum. Dopamine replacement strategies have been the mainstay of treatment for motor symptoms of PD, and nearly 50 years since its introduction, levodopa (the precursor of dopamine) remains the most effective treatment. However, despite its beneficial effects on motor function, dopaminergic therapy has significant limitations, making development of other therapeutic approaches targeting non-dopaminergic pathways a priority [1]. First, neither levodopa nor dopamine agonists have been demonstrated to slow the progression of nigrostriatal cell loss. Second, while initially successful in ameliorating motor symptoms, long-term treatment with levodopa is complicated by the onset of motor fluctuations (with alternating periods of mobility and relative immobility) and involuntary dyskinesias. Last, symptoms that develop at later stages of PD, both motor (e.g., postural instability and freezing of gait) and non-motor, are frequently not responsive to dopaminergic treatments. These symptoms are likely to be caused by the degeneration of neurons in other parts of the nervous system as a result of the same disease process that affects the nigrostriatal system [2].
In this review, we discuss potential non-dopaminergic approaches to treatment of PD symptoms. Multiple neurotransmitters are recognized to play a role in modulating the basal ganglia and other neural circuits thought to be involved in PD. We will focus primarily on neurotransmitter targets in which there have been therapeutic advances in targeting motor symptoms. Agents targeting non-dopaminergic pathways are also being actively explored for treatment of non-motor symptoms.
Neurotransmitter Diversity in the Basal Ganglia and Motor Control
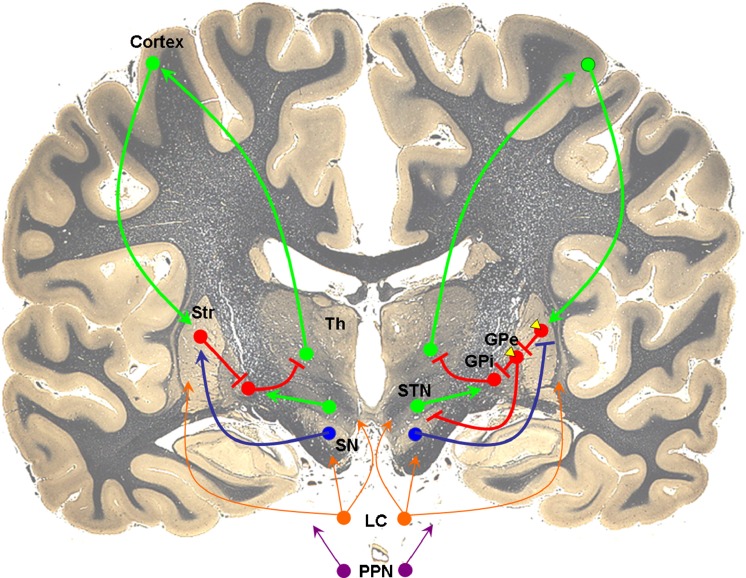
To understand the potential use of pharmacologic agents targeting non-dopaminergic pathways, it is helpful to briefly review the role of these neurotransmitter systems in regulating motor function [3]. In the classic model of basal ganglia organization (Fig. 1), the cerebral cortex sends excitatory glutamatergic inputs to the striatum. Dopamine, via the nigrostriatal pathway, modulates these inputs, either through an excitatory effect on a subpopulation of striatal neurons that contain gamma-aminobutyric acid (GABA) and substance P (direct pathway), or through an inhibitory effect on a separate subpopulation of neurons that co-express GABA and enkephalin (indirect pathway). The effects on the direct and indirect pathways are mediated by dopamine binding to D1 and D2 receptors, respectively, both of which are highly expressed in the striatum (Fig. 2). In the direct pathway, striatal neurons send inhibitory GABAergic inputs directly to the output nuclei of the basal ganglia, the globus pallidus pars interna (GPi) and substantia nigra pars reticulata (SNr), which then send GABAergic fibers to ventral thalamic nuclei. In contrast, axons from striatofugal neurons in the indirect pathway form GABAergic synapses with cells in the globus pallidus pars externa, which then send GABAergic projections to the subthalamic nucleus (STN). The STN then uses glutamate to modulate basal ganglia output from the GPi/SNr. This classic model suggests that dopamine regulates basal ganglia activity by balancing opposing effects on the direct and indirect pathways. Loss of striatal dopamine in PD disrupts this balance, producing a hypokinetic (parkinsonian) state. In contrast, subsequent treatment with dopaminergic agents predisposes to hyperkinetic (dyskinesia) responses. While this model is useful in accounting for some of the phenomenology associated with PD, basal ganglia circuitry is likely to be more complicated. For example, a recent rodent study suggests that both direct and indirect pathways are concurrently activated during initiation of action [4].
Fig. 1.
Neurotransmitter systems involved in basal ganglia circuitry. Excitatory glutamatergic efferents (green) from cortex project to gamma-aminobutyric acid (GABA)ergic (red) striatal neurons. In the direct pathway (left), striatal neurons receive excitatory dopaminergic inputs (blue) from substantia nigra and project directly to globus pallidus interna (GPi). In the indirect pathway (right), dopamine inhibits striatal GABAergic output to the globus pallidus externa (GPe), which then projects to GPi. Adenosine A2A receptors (yellow) are localized to dopamine D2 receptor-containing cells in the indirect pathway. Noradrenergic and cholinergic efferents from the locus coeruleus (orange) and pedunculopontine nucleus (purple), respectively, project widely to multiple brain regions, including cortex and basal ganglia. The coronal brain image is adapted with permission from http://www.brains.rad.msu.edu and http://brainmuseum.org, supported by the US National Science Foundation
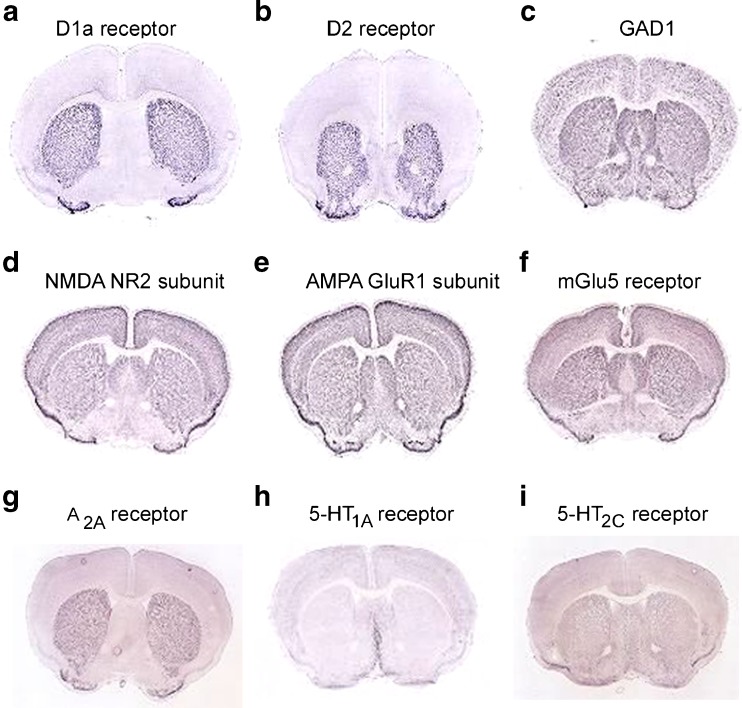
Fig. 2.
Expression patterns of neurotransmitter systems in the rodent brain. Dopamine D1 and D2 receptors and adenosine A2A receptors are localized and highly expressed in the striatum, while glutamic acid decarboxylase [GAD, present in gamma-aminobutryic acid (GABA)ergic neurons], N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid (AMPA), and metabotropic glutamate receptor (mGlu5) subunits, and serotonin (5-HT) receptor subtypes are not concentrated in specific brain regions. In situ hybridization images are obtained from the Allen brain atlas (www.brain-map.org)
Glutamate receptors are expressed at high levels in the striatum. However, unlike dopamine receptors (which are highly enriched in the basal ganglia), they are present at high density throughout the brain (Fig. 2). GABA, which is synthesized from glutamate by the enzyme glutamic acid decarboxylase (GAD), is also expressed widely in the central nervous system (CNS). Given their primary role in basal ganglia circuitry, these neurotransmitter systems are potentially attractive targets to treat parkinsonian symptoms. However, their lack of regional specificity raises the potential challenge of side effects from actions on other brain regions.
