Abstract
Sleep disorders are common in patients with Parkinson’s disease (PD), and preliminary work has suggested viable treatment options for many of these disorders. For rapid eye movement sleep behavior disorder, melatonin and clonazepam are most commonly used, while rivastigmine might be a useful option in patients whose behaviors are refractory to the former. Optimal treatments for insomnia in PD have yet to be determined, but preliminary evidence suggests that cognitive–behavioral therapy, light therapy, eszopiclone, donepezil, and melatonin might be beneficial. Use of the wake-promoting agent modafinil results in significant improvement in subjective measures of excessive daytime sleepiness, but not of fatigue. Optimal treatment of restless legs syndrome and obstructive sleep apnea in PD are not yet established, although a trial of continuous positive airway pressure for sleep apnea was recently completed in PD patients. In those patients with early morning motor dysfunction and disrupted sleep, the rotigotine patch provides significant benefit.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0236-z) contains supplementary material, which is available to authorized users.
Keywords: REM sleep behavior disorder, Insomnia, Excessive daytime sleepiness, Fatigue, Restless legs syndrome, Obstructive sleep apnea
Introduction
Sleep disorders are very common in patients with Parkinson’s disease (PD), and include rapid eye movement (REM) sleep behavior disorder (RBD), sleep fragmentation/insomnia, excessive daytime sleepiness, restless legs syndrome, and obstructive sleep apnea [1–9]. Sleep disorders negatively affect quality of life in patients with PD [10, 11]. Despite the importance of identifying and treating sleep disorders in patients with PD, there is a relative lack of large-scale, randomized controlled trials of sleep treatments in this population. This review will discuss available literature, presenting randomized controlled trials whenever available (Table 1), but future studies providing high quality evidence remain much needed [12].
Table 1.
Randomized controlled studies of sleep disorders in patients with Parkinson’s disease (PD)
| Sleep disorder | Study [ref.] | Intervention | Result |
|---|---|---|---|
| Rapid eye movement behavior disorder (RBD) | Kunz and Bes 2010 [31] | Melatonin, 3 mg (most subjects did not have PD) | Improvement in Clinical Global Impression (CGI)-change scores |
| Di Giacopo et al., 2012 [36] | Rivastigmine patch, 4.6 mg/24 h | Decrease in bedpartner-reported RBD episodes | |
| Insomnia | Rios Romenets et al., 2013 [57] | Group cognitive–behavioral therapy for insomnia, 6 sessions of 90 mins, combined with light therapy | Improvements in some, but not all, subjective measures of insomnia. Motor function and quality of life worsened. |
| Menza et al., 2010 [58] | Eszopiclone, 2 or 3 mg | Total sleep time (by diary) not improved; several secondary measures of insomnia did improve | |
| Rios Romenets et al., 2013 [57] | Doxepin, 10 mg | Improvements in Insomnia Severity Index and Scales for Outcomes in Parkinson’s Disease-night (co-primary outcomes) | |
| Medeiros et al., 2007 [65] | Melatonin, 3 mg | Improvement in subjective primary outcome; no improvement in objective primary outcome | |
| Excessive daytime sleepiness and/or fatigue | Adler et al., 2003 [78] | Modafinil, 200 mg | Improvement in Epworth Sleepiness Score (ESS) scores |
| Hogl et al., 2002 [80] | Modafinil, 200 mg | Improvement in ESS scores; no improvement in Maintenance of Wakefulness Test | |
| Lou et al., 2009 [77] | Modafinil, 100 mg twice a day | No improvement in ESS scores; improvement in motor fatigue but not subjective fatigue ratings | |
| Ondo et al., 2005 [79] | Modafinil, 200–400 mg | No improvement in ESS scores, Multiple Sleep Latency Test, or subjective fatigue ratings | |
| Tyne, 2010 [81] | Modafinil, up to 400 mg | Improvement in ESS scores; improvement on one (not all) subjective fatigue ratings | |
| Postuma, 2012 [83] | Caffeine 200 mg twice daily | No improvement in primary measure of sleepiness (ESS) | |
| Mendonca et al., 2007 [85] | Methylphenidate 10 mg 3 times a day | Significant improvements in fatigue | |
| Early morning motor dysfunction | Trenkwalder et al., 2011 [111] | Rotigotine patch, 2–16 mg/24 h | Significant improvement in PD Sleep Scale-2 scores (co-primary outcome) |
Screening for Sleep Problems in Patients with PD
A recent review of sleep scales for use in PD was completed by a task force of the Movement Disorders Society [13]. Scales were recommended for use in PD if they met 3 criteria: 1) use in PD patients; 2) use by at least 1 investigative group beyond the original developers; and 3) psychometric data showing the scale to be valid and reliable. Based on these criteria, 4 scales were recommended for the assessment of sleep symptoms in PD patients. Two scales [the Epworth Sleepiness Scale (ESS) and the Pittsburgh Sleep Quality Index] are not PD-specific, while the two others [the Parkinson’s Disease Sleep Scale (PDSS) and Scales for Outcomes in Parkinson’s Disease-sleep scale] were specifically developed for PD populations.