Other neurotransmitters have also been implicated in the regulation of basal ganglia function. Adenosine is a purine nucleoside that acts to modulate synaptic function in the CNS. Its action is mediated by 4 G-protein-coupled receptor subtypes: A1, A2A, A2B, and A3. Of these, the A2A receptor has received considerable attention as a potential treatment target because, like dopamine receptors, its expression is highly enriched in the striatum (Fig. 2) [5, 6]. Alterations in the serotonergic system have also been recognized in PD [7]. Of the 14 subtypes of serotonin (5-HT) receptors [8], multiple subtypes, including 5-HT1A and 5-HT2C receptors, are present in striatal neurons (Fig. 2). Serotonergic inputs from the raphe nuclei form widespread connections throughout the brain, including the substantia nigra, striatum, globus pallidus, STN, thalamus, and cortex.
Neuropathological studies have suggested that neurodegeneration in PD is not restricted to dopaminergic neurons and the basal ganglia. According to the Braak staging system [2], inclusion bodies containing α-synuclein are found in caudal brainstem nuclei (stage 1) prior to involvement of the substantia nigra (stage 3). At stage 2, 5-HT-producing raphe nuclei neurons are affected, as are projection neurons in the locus coeruleus that produce noradrenaline. At later stages (through stage 6), acetylcholine (ACh)-producing neurons in the pedunculopontine tegmental nucleus and neocortex also undergo degeneration. The diversity of affected neurotransmitter systems yields a number of symptoms that may only respond to adjunct non-dopaminergic therapies.
Symptomatic Treatment and Motor Fluctuations
The presence of multiple neurotransmitters modulating the basal ganglia circuitry that coordinates movement suggests that non-dopaminergic strategies may be helpful in treating motor symptoms. These approaches offer potential advantages, including providing antiparkinsonian benefits either as monotherapy or in combination with dopamine replacement, allowing reduction in dose of dopaminergic agents to ameliorate treatment-related side effects, or directly reducing motor fluctuations and/or dyskinesias associated with chronic levodopa use.
Adenosine
Adenosine A2A receptors are localized to dendrites, cell bodies, and axon terminals of GABAergic striatopallidal neurons of the indirect pathway, in close association with dopamine D2 receptors [9–11]. By binding to A2A receptors, adenosine activates striatopallidal neurons, opposing the inhibitory effects mediated by D2 receptor binding [12, 13]. These findings suggest that blockade of A2A receptors should inhibit the excessive activity of the indirect pathway that results from dopamine depletion. Indeed, in rodent and non-human primate models of PD, A2A antagonists consistently reversed parkinsonian deficits without development of tolerance to prolonged treatment [14]. The preclinical data have motivated multiple clinical trials investigating whether these agents are effective in treating PD symptoms (Table 1).
Table 1.
Non-dopaminergic therapies for motor symptoms of Parkinson’s disease: Results from clinical trials
| Mechanism | Drug | Phase | Use | n | Dose | Duration | Primary Outcome | Result | Ref |
|---|---|---|---|---|---|---|---|---|---|
| SYMPTOMATIC TREATMENT AND MOTOR FLUCTUATIONS | |||||||||
| Adenosine | |||||||||
| A2A antagonist | Istradefylline (KW-6002) | II | Mono | 176 | 40 mg/day | 12 weeks | Change from baseline in UPDRS motor score | Negative | [15] |
| Adjunct | 15 | 40 or 80 mg/day | 6 weeks | Duration of L-dopa effect | Positive | [16] | |||
| Adjunct | 83 | 5/10/20 or 10/20/40 mg/day | 12 weeks | Reduction in off time | Positive | [17] | |||
| Adjunct | 395 | 20 and 60 mg/day | 12 weeks | Reduction in off time | Positive | [18] | |||
| Adjunct | 196 | 40 mg/day | 12 weeks | Reduction in off time | Positive | [19] | |||
| Adjunct | 363 | 20 or 40 mg/day | 12 weeks | Reduction in off time | Positive | [20] | |||
| III | Adjunct | 231 | 20 mg/day | 12 weeks | Reduction in off time | Positive | [22] | ||
| Adjunct | 373 | 20 or 40 mg/day | 12 weeks | Reduction in off time | Positive | [23] | |||
| Adjunct | 584 | 10, 20, or 40 mg/day | 12 weeks | Reduction in off time | Negative | [24] | |||
| Preladenant | II | Adjunct | 253 | 1, 2, 5, or 10 twice daily | 12 weeks | Reduction in off time | Positive (for 5, 10 mg doses) | [28] | |
| Tozadenant | II | Adjunct | 420 | 60, 120, 180, or 240 mg twice daily | 12 weeks | Reduction in off time | Positive (for 120, 180 mg doses) | [31] | |
| GABA | |||||||||
| GAD gene therapy | AAV2–GAD | II | Adjunct | 45 | Bilateral AAV2–GAD delivery | 6 months | Change from baseline in off state UPDRS motor score | Positive | [42] |
| Serotonin | |||||||||
| 5-HT1A agonist | Pardoprunox | II | Mono | 139 | 9-45 mg/day | 3 weeks | Change from baseline in UPDRS motor score | Positive | [49] |
| III | Mono | 468 | 6, 12, or 12–42 mg/day | 24 weeks | Change from baseline in UPDRS motor score | Positive (high dropout rate) | [50] | ||
| Mono | 334 | 12–42 mg/day (vs pramipexole) | 24 weeks | Change from baseline in UPDRS motor score | Positive (high dropout rate) | [50] | |||
| III | Adjunct | 295 | 12–42 mg/day | 12 weeks | Reduction in off time | Positive (high dropout rate) | [51] | ||
| LEVODOPA-INDUCED DYSKINESIAS | |||||||||
| Glutamate | |||||||||
| NMDA receptor antagonist | Traxoprodil (CP-101,606) | II | Adjunct | 12 | Low, high-dose infusion | Single dose | Change in Dyskinesia Rating Scale score | Positive (dose-related side effects) | [71] |
| Memantine | II | Adjunct | 12 | 30 mg/day | 2 week treatment | Change in dyskinesia score after single levodopa challenge | Negative | [72] | |
| AMPA receptor antagonist | Perampanel | III | Adjunct | 763 | 2 or 4 mg/day | 30 weeks | Reduction in off time and severity of dyskinesias (UPDRS IV) | Negative | [82] |
| Adjunct | 751 | 2 or 4 mg/day | 20 weeks | Reduction in off time and severity of dyskinesias (UPDRS IV) | Negative | [82] | |||
| mGluR5 negative allosteric modulator | Mavoglurant (AFQ056) | II | Adjunct | 31 | 50–300 mg/day | 16 days | Change in LFADLDS | Positive | [95] |
| Adjunct | 28 | 50–300 mg/day | 16 days | Change in mAIMS score | Positive | [95] | |||
| Adjunct | 197 | 20, 50, 100, 150, or 200 mg/day | 13 weeks | Change in mAIMS score | Positive (for 200 mg dose) | [96] | |||
| Dipraglurant (ADX48261) | II | Adjunct | 76 | Dose titration to 300 mg/day | 4 weeks | Change in mAIMS score | Positive (on Days 1, 14) | [99] | |
| Noradrenaline | |||||||||
| α2 Adrenergic receptor antagonist | Fipamezole | II | Adjunct | 179 | 90, 180, or 270 mg/day | 4 weeks | Change in levodopa-induced dyskinesia scale | Negative | [109] |
| Serotonin | |||||||||
| 5-HT1A receptor agonist | Sarizotan | II | Adjunct | 398 | 2, 4, or 10 mg/day | 12 weeks | Change in diary-based on time without dyskinesias | Negative | [110] |
| GAIT AND BALANCE | |||||||||
| Cholinesterase inhibitor | Donepezil | IV | Adjunct | 23 | 5–10 mg/day | 6 weeks | Reduction in fall frequency | Positive | [119] |
| Noradrenergic reuptake inhibitor | Methylphenidate | IV | Adjunct (+ STN DBS) | 69 | 1 mg/kg/day | 90 days | Change in number of steps in stand-walk-sit test | Positive | [125] |
| Adjunct | 23 | Up to 80 mg/day | 12 weeks; 3-week washout | Change in gait composite score | Negative | [126] | |||
| NMDA receptor antagonist | Memantine | IV | Adjunct | 25 | 20 mg/day | 90 days | Change in stride length | Negative | [127] |
GABA = gamma-aminobutyric acid; GAD = glutamic acid decarboxylase; 5-HT = serotonin; NMDA = N-methyl-D-aspartate; AMPA = alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid; mGlu = metabotropic glutamate; AAV = adeno-associated virus; STN = subthalamic nucleus; DBS = deep brain stimulation; UPDRS = Unified Parkinson Disease Rating Scale; LFADLDS = Lang-Fahn Activities of Daily Living Dyskinesia Scale; mAIMS, modified Abnormal Involuntary Movement Scale
Among A2A antagonists that have been investigated clinically, istradefylline (KW-6002) has been studied most extensively. In a phase II randomized clinical trial, istradefylline did not improve motor function when used as monotherapy [15]. However, multiple phase II clinical trials in levodopa-treated PD patients with motor fluctuations demonstrated a significant reduction in off time [16–20]. Several of these trials demonstrated an increase in on time with dyskinesias, although they were not troublesome and did not impair mobility [17–19]. A long-term, open-label study showed persistent improvement in off time over a 52-week treatment period, suggesting a sustained symptomatic benefit [21]. However, despite the initial optimism based on the early studies, subsequent phase III clinical trials have yielded conflicting results. Two studies demonstrated a significant reduction in daily off time with an increased incidence of dyskinesias [22, 23], but istradefylline did not affect off time in another trial [24]. In the latter study, motor function in the on state was improved compared with placebo, and a large placebo response may account for the negative effect on off time [24]. Although the US Food and Drug Administration issued a not approvable letter for istradefylline based on available data in 2008 [25], the drug was later approved for use in Japan as adjunctive treatment for PD [26], and phase III clinical development recently resumed in North America [27].
More recently, preladenant, a second-generation A2A antagonist with higher affinity and greater selectivity, had been moving through the therapeutic pipeline. In a phase II, dose-finding, 12-week randomized, placebo-controlled trial, preladenant at a dose of 5 mg and 10 mg twice daily was well-tolerated and reduced off time without increasing on time with troublesome dyskinesias [28]. In a 36-week open-label extension study, the drug similarly provided a reduction in off time, but with an increased incidence of dyskinesias (33 % vs 9 % in the randomized study) [29]. Three separate phase III randomized, controlled clinical trials have been ongoing, 2 assessing preladenant when added to levodopa in patient with moderate-to-severe PD, and another as monotherapy in early PD. Results have not been presented or published, but a press release from the manufacturer [30] indicated that initial review did not show evidence of efficacy; as a result, extension studies were discontinued and there are no plans to pursue regulatory filings.
A phase IIb randomized clinical trial investigating the safety and efficacy of the A2A antagonist tozadenant (SYN115) to treat end-of-dose wearing off in 420 patients with moderate-to-severe PD patients has been completed, and a preliminary communication reported good tolerability and significant reduction in off time [31]. A previous smaller clinical study of tozadenant in PD patients provided functional magnetic resonance imaging evidence that the drug enters the CNS and engages its putative target of striatopallidal adenosine A2A receptors to reduce the inhibitory influence of the indirect pathway on motor function [32].
Lastly, it is worth noting that the non-specific adenosine receptor antagonist caffeine, likely acting by blocking striatal A2A receptors [33], has recently demonstrated evidence of significant antiparkinsonian actions in a randomized clinical trial. Although the study by Postuma et al. [34] was designed primarily to investigate potential alerting effects, they observed a reduction in Unified Parkinson’s Disease Rating Scale score comparable to that with more specific A2A antagonists and are now pursuing a long-term phase III study to investigate potential disease-modifying benefits, as well as to possibly confirm short-term motor benefits. Convergent epidemiological and laboratory animal data also support the neuroprotective potential of A2A antagonists, including caffeine, in PD [35]. Similarly, clinical, pathological, imaging, and laboratory findings have suggested these agents may help prevent the development of dyskinesias in PD [36–39].
GABA: Glutamic Acid Decarboxylase Gene Therapy
In PD, loss of dopaminergic neurons in the nigrostriatal pathway and reduction of striatal dopamine levels results in disinhibition of the subthalamic nucleus that causes parkinsonian symptoms. The enzyme GAD converts glutamate into GABA, the major inhibitory neurotransmitter in the brain. GAD gene transfer using an adeno-associated virus (AAV) has been explored as an approach to convert STN neurons from being excitatory to inhibitory [40]. In an initial phase I, open-label study, 12 patients with advanced PD were followed for 12 months after unilateral injection of AAV–GAD into the STN. Improvements in contralateral on and off motor function were observed 3 months after the injection and persisted for 12 months [41]. In a phase II double-blind, randomized trial comparing bilateral delivery of AAV2–GAD to sham surgery, patients receiving active gene therapy demonstrated a significant improvement in motor Unified Parkinson’s Disease Rating Scale score in the off state, but not the on state, at 6 months [42]. Despite these initial promising proof-of-principle findings for non-dopaminergic modulation of STN neurotransmission and for gene therapy in PD, the long-term follow-up study has been terminated owing to financial reasons [43] and there are no plans for phase III studies.
Serotonin
In PD, serotonergic neurons in the raphe nuclei degenerate, leading to a reduction in 5-HT levels [7]. Loss of 5-HT is thought to contribute to both motor and non-motor symptoms. In preclinical models, several 5-HT1A receptor agonists have shown efficacy in improving motor activity and reducing dyskinesias. However, interpretation of these results is complicated in that these agents can also interact with other receptors. In levodopa-treated parkinsonian rats, the partial 5-HT1A receptor agonist piclozotan improved motor function [44]. A randomized pilot study using piclozotan in a small number of PD patients on levodopa was also reported to show improvements in off and on time without dyskinesias [45, 46]. However, results have been published only in abstract form and additional trials have not been registered.
Pardoprunox (SLV308) is a full 5-HT1A agonist that also has partial dopamine D2/D3 agonist properties. As monotherapy in animal models it reduced parkinsonian symptoms and induced only mild dyskinesias [47, 48]. In a double-blind study of pardoprunox in early PD, treatment resulted in improvement in motor function and activities of daily living [49]. Two large, randomized, phase III dose-finding trials also showed significant improvement in motor function, although dropout rates were high owing to treatment-emergent adverse events (e.g., nausea, somnolence, and dizziness) at higher doses [50]. As adjunctive therapy to levodopa, pardoprunox reduced off time and improved on time without troublesome dyskinesias in a phase III study [51]. However, a high dropout rate was similarly noted with the selected dose range, and the most recent registered clinical trial of pardoprunox was terminated “due to strategic considerations” [52].
Levodopa-induced Dyskinesias
Repeated administration of levodopa results in the development of motor complications, including involuntary dyskinesias. Age of PD onset, disease severity, and high levodopa dose are risk factors for the development of levodopa-induced dyskinesias (LID). Based on a literature review, the rate of development of dyskinesias has been reported to be about 35–40 % by 4–6 years of treatment, and nearly 90 % within a decade [53]. The mean time to onset of dyskinesias in a recent community-based study was 6.6 years [54]. LID can be clinically expressed in a variety of ways, occurring when levodopa effects are maximal (peak-dose dyskinesias, generally choreiform, but may be dystonic), with rising or falling levels of medication (diphasic dyskinesias), or at low levels of levodopa (off-period dystonia) [55, 56].
Evidence from postmortem and pharmacological preclinical studies supports a role for multiple non-dopaminergic systems in the development of LID [57–59]. These studies have led to exploratory trials investigating drugs targeting other neurotransmitters as adjunctive therapy with the goal of decreasing LID without compromising motor function.
Glutamate
Glutamate is the most abundant excitatory neurotransmitter in the brain and is directly involved in activating basal ganglia motor circuits. Loss of nigrostriatal dopamine input is believed to induce changes in synaptic connectivity in the striatum [60]. Repeated exposure to dopaminergic drugs, particularly in a hypodopaminergic parkinsonian state, results in maladaptive plasticity in glutamatergic synapses that contributes to the expression of dyskinesias [57, 61, 62].