Treatment of RBD
RBD is a parasomnia that occurs in 15–59 % of PD patients, more commonly in those who are older, male, have sudden sleep attacks, experience orthostatic hypotension, and have PD not associated with prominent tremor [1–4, 14–16]. Considered to be a disorder of both nocturnal movement and dream content, RBD is associated with a lack of atonia in REM sleep, and patients act out vivid and frequently violent dreams [17]. Treatment for RBD is generally recommended when there is substantial risk of injury to the patient or bed partner, or when there is severe sleep disruption [18]. Because RBD frequently manifests in patients who will ultimately develop a synucleinopathy [19–21], and because most treatment studies of RBD have included a mix of patients with and without diagnosed PD, this section will consider RBD treatments used in patients with and without comorbid PD.
Nonpharmacologic therapy revolves around safety measures, for example use of a hospital bed with padded bed rails or removing potentially dangerous objects from near the bed. A customized bed alarm that plays a soothing message from a familiar person, triggered by patient movement, has shown preliminary benefit in a small series of RBD patients with treatment-refractory disease [22]. Although such a device is not yet widely available, a bed partner may sometimes be similarly able to halt an episode of dream enactment by gently reassuring the patient that they are dreaming and suggesting they return to sleep. Until recently, there were no randomized controlled trials for the treatment of RBD, and clonazepam was considered first-line therapy, based on published clinical experience. Several large case series (totaling >250 patients) using clonazepam in patients with RBD (with or without PD) have reported partial or complete response rates of 87–90 %, with typical doses of 0.5–2.0 mg at bedtime [23–26]. Although development of tolerance and substance abuse appear to be low when clonazepam is used for RBD and other sleep disorders [24, 27], other benzodiazepine side effects may be especially problematic in a PD population. In particular, excessive daytime sleepiness, confusion, and cognitive impairment may be seen in up to 58 % of patients using clonazepam for the treatment of RBD [28], although other patient series have yielded lower rates of adverse events [27]. The presence of sleep apnea, dementia, or a high baseline risk of falling are relative contraindications to the use of clonazepam [29], and the common occurrence of these problems in PD patients may further limit the usefulness of clonazepam in this population.
Recently, 2 small randomized controlled trials have been performed to assess different pharmacologic treatment strategies in RBD. Building on several small case series of RBD patients treated successfully with up to 12 mg of melatonin [30–32], Kunz et al. [33] performed a double-blind, placebo-controlled, cross-over trial of melatonin. Of the 8 men enrolled in the trial, 1 also had PD. The RBD was judged to be relatively mild in most cases. A significant improvement in global improvement scores was found with 4 weeks of 3-mg melatonin treatment, with complete resolution of behaviors reported by 4 of 8 patients, partial reduction in 2 of 8, slight reduction in 1, and no change in 1 [33]. None of the patients in the trial reported adverse reactions, although prior open-label use of melatonin for RBD has been associated with headache (13 %) and carryover sleepiness (13 %) [32]. In a survey of 45 patients with RBD who were assigned to treatment with either melatonin or clonazepam based on physician preference, over half of whom had comorbid neurodegenerative diseases, both treatments appeared reasonably effective [34]. For overall subjective ratings of improvement, moderate or better improvement was noted in 48 % of patients treated with melatonin (median effective dose 6 mg) and 78 % of patients treated with clonazepam (median effective dose 0.5 mg; p = 0.06 for the comparison). Considering individual features of treatment response, a statistically significant improvement from pre-treatment was noted only for melatonin therapy with respect to the frequency of injury, falls, and severity of injury (although a non-significant trend toward improvement with clonazepam was noted for all but the latter). Overall, side effects were not different between groups (61 % on clonazepam vs 33 % on melatonin; p = 0.07), nor were they different when specifically considering unsteadiness (39 % on clonazepam, 7 % on melatonin; p = 0.07) or dizziness (22 % on clonazepam, 4 % on melatonin; p = 0.08). Yet, among patients who discontinued therapy, the main reason for doing so differed between groups, with side effects reported as the main reason in clonazepam-treated patients and lack of benefit reported in melatonin-treated patients. Average duration of treatment prior to participation in this study was 27 months for melatonin-treated patients and 54 months for clonazepam patients (not significantly different), providing some support for the chronic use of these medications for RBD [34].
As impairment of cholinergic pathways has been implicated in the development of RBD [35], cholinesterase inhibitors might be useful in treating RBD. A randomized, double-blind, crossover trial of the cholinesterase inhibitor rivastigmine in 10 patients with PD provided support for this hypothesis [36]. In this trial, use of rivastigmine in a 4.6 mg/24 h transdermal patch resulted in a significant decrease in bed partner-reported RBD episodes compared to placebo. All patients enrolled in this study had previously failed to respond to both melatonin and clonazepam. It is unclear at present if the beneficial effect of rivastigmine represents a drug-specific effect or would be seen with the entire class of cholinesterase inhibitors. Donepezil has been reported to reduce the frequency and severity of RBD events in 4 of 6 patients [37, 38], but others have suggested anecdotally that donepezil is ineffective in RBD associated with DLB [32].
Although not designed primarily to measure the effect on RBD, a randomized controlled trial of memantine in patients with PD dementia or dementia with Lewy bodies used a single question (as part of the Stavanger Sleepiness Questionnaire) to measure the severity of physical activity during sleep [39]. Patients randomized to the memantine arm (final dose of 10 mg twice daily) had a significant decrease in severity of nocturnal movement compared with controls, suggesting that memantine might be considered in refractory RBD cases.
Conflicting data exist regarding the efficacy of pramipexole for RBD. In 3 open-label case series, pramipexole was reported to be effective in 63–89 % of treated patients based on subjective reports of RBD behaviors [40–42]. In all of these series, patients either had idiopathic RBD or their associated neurodegenerative disease was mild (i.e., mild PD or mild cognitive impairment). In contrast, in a series of PD patients with RBD, who had an average Hoehn and Yahr score of 1.86 at study entry, there were no differences in either measured RBD behaviors on polysomnography or subjective reports of behaviors when pramipexole was added to a stable levodopa regimen [43]. Those patients who respond to pramipexole tend to have less severe RBD [44], which might explain the apparent discrepancy between these series. Thus, while prampexole might have a role in the treatment of idiopathic RBD, it may be less likely to benefit PD patients with severe or advanced disease.
Levodopa has been anecdotally reported to decrease the frequency or severity of RBD events in a few patients with PD or DLB [45, 46]. However, these isolated reports are not supported by any additional literature suggesting that RBD might be improved by levodopa. In particular, an early study of levodopa in PD patients, prior to the definition of REM behavior disorder in the medical literature, suggested that levodopa was associated with nightmares and vivid dreams [47]. More recently, in a series of 351 patients with PD, the presence of RBD was associated with higher levodopa dose [48]. Levodopa use by PD patients does not appear to affect either the percentage of time spent in REM sleep [48], nor the presence of REM without atonia [49]. Therefore, it does not seem likely that levodopa would beneficially affect RBD symptoms.
Because of the potential for harm from untreated severe RBD, other agents may be considered when the above treatments are ineffective or contraindicated. In a case series, zopiclone was effective in controlling RBD symptoms in 8 of 11 patients [28]; only its s-enantiomer, eszopiclone, is available in the USA. Based on single published cases, benefit in RBD has been reported with use of sodium oxybate [50], carbamazepine [51], desipramine [52], and clonidine [53].
Treatment of Insomnia
Sleep fragmentation and sleep maintenance insomnia are among the most frequent sleep complaints reported by PD patients [5]. Before embarking on pharmacologic or behavioral treatments for insomnia in PD patients, a careful evaluation of the type of sleep disturbance that is occurring is needed. If the sleep disturbance is associated with uncontrolled motor symptoms in the night, changes in dopaminergic therapy may be beneficial (see “Effects of Dopaminergic Medications” below). Nocturia (defined as voiding ≥2 times per night) is present in nearly two thirds of PD patients, and is associated with severe reports of bother, as well as disrupted sleep architecture, in 20 % [54]. In these patients, treatments targeted at nocturia, rather than insomnia itself, may be beneficial. Untreated depression, another common feature of PD, can exacerbate insomnia symptoms, and its treatment may improve comorbid insomnia [55].