Glutamate signaling in the CNS is mediated by a variety of receptors, including ionotropic receptors (those that directly conduct ion flow in response to glutamate binding) and metabotropic receptors (those whose actions are mediated via intracellular signaling pathways). Among ionotropic receptors, N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid (AMPA) receptors have been most extensively studied for a possible role in LID [59, 63].
Changes in NMDA receptor levels, phosphorylation state, and cellular distribution have been identified in the dyskinetic state in animal models [59]. Highlighting the complexity of antidyskinetic strategies targeting these receptors, preclinical studies using NMDA antagonists directed at specific receptor subunits in nonhuman primate models have yielded conflicting results. In one study, a negative allosteric modulator (Co-101,244/PD-174,494) acting on NR2B receptors decreased LID, while antagonists with increased specificity for NR1A/NR2A receptors exacerbated dyskinesias [64]. In contrast, another NR2B-specific antagonist (traxoprodil, CP-101,606) increased severity of dyskinesias in levodopa-treated animals [65].
As clinical support for a role for NMDA receptor modulation as an approach to treat LID, the nonselective NMDA antagonist amantadine is currently the only accepted treatment for dyskinesia in PD [66]. It has been recommended by the American Academy of Neurology for the treatment of dyskinesias (level C evidence) [67], and has also been suggested to be efficacious in treatment of LID in an evidence-based review by the Movement Disorders Society [68]. In a recent double-blind, randomized, placebo-controlled cross-over study of 36 patients with PD and dyskinesias, 64 % of patients showed improvement in LID [69]. Treatment with amantadine can also be limited by neuropsychiatric and other side effects. Remacemide, another nonselective NMDA antagonist, did not show a significant benefit in reducing dyskinesias in a randomized, controlled trial [70]. Hence, there is a need to develop better NMDA receptor antagonists with antidyskinetic properties.
To date, several small pilot studies have been conducted investigating other NMDA receptor blockers in PD. Traxoprodil, an antagonist selective for NR2B subunits, reduced the maximum severity of acute LID by approximately 30 % in response to a 2-h levodopa infusion in 12 PD patients with motor fluctuations and dyskinesias, but did not improve parkinsonism and caused dose-related neuropsychiatric side effects [71]. However, memantine, an NMDA receptor antagonist approved for treatment of dementia, did not improve dyskinesias in a small cross-over study [72], although there are case studies reporting a positive response [73–75]. An early small, double-blind, cross-over study suggested that the NMDA antagonist dextromethorphan may be effective in reducing LID [76]. AVP-923, a combination agent combining dextromethorphan and quinidine that has been approved for treatment of pseudobulbar affect [77], is currently being studied to assess its efficacy in reducing dyskinesias in a small phase IIa study [78]. Interestingly, recent preclinical data suggests that the potential antidyskinetic effect may be mediated by indirect 5-HT1A agonism rather than through an NMDA antagonist effect [79].
The role of AMPA receptors in the development of LID has received less attention, although the AMPA antagonists talampanel (LY-300,164) [80] and topiramate [81] reduced LID in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primates. To date, 2 phase III human clinical trials using the AMPA antagonist perampanel as potential treatment for PD motor fluctuations have been published, but the drug did not show benefit either in reducing dyskinesias or “off” time [82]. Two small phase II studies investigating talampanel as an antidyskinetic agent have been completed, but results have not been published [83, 84].
Currently, metabotropic glutamate (mGlu) receptors are receiving significant attention as potential therapeutic targets [85, 86]. In particular, mGlu5 receptors are highly expressed in the striatum and globus pallidus. Expression of mGlu5 is upregulated in MPTP-lesioned primates treated with levodopa, and this increase is associated with the development of LID [87]. Administration of mGlu5 antagonists has been shown to attenuate abnormal involuntary movements in rodent models [88, 89] and LIDs in primates [90–93].
Negative allosteric modulators (NAM) of G-protein-coupled receptors target binding sites distinct from the active site and inhibit the response to endogenous ligand. Drugs targeting allosteric sites may provide greater receptor selectivity and potentially decrease adverse side effects [94]. Clinically, the selective mGlu5 NAM mavoglurant (AFQ056) was demonstrated to show a significant antidyskinetic effect in 2 small phase II randomized clinical trials [95]. Findings from a larger dose-finding study of 197 patients with PD and dyskinesias provided further evidence of anti-dyskinetic benefit without worsening of parkinsonism [96]. A phase II study exploring the efficacy and safety of a modified release form was also recently completed [97]. Another mGlu5 NAM, dipraglurant (ADX48261), has similarly been under investigation as a putative antidyskinetic agent [98]. Although not yet published, preliminary results presented in abstract form suggest a significant reduction in peak dose LIDs without affecting levodopa efficacy [99]. Together, these results suggest that mGlu5 antagonists offer promise for the treatment of LID.
Noradrenaline
Noradrenaline exerts its action by binding to G-protein-coupled adrenergic receptors, which are expressed in the striatum, STN, and substantia nigra [100]. Of particular interest are α2 adrenergic receptors, which may act to modulate GABA [101, 102] and dopamine release [103]. In pharmacological studies using primate models, α2 antagonists have been shown to reduce LID [104, 105], possibly through preferential effects on the direct pathway [57]. These preclinical findings have motivated clinical trials exploring these agents as potential antidyskinetic therapies.
Pilot studies using idazoxan yielded conflicting results [106, 107], and this drug is no longer in clinical development for PD. Currently, the selective α2A/2C receptor antagonist fipamezole is being studied for LID. An initial small pilot study demonstrated good tolerability and suppression of LID without exacerbating parkinsonian symptoms [108]. In a phase II study conducted in the USA and India, fipamezole failed to show a statistically significant reduction in dyskinesias [109]. However, separate outcome analysis of the US patients did show a benefit at the highest dose used; it has been proposed that this differential result may be owing to heterogeneity between the US and Indian study populations. An additional clinical trial may be helpful to determine whether fipamezole is indeed useful for treatment of LID.
Serotonin
Serotonin has also been implicated to play a role in LID, and 5-HT1A receptor agonists and 5-HT2A receptor antagonists have been explored as promising antidyskinetic agents. In a large phase IIb study, sarizotan, a full 5-HT1A agonist with additional affinity for dopamine D3/D4 receptors, did not show benefit in increasing on time without dyskinesias, and resulted in increased off time at higher doses [110]. Eltoprazine, a mixed 5-HT1A/1B receptor agonist, is effective in suppressing LID in animal models [111]. A small, human, randomized clinical trial has been completed in Sweden, but results have not yet been published [112]. It has been proposed that drugs aimed at reducing LID by modulating serotonergic function may need to demonstrate anatomic selectivity, as well as receptor selectivity [113].
Gait and Balance
Postural instability and gait difficulty are cardinal features of idiopathic PD, but typically do not cause prominent functional disability until later stages of disease. In particular, patients at more advanced stages of PD may become unable to initiate locomotion and develop freezing of gait (FOG) [114]. FOG is often associated with gait imbalance and can result in falls [115]. In some cases, FOG may respond to dopaminergic therapy at earlier stages [116]; however, PD-associated gait disorders may become progressively resistant to dopamine replacement or can be unresponsive from the start. Neurodegeneration in non-dopaminergic brainstem structures may contribute directly to this lack of response [117]. Cholinergic neurons in the pedunculopontine tegmental nucleus (PPN) and the prefrontal and frontal cortex are thought to be involved in gait control. Noradrenaline-producing cells in the locus coeruleus are also severely affected in PD. As a result of striatal dopamine depletion, excessive glutamatergic activity at projections from STN to PPN may also contribute to locomotor dysfunction. Interest in the role of PPN in PD gait disorders has been supported by the finding that low-frequency deep brain stimulation may reduce falls and FOG, either alone or in combination with high-frequency STN stimulation [118].
Strategies to increase ACh transmission have been used to target gait and balance symptoms unrelated to FOG. A small, randomized, placebo-controlled, crossover study in PD patients with falls showed that the centrally-acting cholinesterase inhibitor donepezil reduced falls by approximately half [119]. A single-center study in the UK is similarly exploring the effects of rivastigmine on gait and balance [120]. In the striatum, nicotinic ACh receptors are located presynaptically, and include subtypes α4β2, α6β2, and α7 receptors. A single-site study investigating the use of varenicline, a partial α4β2 and full α7 agonist used as an aid for smoking cessation, to improve balance is ongoing [121].