In patients with chronic insomnia, regardless of etiology, treatment with behavioral interventions is recommended with the highest level of recommendation by the American Academy of Sleep Medicine [56]. This reflects the high-grade evidence supporting the use of cognitive–behavioral therapy for insomnia (CBT-I) in patients with or without comorbid conditions [56], although there is a shortage of trained providers and insurance coverage is variable. However, this treatment modality has yet to be evaluated as a single intervention in a randomized controlled trial of PD patients, but one study has evaluated CBT-I in combination with bright light therapy [57]. In this randomized, but unblinded, study, CBT-I was administered in a group setting, consisting of 6 weekly 90-min sessions. In addition, patients received light therapy with 10,000 lux lightboxes, with timing of administration depending on whether the patient had primarily sleep onset insomnia (light therapy in the morning) or primarily sleep maintenance insomnia (light therapy for the 30 mins before bedtime). The placebo condition was 30 mins of light therapy with sub-threshold red light. The CBT-I/light therapy group demonstrated a significant improvement in several subjective measures of insomnia severity (including 1 of 2 primary outcome measures), but improvements were not seen in all outcomes. In particular, the total Unified Parkinson’s Disease Rating Scale (UPDRS) and the Parkinson’s Disease Questionnaire (PDQ) 39-item were both worsened in the active therapy group relative to the placebo group [57]. The authors hypothesized that the increased “awareness of self” developed during CBT may have made patients more aware of their disease-related limitations and thus more likely to rate their quality of life as poor, but did not offer a mechanistic hypothesis for the deterioration in UPDRS scores. Further work is needed to separate the effects of bright light therapy and group CBT-I, as well as the potential benefit of individual CBT-I or other behavioral interventions in PD.
If pharmacologic therapy for insomnia is chosen, there are a variety of options that are US Food and Drug Administration-approved for the treatment of insomnia. However, the majority of these approved medications, as well as those frequently used off-label for the treatment of primary insomnia, have not been studied in patients with PD. The exceptions to this are eszopiclone, doxepin, and melatonin. Eszopiclone is a gamma-aminobutyric acid-A receptor agonist approved for the treatment of insomnia. Thirty PD patients with either sleep maintenance or sleep onset insomnia were randomized to eszopiclone or placebo, with an age-based dosing of medication (3 mg for those under 65 years old and 2 mg for those aged 65 years or older) [58]. Patients with a Mini Mental Status Exam score of <26 were excluded, as were patients with comorbid sleep disorders, including sleep apnea, periodic limb movements, or RBD. Dropouts were common (3 in the eszopiclone arm and 8 in the placebo arm). The primary end-point, total sleep time measured by sleep diary, was not different between groups (19.5 additional mins in the eszopiclone group; p = 0.11). However, several secondary endpoints were improved on active drug, including decreased number of awakenings, improved quality of sleep, and improved clinician’s global impression of sleep improvement. Side effects thought possibly to be related to eszopiclone were present in 2 patients (13 %), and included daytime sleepiness and dizziness. Zolpidem, a commonly prescribed sedative-hypnotic in the same medication class as eszopiclone, has not been studied for insomnia in PD patients, but may improve motor symptoms [59].
Doxepin, dosed at 10 mg at bedtime, was compared with an inactive red light placebo treatment in a third arm of the CBT-I/light therapy study discussed above [57]. Treatment allocation was randomized, but not blinded. In both primary outcome measures (the Insomnia Severity Index and the Scales for Outcomes in Parkinson’s Disease-night scale) at 6 weeks, there was a significant benefit of doxepin. Several secondary outcome measures also showed a beneficial effect of doxepin, including the Fatigue Severity Scale (FSS). There was no significant worsening in measures of daytime sleepiness and a significant improvement in MoCA scores in the doxepin-treated patients (with MoCA improving 3.4 points relative to the placebo group) [57]. Other tricyclic antidepressants, for example amitriptyline, are sometimes used off label to treat insomnia, but have not been systematically studied in PD patients. Trazodone, another sedating antidepressant, is widely prescribed for insomnia. It is sometimes posited to be safer than sedative-hypnotics, although scant data are available to support or refute this assertion [60]. A small, randomized, controlled study evaluating the motor effects of trazodone in Parkinson’s patients demonstrated an improvement in UPDRS scores (decreasing 5.5 points with trazodone use vs increasing 4.0 points in the no-treatment control group), which was strongly correlated with improvement in depression (Spearman r = 0.74; p = 0.034) [61]. Despite this potentially encouraging result, studies in healthy adults, older adults, and depressed adults have suggested that there may be risks of trazodone use that could be clinically meaningful in the PD population. These include increased rates of hip fracture or falls, dysequilibrium, impairments in short-term memory, and worsening verbal memory [60, 62–64]. Studies evaluating the magnitude of these effects relative to those accompanying other pharmacologic treatments for insomnia are needed, both within the PD population and in insomnia patients in general.
Melatonin (3 mg) was compared with matched placebo in 18 PD patients in a randomized, double-blind trial [65]. The authors found significant improvement in subjective sleep quality (as rated by the Pittsburgh Sleep Quality Index, mean change from baseline 3.8 vs 1.2 on placebo; p = 0.03). Their co-primary outcome, objectively measured sleep quality by polysomnography, did not demonstrate any difference between groups. There were no adverse events and melatonin therapy had no impact on motor function [65]. The lack of objectively measured improvement is broadly consistent with meta-analyses of melatonin for sleep in non-PD populations, which have suggested a statistically significant, but clinically modest, improvement in objectively measured sleep, for example decreasing sleep latencies by 4.0 to 7.2 mins [66, 67]. Individual patients, however, may appreciate a symptomatic improvement in sleep quality even in the absence of objective improvement, so melatonin may be considered on a case-by-case benefit pending more definitive studies of melatonin in PD.
Quetiapine has been preliminarily evaluated for insomnia in PD patients without psychosis in an open-label study of 14 patients [68]. Following 3 months of treatment (mean quetiapine dose of 32 mg), 11 of 14 patients had improvement in insomnia severity, as measured by the Pittsburgh Sleep Quality Index. Two of 14 patients discontinued therapy for adverse events. Given potential safety concerns with antipsychotic medications, especially in those patients with comorbid dementia, we do not consider quetiapine to be a first-line treatment for insomnia.
Treatment of Excessive Daytime Sleepiness and Fatigue
Daytime sleepiness and fatigue are common problems in patients with PD [69]. Typically, poor-quality or short duration nocturnal sleep results in daytime sleepiness or fatigue through the actions of the sleep homeostat. Also known as Process S, the sleep homeostat increases the drive to sleep in the presence of sustained wakefulness and decreases the drive to sleep as sleep is obtained [70]. In patients with PD, however, this homeostatic regulation of sleep may be impaired. In particular, while fragmented nocturnal sleep is a common problem in PD patients, multiple studies have shown that in PD patients, daytime sleepiness and nocturnal sleep time or sleep efficiency are positively correlated (i.e., the patients with the most nocturnal sleep also demonstrate the highest levels of daytime sleepiness) or unrelated [71–75]. Thus, while interventions to improve nocturnal sleep may still be beneficial in PD patients, it is not uncommon for sleepiness to be present in patients with sufficient nocturnal sleep or after the successful treatment of any comorbid nocturnal sleep disorders. Interventions that have been studied in randomized controlled trials for the treatment of excessive daytime sleepiness and/or fatigue in PD include modafinil, amphetamines, caffeine, and memantine.
The most commonly studied agent for any sleep disorder in PD patients is the wake-promoting agent modafinil. While the mechanism of action is unknown, it is thought possibly to reflect activity through noradrenergic and dopaminergic systems [76]. Five placebo-controlled, double-blind, randomized trials have been performed evaluating modafinil, 200–400 mg/day, for sleepiness and/or fatigue in PD [77–81]. Although several of the studies did not individually show a significant benefit of modafinil on subjective sleepiness (as measured by the ESS, a 24-point measure of likelihood of dozing), combining data from 4 of the studies yields an estimated decrease of 2.3 points on the ESS on modafinil versus placebo (p < 0.05; Fig. 1), a modest, but clinically meaningful, decrease in sleepiness. The fifth study could not be included in meta-analysis, but found a significant decrease in ESS scores on modafinil versus placebo (decrease in median ESS by 9 points in the modafinil group and by 0 in the placebo group) [81]. Four of these studies also included at least one subjective measure of fatigue, which was not improved in any of the studies with modafinil [77–79, 81], except for the Clinical Global Improvement scale of fatigue in 1 [81]. Objectively-measured motor fatigabilty (finger-tapping) was improved in the one study that examined it [77]. Importantly, the studies that used objective measures of sleepiness, either the maintenance of wakefulness or the multiple sleep latency tests, did not find an objective benefit of modafinil on sleepiness [79, 80]. While this may represent a lack of power, it is also possible that modafinil results in subjective, but not objective, improvements in sleepiness [82]. This disconnect between symptoms and performance is particularly important for patients participating in activities for which objective sleepiness may cause impairments, for example driving.
Fig. 1.