Methylphenidate is an amphetamine-like stimulant that inhibits presynaptic noradrenaline and dopamine transporters. Three small pilot studies using different dosing protocols demonstrated improvement in various gait measures, including gait speed and freezing [122–124]. Two subsequent randomized studies have been completed and reported conflicting results. In a study of 69 PD patients treated with STN–DBS, methylphenidate treatment improved the number of steps in the stand-walk-sit test; the treated group experienced significantly more adverse events [125]. However, another trial of 23 patients did not show any improvement in a gait composite score of stride length and velocity [126].
A recent study of 25 patients explored the use of the NMDA receptor antagonist memantine as treatment for axial symptoms of PD. Although the treated group showed improvement in axial motor symptoms and dyskinesias, no improvement was noted in stride length [127]. Similarly, a randomized, double-blind, placebo-controlled crossover trial of the non-specific NMDA antagonist amantadine failed to show benefit against FOG resistant to dopaminergic therapy [128].
Conclusions
Dopamine deficiency due to degeneration of the nigrostriatal pathway is the primary cause of motor symptoms in PD. Nevertheless, multiple other neurotransmitter systems play an important role in modulating basal ganglia function and motor control. Targeting these systems, in particular, offers potential approaches to treating motor complications of dopamine replacement and symptoms that are resistant to dopaminergic therapy. At first glance, candidate non-dopaminergic agents would appear to be the proverbial “low-hanging” fruit in the PD pipeline. Receptors for neurotransmitters, including adenosine, GABA, serotonin, glutamate, and noradrenaline, have been well characterized biochemically with extensive knowledge of their neuroanatomic distribution and intracellular signaling pathways. Moreover, preclinical studies in rodent and nonhuman primate models have demonstrated effectiveness in reducing parkinsonian symptoms.
Based on the promise of the animal studies and early phase clinical studies, a number of randomized clinical trials directed at a variety of neurotransmitter targets have been completed [129]. Unfortunately, no compound specifically targeting non-dopaminergic pathways has yet received broad regulatory approval of an indication for use in the therapeutic armamentarium for PD. Drawing on previous studies, a potential hurdle may be finding agents that show high receptor specificity and also target specific brain regions. For example, the presence of multiple glutamate and serotonin receptor subtypes offers the potential for designing drugs that act on one, or a narrow, subset of receptors. However, the widespread distribution of these receptors throughout the CNS poses the challenge of finding doses that do not cause limiting side effects through action at undesired neuroanatomic sites.
Adenosine A2A receptors have been an attractive potential target, as they are highly enriched in the striatum. Phase III studies investigating 2 A2A antagonists, istradefylline and preladenant, have been conducted, and while phase II studies have consistently shown benefit in reducing motor fluctuations, the large phase III studies have yielded conflicting results, slowing progression through the therapeutic pipeline. These discrepancies raise questions about the design of clinical trials addressing motor fluctuations. Determination of on/off fluctuations relies on patient diaries, which may be subject to variability despite appropriate training. Also, while there are multiple dyskinesia rating scales [130], the clinical variability in the types of dyskinesias and their timing offers challenges in quantifying response to treatment. There is similarly a need to define the most appropriate outcome measures for trials focusing on PD gait symptoms. The recent approval of istradefylline in Japan [26] will hopefully provide additional experience about the effect of A2A antagonists as adjunctive therapy. Additional phase III studies with mGlu5 receptor antagonists will be necessary to confirm the initial promising results from phase I/II studies.
Given the complexity of the pharmacology of dopamine-induced and dopamine-refractory PD symptoms, it may be necessary to target multiple non-dopaminergic systems in order to optimize clinical response while minimizing side effects from any particular pathway. This presents obvious obstacles to clinical trial design. However, despite the challenges thus far, ongoing development of these strategies remains a critical and hopeful pursuit toward improved treatment of PD.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1225 kb)
Acknowledgments
Work on this review was supported by NIH (5K24NS060991) and DoD (W81XWH-11-1-0150) (M.A.S.).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Lang AE, Obeso JA. Challenges in Parkinson’s disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol. 2004;3:309–316. doi: 10.1016/S1474-4422(04)00740-9. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 3.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 4.Cui G, Jun SB, Jin X, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Hickey P, Stacy M. Adenosine A2A antagonists in Parkinson’s disease: What’s next? Curr Neurol Neurosci Rep. 2012;12:376–385. doi: 10.1007/s11910-012-0279-2. [DOI] [PubMed] [Google Scholar]
- 7.Huot P, Fox SH, Brotchie JM. The serotonergic system in Parkinson’s disease. Prog Neurobiol. 2011;95:163–212. doi: 10.1016/j.pneurobio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Nichols DE, Nichols CD. Serotonin rceptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 9.Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- 11.Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Ferré S, O’Connor WT, Fuxe K, Ungerstedt U. The striopallidal neuron: a main locus for adenosine-dopamine interactions in the brain. J Neurosci. 1993;13:5402–5406. doi: 10.1523/JNEUROSCI.13-12-05402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori A, Shindou T. Modulation of GABAergic transmission in the striatopallidal system by adenosine A2A receptors: a potential mechanism for the antiparkinsonian effects of A2A antagonists. Neurology. 2003;61(11 Suppl. 6):S44–S48. doi: 10.1212/01.wnl.0000095211.71092.a0. [DOI] [PubMed] [Google Scholar]
- 14.Jenner P. A2A antagonists as novel non-dopaminergic therapy for motor dysfunction in PD. Neurology. 2003;61(11 Suppl. 6):S32–S38. doi: 10.1212/01.wnl.0000095209.59347.79. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez HH, Greeley DR, Zweig RM, et al. Istradefylline as monotherapy for Parkinson disease: Results of the 6002-US-051 trial. Parkinsonism Rel Disord. 2010;16:16–20. doi: 10.1016/j.parkreldis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Bara-Jimenez W, Sherzai A, Dimitrova T, et al. Adenosine A(2A) receptor antagonist treatment of Parkinson’s disease. Neurology. 2003;61:293–296. doi: 10.1212/01.wnl.0000073136.00548.d4. [DOI] [PubMed] [Google Scholar]
- 17.Hauser RA, Hubble JP, Truong DD. Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology. 2003;61:297–303. doi: 10.1212/01.wnl.0000081227.84197.0b. [DOI] [PubMed] [Google Scholar]
- 18.Stacy M, Silver D, Mendis T, et al. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology. 2008;70:2233–2240. doi: 10.1212/01.wnl.0000313834.22171.17. [DOI] [PubMed] [Google Scholar]
- 19.LeWitt PA, Guttman M, Tetrud JW, et al. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005) Ann Neurol. 2008;63:295–302. doi: 10.1002/ana.21315. [DOI] [PubMed] [Google Scholar]
- 20.Mizuno Y, Hasegawa K, Kondo T, Kuno S, Yamamoto M. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Mov Disord. 2010;25:1437–1443. doi: 10.1002/mds.23107. [DOI] [PubMed] [Google Scholar]
- 21.Factor S, Mark MH, Watts R, et al. A long-term study of istradefylline in subjects with fluctuating Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:423–426. doi: 10.1016/j.parkreldis.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Hauser RA, Shulman LM, Trugman JM, et al. Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Mov Disord. 2008;23:2177–2185. doi: 10.1002/mds.22095. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno Y, Kondo T, the Japanese Istradefylline Study Group Adenosine A2A receptor antagonist istradefylline reduces daily off time in Parkinson’s disease. Mov Disord. 2013;28:1138–1141. doi: 10.1002/mds.25418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pourcher E, Fernandez HH, Stacy M, Mori A, Ballerini R, Chaikin P. Istradefylline for Parkinson’s disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study. Parkinsonism Relat Disord. 2012;18:178–184. doi: 10.1016/j.parkreldis.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Park A, Stacy M. Istradefylline for the treatment of Parkinson’s disease. Expert Opin Pharmacother. 2012;13:111–114. doi: 10.1517/14656566.2012.643869. [DOI] [PubMed] [Google Scholar]
- 26.Dungo R, Deeks ED. Istradefylline: first global approval. Drugs. 2013;73:875–882. doi: 10.1007/s40265-013-0066-7. [DOI] [PubMed] [Google Scholar]
- 27.Kyowa Hakka Kirin Pharma, Inc. A 12-week randomized study to evaluate oral istradefylline in subjects with moderate to severe Parkinson’s disease (KW-6002). Available at: http://clinicaltrials.gov/show/NCT01968031. Accessed 5 Nov 2013.
- 28.Hauser RA, Cantillon M, Pourcher E, et al. Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomized trial. Lancet Neurol. 2011;10:221–229. doi: 10.1016/S1474-4422(11)70012-6. [DOI] [PubMed] [Google Scholar]
- 29.Factor SA, Wolski K, Togasaki DM, et al. Long-term safety and efficacy of preladenant in subjects with fluctuating Parkinson’s disease. Mov Disord. 2013;28:817–820. doi: 10.1002/mds.25395. [DOI] [PubMed] [Google Scholar]
- 30.Merck. Merck provides update on phase III clinical program for preladenant, the company’s investigational Parkinson’s disease medicine. Available at: http://www.mercknewsroom.com/press-release/research-and-development-news/merck-provides-update-phase-iii-clinical-program-prelade. Accessed May 23, 2013.
- 31.Olanow C, Hauser R, Kieburtz K, et al. A phase 2, placebo-controlled, randomized, double-blind trial of tozadenant (SYN-115) in patients with Parkinson’s disease with wearing-off fluctuations on levodopa. Neurology 2013;Emerging Science Abstracts:005.
- 32.Black KJ, Koller JM, Campbell MC, Gusnard DA, Bandak SI. Quantification of indirect pathway inhibition by the adenosine A2a antagonist SYN115 in Parkinson disease. J Neurosci. 2010;30:16248–16292. doi: 10.1523/JNEUROSCI.2590-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarzschild MA. Caffeine in Parkinson disease: better for cruise control than snooze patrol? Neurology. 2012;79:616–618. doi: 10.1212/WNL.0b013e318263580e. [DOI] [PubMed] [Google Scholar]
- 34.Postuma RB, Lang AE, Munhow RP, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79:651–658. doi: 10.1212/WNL.0b013e318263570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, Chen JF. Adenosine, adenosine A2A antagonists, and Parkinson’s disease. Parkinsonism Rel Disord. 2009;15:406–413. doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Calon F, Dridi M, Hornykiewicz O, Bédard PJ, Rajput AH, Di Paolo T. Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain. 2004;127:1075–1084. doi: 10.1093/brain/awh128. [DOI] [PubMed] [Google Scholar]
- 37.Xiao D, Bastia E, Xu YH, et al. Forebrain adenosine A2A receptors contribute to L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice. J Neurosci. 2006;26:13548–13555. doi: 10.1523/JNEUROSCI.3554-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramlackhansingh AF, Bose SK, Ahmed I, Turkheimer FE, Pavese N, Brooks DJ. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology 2011;76:1811-1816. [DOI] [PMC free article] [PubMed]
- 39.Wills AM, Eberly S, Tennis M, et al. Caffeine consumption and risk of dyskinesia in CALM-PD. Mov Disord. 2013;28:380–383. doi: 10.1002/mds.25319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo J, Kaplitt MG, Fitzsimmons HL, et al. Subthalamic GAD gene therapy in a Parkinson’s disease rat model. Science. 2002;298:425–429. doi: 10.1126/science.1074549. [DOI] [PubMed] [Google Scholar]
- 41.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 42.LeWitt PA, Rezai AR, Leehey MA, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 43.Neurologix, Inc. Long term follow-up study for rAAV-GAD treated subjects. Available at: http://clinicaltrials.gov/ct2/show/NCT01301573. Accessed 20 Feb /2012.
- 44.Tani Y, Ogata A, Koyama M, Inoue T. Effects of piclozotan (SUN N4057), a partial serotonin 1A receptor agonist, on motor complications induced by repeated administration of levodopa in parkinsonian rats. Eur J Pharmacol. 2010;649:218–223. doi: 10.1016/j.ejphar.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Hauser RA, Gertner JM, Okamoto M, Reed RF, Sage JI. Piclozotan reduces dyskinesia and OFF time in Parkinson’s disease (PD) patients with L-dopa induced motor complications. Parkinsonism Relat Disord. 2009;15(Suppl. 2):S118. [Google Scholar]
- 46.Sage JI, Hauser RA, Cordon ME, et al. Pilot study of the efficacy and safety of piclozotan in Parkinson’s disease with L-dopa induced motor complications. Mov Disord. 2009;24(Suppl. 2):S277. [Google Scholar]
- 47.Jones CA, Johnston LC, Jackson MJ, et al. An in vivo pharmacological evaluation of pardoprunox (SLV308)--a novel combined dopamine D(2)/D(3) receptor partial agonist and 5-HT(1A) receptor agonist with efficacy in experimental models of Parkinson's disease. Eur Neuropsychopharmacol. 2010;20:582–593. doi: 10.1016/j.euroneuro.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Johnston LC, Jackson MJ, Rose S, McCreary AC, Jenner P. Pardoprunox reverses motor deficits but induces only mild dyskinesia in MPTP-treated common marmosets. Mov Disord. 2010;25:2059–2068. doi: 10.1002/mds.23249. [DOI] [PubMed] [Google Scholar]
- 49.Bronzova J, Sampaio C, Hauser RA, et al. Double-blind study of pardoprunox, a new partial dopamine agonist, in early Parkinson’s disease. Mov Disord. 2010;25:738–746. doi: 10.1002/mds.22948. [DOI] [PubMed] [Google Scholar]
- 50.Sampaio C, Bronzova J, Hauser RA, et al. Pardoprunox in early Parkinson’s disease: results from 2 large, randomized double-blind trials. Mov Disord. 2011;26:1464–1476. doi: 10.1002/mds.23590. [DOI] [PubMed] [Google Scholar]
- 51.Rascol O, Bronzova J, Hauser RA, et al. Pardoprunox as adjunct therapy to levodopa in patients with Parkinson’s disease experiencing motor fluctuations: results of a double-blind, randomized, placebo-controlled trial. Parkinsonism Relat Disord. 2012;18:370–376. doi: 10.1016/j.parkreldis.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Abbott Products. A pilot study to assess efficacy and safety of pardoprunox as adjunct therapy to L-dopa in the treatment of patients with Parkinson’s disease experiencing motor fluctuations and dyskinesia. Available at: http://clinicaltrials.gov/ct2/show/NCT00903838. Accessed 25 Aug 2011.