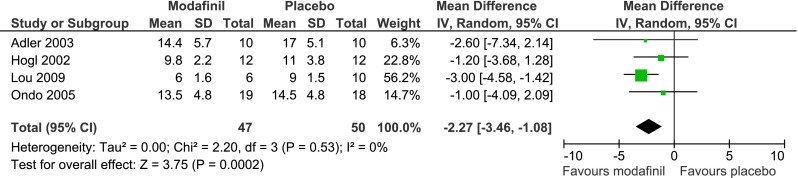
Meta-analysis of studies assessing modafinil versus placebo for excessive sleepiness in Parkinson’s disease. Mean ± S.D. reflect the Epworth Sleepiness Scale scores on modafinil and placebo, respectively. For the Hogl study, S.D. were not provided for the on-treatment scores, so S.D. for baseline scores were used as an approximation for the purpose of this meta-analysis. The Adler and Hogl studies used crossover designs, but data presented in the Adler study were from the first treatment period only (i.e., treating the study as a parallel group design) and in the Hogl study appeared to represent both treatment periods combined by medication ([77–80]). Analysis was performed using RevMan version 5.2
A randomized, placebo-controlled, double-blind trial of caffeine was recently conducted to determine the effects on sleepiness and motor function in PD patients [83]. Patients were dosed with 100 mg twice daily (upon awakening and after lunch) for 3 weeks, then 200 mg twice daily at the same time points. At the primary endpoint (the ESS), there was no significant improvement with caffeine at either dose using an intention-to-treat analysis, although there was a significant decrease in ESS of 1.97 points using a per-protocol analysis. Of the secondary outcomes related to sleepiness, only the Clinical Global Impression of Change (CGI-C) of somnolence was significantly improved with caffeine. Interestingly, there was a significant improvement in UPDRS total and motor scales in the caffeine group. The authors concluded that there was no significant benefit for sleepiness of caffeine, although the motor improvement may warrant further investigation [83].
Reports of clinical use of amphetamines for the treatment of sleepiness associated with parkinsonian syndromes date back to at least the early 1950s [84]. Methylphenidate, dosed as 10 mg 3 times per day, was compared with a matched placebo in a double-blind, parallel group trial for fatigue in PD patients [85]. Using two different measures of subjective fatigue, the FSS and the Multidimensional Fatigue Inventory, patients in the methylphenidate group had significant reductions in fatigue severity. Three patients dropped out of each arm for side effects, and side effects were more commonly reported in the placebo group than the methylphenidate group. Therefore, methylphenidate might be useful for PD-related fatigue. However, consensus guidelines have highlighted the potential for abuse of methylphenidate, which theoretically might be of higher concern in a PD population at risk for dopamine dysregulation syndrome or impulse control disorders [82].
Memantine was tested in a pilot controlled trial for a variety of non-motor symptoms of PD, which included subjective sleepiness and fatigue. Patients were titrated to a dose of 20 mg per day or matched placebo and reassessed at 8 weeks. There were no significant improvements from memantine for either sleepiness or fatigue [86].
Sodium oxybate was used in an open-label, non-randomized study of 30 PD patients with excessive daytime sleepiness [87]. Patients were excluded for mini-mental status examination scores <25 or for clinically meaningful sleep apnea. Dose was titrated from 2.25 g twice nightly up to a maximum of 4.5 g twice nightly, based on clinical response. Patients reported significant improvements in both ESS and FSS (p < 0.001 for both), and polysomnographically-measured sleep demonstrated an increase in stage N3 and decrease in REM sleep. Three patients (10 %) dropped out of the study owing to side effects, which included dizziness and depressive symptoms, and average apnea–hypopnea index per hour of sleep increased from 7 to 13 (where 5–15 events/h define mild sleep apnea) [87]. Although this is promising preliminary data, sodium oxybate does have two US Food and Drug Administration “black box” warnings, one for being a central nervous system and respiratory depressant, causing obtundation in clinical trials, and one for having abuse potential, as it is a sodium salt of gamma hydroxybutyrate. Careful consideration of the risk:benefit ratio in individual Parkinson’s patients would therefore be needed before using this agent.
Treatment of Restless Legs Syndrome/Periodic Limb Movements of Sleep
When restless legs syndrome (RLS) and PD co-exist in the same patient, the RLS symptoms may be adequately treated with the dopaminergic therapy used for PD, as dopamine agonists are effective for treatment of RLS [88, 89]. Several other drug classes are effective for RLS symptoms in patients without PD, and may be considered in PD patients if needed. These include gabapentin and related medications (i.e., pregabalin, gabapentin enacarbil) and opiates [88, 89]. Studies evaluating the effect of subthalamic nucleus deep brain stimulation on comorbid RLS in patients with PD have been mixed. Development of new RLS symptoms was noted in 11 of 195 implanted patients in one study, which was attributed to medication reduction by the authors [90]. In contrast, 2 other groups found improvements in RLS severity in PD patients after subthalamic nucleus stimulation, even with reduction in dopaminergic medications in some patients [91, 92]. To our knowledge, there have been no clinical trials of RLS treatment in PD patients.