- 53.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 54.Evans JR, Mason SL, Williams-Gray CH, et al. The natural history of treated Parkinson’s disease in an incident, community based cohort. J Neurol Neurosurg Psychiatry. 2011;82:1112–1118. doi: 10.1136/jnnp.2011.240366. [DOI] [PubMed] [Google Scholar]
- 55.Nutt JG. Levodopa-induced dyskinesia: review, observations, and speculations. Neurology. 1990;40:340–345. doi: 10.1212/wnl.40.2.340. [DOI] [PubMed] [Google Scholar]
- 56.Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA. Levodopa-induced dyskinesias in Parkinson’s disease: clinical and pharmacological classification. Mov Disord. 1992;7:117–124. doi: 10.1002/mds.870070204. [DOI] [PubMed] [Google Scholar]
- 57.Brotchie JM. Nondopaminergic mechanisms in levodopa-induced dyskinesias. Mov Disord. 2005;20:919–931. doi: 10.1002/mds.20612. [DOI] [PubMed] [Google Scholar]
- 58.Brotchie J, Jenner P. New approaches to therapy. Int Rev Neurobiol. 2011;98:123–150. doi: 10.1016/B978-0-12-381328-2.00005-5. [DOI] [PubMed] [Google Scholar]
- 59.Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of L-dopa-induced dyskinesia in Parkinson’s disease. Pharmacol Rev. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- 60.Day M, Wang Z, Ding J, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 61.Jenner P. Molecular mechanisms of L-dopa-induced dyskinesia. Nat Rev Neurosci. 2008;9:665–677. doi: 10.1038/nrn2471. [DOI] [PubMed] [Google Scholar]
- 62.Calabresi P, Di Fillippo M, Ghiglieri V, et al. Levodopa-induced dyskinesia in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol. 2010;9:1106–1117. doi: 10.1016/S1474-4422(10)70218-0. [DOI] [PubMed] [Google Scholar]
- 63.Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms, and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs. 2012;26:1017–1032. doi: 10.1007/s40263-012-0016-z. [DOI] [PubMed] [Google Scholar]
- 64.Blanchet PJ, Konitsiotis S, Whittemore EB, et al. Differing effects of N-methyl-D-aspartate receptor subtype selective antagonists on dyskinesias in levodopa-treated 1-methyl-4-phenyl-tetrahydropyridine monkeys. J Pharmacol Exp Ther. 1999;290:1034–1040. [PubMed] [Google Scholar]
- 65.Nash JE, Ravenscroft P, McGuire S, et al. The NR2B-selective NMDA receptor antagonist CP-101,606 exacerbates L-dopa-induced dyskinesia and provides mild potentiation of anti-parkinsonian effects of L-dopa in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp Neurol. 2004;188:471–479. doi: 10.1016/j.expneurol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Verhagen Metman L, Del Dotto P, van den Munckhof P, et al. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998;50:1323–1326. doi: 10.1212/wnl.50.5.1323. [DOI] [PubMed] [Google Scholar]
- 67.Pahwa R, Factor SA, Lyons KE, et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:983–995. doi: 10.1212/01.wnl.0000215250.82576.87. [DOI] [PubMed] [Google Scholar]
- 68.Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society evidence-based medicine review update; treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(Suppl. 3):S2–S41. doi: 10.1002/mds.23829. [DOI] [PubMed] [Google Scholar]
- 69.Sawada H, Oeda T, Kuno S, et al. Amantadine for dyskinesias in Parkinson’s disease: a randomized controlled trial. PLoS One. 2010;5:e15298. doi: 10.1371/journal.pone.0015298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shoulson I, Penney J, McDermott M, et al. A randomized, controlled trial of remacemide for motor fluctuations in Parkinson’s disease. Neurology. 2001;56:455–462. doi: 10.1212/wnl.56.4.455. [DOI] [PubMed] [Google Scholar]
- 71.Nutt JG, Gunzler SA, Kirchhoff T, et al. Effects of a NR2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov Disord. 2008;23:1860–1866. doi: 10.1002/mds.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merello M, Nouzeilles MI, Cammarota A, Leiguarda R. Effect of memantine (NMDA antagonist) on Parkinson’s disease: a double-blind crossover randomized study. Clin Neuropharmacol. 1999;22:273–276. [PubMed] [Google Scholar]
- 73.Hanagasi HA, Kaptanoglu G, Sahin HA, Emre M. The use of NMDA antagonist memantine in drug-resistant dyskinesias resulting from L-dopa. Mov Disord. 2000;15:1016–1017. doi: 10.1002/1531-8257(200009)15:5<1016::aid-mds1042>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 74.Varanese S, Howard J, DiRocco A. NMDA antagonist memantine improves levodopa-induced dyskinesias and “on-off” phenomena in Parkinson’s disease. Mov Disord. 2010;25:508–510. doi: 10.1002/mds.22917. [DOI] [PubMed] [Google Scholar]
- 75.Vidal EI, Fukushima FB, Valle AP, Villas Boas PJ. Unexpected improvement in levodopa-induced dyskinesia and on-off phenomena after introduction of memantine for treatment of Parkinson’s disease dementia. J Am Geriatr Soc. 2013;61:170–172. doi: 10.1111/jgs.12058. [DOI] [PubMed] [Google Scholar]
- 76.Verhagen Metman L, Del Dotto P, Natté R, van den Munckhof P, Chase TN. Dextromethorphan improves levodopa-induced dyskinesias in Parkinson’s disease. Neurology. 1998;51:203–206. doi: 10.1212/wnl.51.1.203. [DOI] [PubMed] [Google Scholar]
- 77.Olney N, Rosen H. AVP-923, a combination of dextromethorphan hydrobromide and quinidine sulfate for the treatment of pseudobulbar affect and neuropathic pain. IDrugs. 2010;13:254–265. [PubMed] [Google Scholar]
- 78.Avanir Pharmaceuticals. Safety and Efficacy of AVP-923 in the treatment of levodopa-induced dyskinesia in Parkinson’s disease patients (LID in PD). Available at: http://clinicaltrials.gov/show/NCT01767129. Accessed 28 Oct 2013.
- 79.Paquette MA, Martinez AA, Macheda T, et al. Anti-dyskinetic mechanisms of amantadine and dextromethorphan in the 6-OHDA rat model of Parkinson’s disease: role of NMDA vs. 5-HT1A receptors. Eur J Neurosci. 2012;36:3224–3234. doi: 10.1111/j.1460-9568.2012.08243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Konitsiotis S, Blanchet PJ, Verhagen L, Lamers E, Chase TN. AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology. 2000;54:1589–1595. doi: 10.1212/wnl.54.8.1589. [DOI] [PubMed] [Google Scholar]
- 81.Silverdale MA, Nicholson SL, Crossman AR, Brotchie JM. Topiramate reduces levodopa-induced dyskinesia in the MPTP-lesioned marmoset model of Parkinson’s disease. Mov Disord. 2005;20:403–409. doi: 10.1002/mds.20345. [DOI] [PubMed] [Google Scholar]
- 82.Lees A, Fahn S, Eggert KM, et al. Perampanel, an AMPA antagonist, found to have no benefit in reducing “off” time in Parkinson’s disease. Mov Disord. 2012;27:284–288. doi: 10.1002/mds.23983. [DOI] [PubMed] [Google Scholar]
- 83.National Institute of Neurological Disorders and Stroke (NINDS). Talampanel to treat Parkinson’s disease. Available at: http://clinicaltrials.gov/show/NCT00108667. Accessed 3 March 2008.
- 84.Teva Pharmaceutical Industries. Effects of talampanel on patients with advanced Parkinson’s disease. Available at: http://clinicaltrials.gov/ct2/show/NCT00036296. Accessed 11 Apr 2011.
- 85.Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- 86.Dickerson JW, Conn PJ. Therapeutic potential of targeting metabotropic glutamate receptors for Parkinson’s disease. Neurodegener Dis Manag. 2012;2:221–232. doi: 10.2217/nmt.12.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Samadi P, Gregoire L, Morissette M, et al. mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiol Aging. 2008;29:1040–1051. doi: 10.1016/j.neurobiolaging.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA. Antagonism of metabotropic glutamate receptor type 5 attenuates L-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem. 2007;101:483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- 89.Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci MA. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther. 2009;330:227–235. doi: 10.1124/jpet.108.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gregoire L, Morin N, Ouattara B, et al. The acute antiparkinsonian and antidyskinetic effect of AFQ056, a novel metabotropic glutamate receptor type 5 antagonist, in L-Dopa-treated parkinsonian monkeys. Parkinsonism Relat Disord. 2011;17:270–276. doi: 10.1016/j.parkreldis.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 91.Rylander D, Iderberg H, Li Q, et al. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis. 2010;39:352–361. doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Morin M, Gregoire L, Gomez-Mancilla B, Gasparini F, DiPaolo T. Effect of the metabotropic glutamate receptor type 5 antagonists MPEP and MTEP in parkinsonian monkeys. Neuropharmacology. 2010;58:981–986. doi: 10.1016/j.neuropharm.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 93.Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM. Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther. 2010;333:865–873. doi: 10.1124/jpet.110.166629. [DOI] [PubMed] [Google Scholar]
- 94.Nickols HH, Conn PJ. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis 2013 Sep 27 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 95.Berg D, Godau J, Trenkwalder C, et al. AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov Disord. 2011;26:1243–1250. doi: 10.1002/mds.23616. [DOI] [PubMed] [Google Scholar]
- 96.Stocchi F, Rascol O, Destee A, et al. AFQ056 in Parkinson patients with levodopa-induced dyskinesias: 13-week, randomized, dose-finding study. Mov Disord. 2013;28:1838–1846. doi: 10.1002/mds.25561. [DOI] [PubMed] [Google Scholar]
- 97.Novartis Pharmaceuticals. Evaluation of the efficacy and safety of modified release AFQ056 in Parkinson’s patients with L-dopa induced dyskinesias. Available at: http://clinicaltrials.gov/ct2/show/NCT01491529. Accessed 23 Aug 2013.
- 98.Addex Pharma S.A. ADX48621 for the treatment of levodopa induced dyskinesia in patients with Parkinson’s disease. Available at: http://clinicaltrials.gov/show/NCT01336088. Accessed 13 Jul 2012.
- 99.Tison F, Durif F, Corval JC, et al. Safety, tolerability and anti-dyskinetic efficacy of dipraglurant, a novel mGluR5 negative allosteric modulator (NAM) in Parkinson’s disease (PD) patients with levodopa-induced dyskinesia (LID) Neurology. 2013;80:S23.004. [Google Scholar]
- 100.Scheinen M, Lomasney JW, Hayden-Hixon DM, et al. Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 101.Fox SH, Henry B, Hill MP, Peggs D, Crossman AR, Brotchie JM. Neural mechanisms underlying peak-dose dyskinesia induced by levodopa and apomorphine are distinct: evidence from the effects of the alpha(2) adrenoceptor antagonist idazoxan. Mov Disord. 2001;16:642–650. doi: 10.1002/mds.1148. [DOI] [PubMed] [Google Scholar]
- 102.Johnston TH, Fox SH, Piggott MJ, Savola JM, Brotchie JM. The α2 adrenergic antagonist fipamezole improves quality of levodopa action in Parkinsonian primates. Mov Disord. 2010;25:2084–2093. doi: 10.1002/mds.23172. [DOI] [PubMed] [Google Scholar]
- 103.Buck K, Voehringer P, Ferger B. The alpha(2) adrenoceptor antagonist idazoxan alleviates L-DOPA-induced dyskinesia by reduction of striatal dopamine levels: an in vivo microdialysis study in 6-hydroxydopamine-lesioned rats. J Neurochem. 2010;112:444–452. doi: 10.1111/j.1471-4159.2009.06482.x. [DOI] [PubMed] [Google Scholar]
- 104.Savola JM, Hill M, Engstrom M, et al. Fipamezole (JP-1730) is a potent alpha2 adrenergic receptor antagonist that reduces levodopa-induced dyskinesia in the MPTP-lesioned primate model of Parkinson's disease. Mov Disord. 2003;18:872–883. doi: 10.1002/mds.10464. [DOI] [PubMed] [Google Scholar]
- 105.Henry B, Fox SH, Peggs D, Crossman AR, Brotchie JM. The alpha2-adrenergic receptor antagonist idazoxan reduces dyskinesia and enhances anti-parkinsonian actions of L-dopa in the MPTP-lesioned primate model of Parkinson's disease. Mov Disord. 1999;14:744–753. doi: 10.1002/1531-8257(199909)14:5<744::aid-mds1006>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 106.Rascol O, Arnulf I, Peyro-Saint Paul H, et al. Idazoxan, an alpha-2 antagonist, and L-dopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2001;16:708–713. doi: 10.1002/mds.1143. [DOI] [PubMed] [Google Scholar]
- 107.Manson AJ, Iakovidou E, Lees AK. Idazoxan is ineffective for levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2000;15:336–337. doi: 10.1002/1531-8257(200003)15:2<336::aid-mds1023>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 108.Dimitrova TD, Bara-Jimenez W, Savola JM, et al. Alpha2-adrenergic antagonist effects in Parkinson’s disease. Mov Disord. 2009;24(suppl 1):S261. [Google Scholar]
- 109.LeWitt P, Hauser RA, Lu M, et al. Randomized clinical trial of fipamezole for dyskinesia in Parkinson disease (FJORD study) Neurology. 2012;79:163–169. doi: 10.1212/WNL.0b013e31825f0451. [DOI] [PubMed] [Google Scholar]
- 110.Goetz CG, Damier P, Hicking C, et al. Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov Disord. 2007;22:179–186. doi: 10.1002/mds.21226. [DOI] [PubMed] [Google Scholar]
- 111.Bezard E, Tronci E, Pioli EY, et al. Study of the antidyskinetic effect of eltoprazine in animal models of levodopa-induced dyskinesia. Mov Disord. 2013;28:1088–1096. doi: 10.1002/mds.25366. [DOI] [PubMed] [Google Scholar]
- 112.PsychoGenics, Inc. A double-blind, randomized, placebo controlled, dose finding study of oral eltoprazine for treatment of levodopa-induced dyskinesias (LID) in a levodopa challenge-dose setting in Parkinson Disease. Available at http://www.clinicaltrialsregister.eu/ctr-search/trial/2009-015928-28/SE. Accessed 13 Aug 2010.
- 113.Huot P, Fox SH, Newman-Tancredi A, Brotchie JM. Anatomically selective serotonergic type 1A and serotonergic type 2A therapies for Parkinson’s disease: an approach to reducing dyskinesia without exacerbating Parkinsonism? J Pharmacol Exp Ther. 2011;339:2–8. doi: 10.1124/jpet.111.184093. [DOI] [PubMed] [Google Scholar]
- 114.Okuma Y, Yanagisawa N. The clinical spectrum of freezing of gait in Parkinson’s disease. Mov Disord. 2008;23(Suppl. 2):S426–430. doi: 10.1002/mds.21934. [DOI] [PubMed] [Google Scholar]
- 115.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 116.Giladi N. Medical treatment of freezing of gait. Mov Disord. 2008;23(Suppl. 2):S482–488. doi: 10.1002/mds.21914. [DOI] [PubMed] [Google Scholar]
- 117.Devos D, Defebvre L, Bordet R. Dopaminergic and non-dopaminergic pharmacological hypotheses for gait disorders in Parkinson’s disease. Fundamen Clin Phamacol. 2010;24:407–421. doi: 10.1111/j.1472-8206.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 118.Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology. 2013;80:1148–1155. doi: 10.1212/WNL.0b013e3182886a76. [DOI] [PubMed] [Google Scholar]
- 119.Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75:1263–1269. doi: 10.1212/WNL.0b013e3181f6128c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Research and Enterprise Department, UK. A randomised, double blind, placebo controlled trial to evaluate the effect of Rivastigmine on gait in people with Parkinson’s disease who have fallen. Available at http://www.clinicaltrialsregister.eu/ctr-search/trial/2011-003053-25/GB. Accessed 11 May 2012.
- 121.Rush University Medical Center. Varenicline for gait and balance impairment in Parkinson disease. Available at: http://clinicaltrials.gov/show/NCT01341080. Accessed 26 Sep 2012.
- 122.Aureil E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2006;29:15–17. doi: 10.1097/00002826-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 123.Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:470–475. doi: 10.1136/jnnp.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pollak L, Dobronevsky Y, Prohorov T, Bahunker S, Rabey JM. Low dose methylphenidate improves freezing in advanced Parkinson’s disease during off-state. J Neural Transm Suppl. 2007;72:145–148. doi: 10.1007/978-3-211-73574-9_17. [DOI] [PubMed] [Google Scholar]
- 125.Moreau C, Delval A, Defebvre L, et al. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson’s disease undergoing subthalamic stimulation : a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol. 2012;11:589–596. doi: 10.1016/S1474-4422(12)70106-0. [DOI] [PubMed] [Google Scholar]
- 126.Espay AJ, Dwivedi AK, Payne M, et al. Methylphenidate for gait impairment in Parkinson disease: a randomized clinical trial. Neurology. 2011;76:1256–1262. doi: 10.1212/WNL.0b013e3182143537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moreau C, Delval A, Tiffreau V, et al. Memantine for axial signs in Parkinson’s disease : a randomised, double-blind, placebo-controlled pilot study. J Neurol Neurosurg Psychiatry. 2013;84:552–555. doi: 10.1136/jnnp-2012-303182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim YE, Yun JY, Yang HJ, et al. Intravenous amantadine for freezing of gait resistant to dopaminergic therapy: a randomized, double-blind, placebo-controlled, cross-over clinical trial. PLoS One. 2012;7:e48890. doi: 10.1371/journal.pone.0048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kalia LV, Brotchie JM, Fox SH. Novel nondopaminergic targets for motor features of Parkinson’s disease: review of recent trials. Mov Disord. 2013;28:131–144. doi: 10.1002/mds.25273. [DOI] [PubMed] [Google Scholar]
- 130.Colosimo C, Martínez-Martin P, Fabbrini G, et al. Task force report on scales to assess dyskinesia in Parkinson’s disease: critique and recommendations. Mov Disord. 2010;25:1131–1142. doi: 10.1002/mds.23072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1225 kb)