Treatment of Obstructive Sleep Apnea
Obstructive sleep apnea (OSA), a disorder characterized by repetitive cessations or reductions of respiration during sleep, does not appear to be more common in patients with PD than in age-matched controls [93–96], although studies have not universally supported this [97]. However, OSA is common in the middle-aged-to-elderly general population [98], and thus is still frequently encountered in PD patients. OSA is typically treated with continuous positive airway pressure (CPAP), which is beneficial in reducing sleepiness in OSA patients [99]. Clinical experience suggests that individual PD patients may experience improvement in daytime sleepiness with CPAP use, although some patients may not have the motor dexterity required to affix and position CPAP equipment. A randomized controlled trial of continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea in patients with PD has recently completed, but results are not yet available [100]. Mandibular advancement devices have recently been shown to be similarly beneficial to CPAP in improving daytime sleepiness and driving performance in OSA patients without PD [101], and thus might be a reasonable alternative for PD patients.
Effects of Dopaminergic Medications for PD on Sleep Symptoms
The treatments used for PD motor symptoms may have a substantial impact on comorbid sleep disorders and symptoms. For example, dopamine agonists are known to be sedating, even in healthy controls [102]. A meta-analysis of treatment trials in early PD suggests that non-ergot dopamine agonists increase sleepiness, with odds ratios of 2.16 (confidence interval 1.53–3.03) for pramipexole and 3.75 (confidence interval 2.52–5.59) for ropinirole [103]. There may be a divergent effect on sleepiness of dopamine agonists versus levodopa, such that patients medicated with either are sleepier than unmedicated patients, but higher doses of dopamine agonists worsen objectively measured sleepiness, while higher doses of levodopa lessen objectively measured sleepiness [74]. Levodopa has been associated with at least transient sleep-onset insomnia [104]. Therefore, it is important to consider these direct medication effects on sleep symptomatology in the PD patient.
Several randomized controlled trials of levodopa preparations have considered subjective or objective sleep variables [105–108]. An early study of 11 PD patients suggested a significant improvement in subjective rating of sleep quality on a visual analog scale with carbidopa–levodopa, dosed either as 50/200 at bedtime or 25/100 at bedtime, and again at 03:00 AM, versus placebo [105]. In contrast, a polysomnographic study of 32 patients with akinetic-rigid PD did not demonstrate any acute benefit on objectively measured sleep variables from 50/200 carbidopa-levodopa extended release versus placebo [108]; in this study, patients were not blinded (although polysomnography scorers were), and subjective ratings of sleep were not reported. The difference between the results of these 2 studies is not likely to represent an inherent difference between immediate- and controlled-release preparations of levodopa, as 2 relatively large studies (totaling 239 patients) randomizing PD patients to 1 of the 2 preparations found no difference in any subjective measure of sleep quality [106, 107]. Importantly, none of these studies selected patients specifically because of the presence of any sleep symptoms. In clinical practice, bedtime dosing of dopaminergics is sometimes considered for patients reporting motor symptoms that interrupt sleep; the suitability of levodopa for such purposes has yet to be evaluated within a randomized trial.
Dopamine agonists have been specifically evaluated with respect to effects on sleep in several randomized controlled trials. The EASE-PD Adjunct study randomized 393 advanced PD patients with insufficient response to levodopa to adjunctive therapy with extended release ropinirole or placebo, considering the PDSS and the ESS as secondary outcomes [109]. There was a statistically significant, but likely not clinically meaningful, improvement in PDSS scores in the ropinirole-treated group (i.e., 1.2 points on a 150-point scale), and no significant differences in ESS scores (7.8 in the ropinirole group vs 7.7 in the placebo group) [109]. However, a post hoc analysis of the EASE-PD data separated the study population by baseline PDSS scores into those with and without sleep symptoms, and found a significant and more clinically meaningful improvement in PDSS scores (adjusted mean difference of 9 points; p = 0.005) in those with sleep symptoms at baseline [110]. The RECOVER study was designed specifically to evaluate the effects of dopamine agonist therapy on PD patients with problematic early morning motor symptoms, with the PDSS-2 serving as a co-primary endpoint. The 287-patient study showed a significant improvement in PDSS-2 scores (4.3 points improvement vs placebo, on the 60-point PDSS-2 scale; p < 0.0001) in the group treated with rotigotine transdermal patch [111]. Taken together, these 2 studies suggest that 24-h dopamine agonist therapy may benefit subjective measures of sleep, particularly in those patients with sleep complaints or early morning motor dysfunction.
Conclusions and Future Directions
Sleep disorders are common and problematic in patients with PD. These disorders are readily treatable in the general population, although further studies are clearly needed to better delineate the most effective approaches to these disorders in patients with PD. Future studies should evaluate not only improvements in subjective symptoms and quality of life, but also possible improvements in motor function or disease course with the treatment of comorbid sleep disorders in PD.
Electronic supplementary material
(PDF 1225 kb)
Acknowledgments
Conflict of Interest
Dr. Trotti has served as a consultant for UCB Pharma. Dr. Bliwise has served or is serving as a consultant for Ferring Pharmaceuticals, Vantia Therapeutics, and the New England Research Institute. Full conflict of interest disclosures are available in the electronic supplementary material for this article.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Yoritaka A, Ohizumi H, Tanaka S, Hattori N. Parkinson's disease with and without REM sleep behaviour disorder: are there any clinical differences? Eur Neurol. 2009;61:164–170. doi: 10.1159/000189269. [DOI] [PubMed] [Google Scholar]
- 2.De Cock V, Vidailhet M, Leu S, et al. Restoration of normal motor control in Parkinson's disease during REM sleep. Brain. 2007;2007:450–456. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- 3.Gagnon JF, Bedard MA, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology. 2002;59:585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 4.Gjerstad MD, Boeve B, Wentzel-Larsen T, Aarsland D, Larsen JP. Occurrence and clinical correlates of REM sleep behaviour disorder in patients with Parkinson's disease over time. J Neurol Neurosurg Psychiatry. 2008;79:387–391. doi: 10.1136/jnnp.2007.116830. [DOI] [PubMed] [Google Scholar]
- 5.Bliwise DL, Trotti LM, Rye DB. Movement disorders specific to sleep and the nocturnal manifestations of waking movement disorders. In: Watts RL, Standaert DG, Obeso J (eds). Movement disorders. 3rd ed. McGraw-Hill, 2011, pp. 935-974.
- 6.Lees A, Blackburn N, Campbell V. The nightime problems of Parkinson's disease. Clin Neuropharmacol. 1988;11:512–519. doi: 10.1097/00002826-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Nausieda P, Glantz R, Weber S, Baum R, Klawans H. Psychiatric complications of levodopa therapy of Parkinson' disease. In: Hassler R, Christ J, editors. Advances in neurology. New York: Raven Press; 1984. pp. 271–277. [PubMed] [Google Scholar]
- 8.Factor S, McAlarney T, Sanchez-Ramos J, Weiner W. Sleep disorders and sleep effect in Parkinson's Disease. Mov Disord. 1990;5:280–285. doi: 10.1002/mds.870050404. [DOI] [PubMed] [Google Scholar]
- 9.van Hilten J, Weggeman M, Velde E, Kerkhof G, Dijk J, Roos R. Sleep, excessive daytime sleepiness and fatigue in Parkinson's disease. J Neural Transm [P-D Sect] 1993;5:235–244. doi: 10.1007/BF02257678. [DOI] [PubMed] [Google Scholar]
- 10.Karlsen KH, Larsen JP, Tandberg E, Maeland JG. Influence of clinical and demographic variables on quality of life in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;66:431–435. doi: 10.1136/jnnp.66.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsaa EB, Larsen JP, Wentzel-Larsen T, Herlofson K, Alves G. Predictors and course of health-related quality of life in Parkinson's disease. Mov Disord. 2008;23:1420–1427. doi: 10.1002/mds.22121. [DOI] [PubMed] [Google Scholar]
- 12.Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl. 3):S42–80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogl B, Arnulf I, Comella C, et al. Scales to assess sleep impairment in Parkinson's disease: critique and recommendations. Mov Disord. 2010;25:2704–2716. doi: 10.1002/mds.23190. [DOI] [PubMed] [Google Scholar]
- 14.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord. 2008;23:1665–1672. doi: 10.1002/mds.22099. [DOI] [PubMed] [Google Scholar]
- 15.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79:1117–1121. doi: 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- 16.Kumru H, Santamaria J, Tolosa E, Iranzo A. Relation between subtype of Parkinson's disease and REM sleep behavior disorder. Sleep Med. 2007;8:779–783. doi: 10.1016/j.sleep.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Trotti LM. REM sleep behaviour disorder in older individuals: epidemiology, pathophysiology and management. Drugs Aging. 2010;27:457–470. doi: 10.2165/11536260-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iranzo A, Santamaria J, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009;13:385–401. doi: 10.1016/j.smrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 20.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 21.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell MJ, Arneson PA, Schenck CH. A novel therapy for REM sleep behavior disorder (RBD) J Clin Sleep Med. 2011;7:639–644A. doi: 10.5664/jcsm.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123:331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 24.Wing YK, Lam SP, Li SX, et al. REM sleep behaviour disorder in Hong Kong Chinese: clinical outcome and gender comparison. J Neurol Neurosurg Psychiatry. 2008;79:1415–1416. doi: 10.1136/jnnp.2008.155374. [DOI] [PubMed] [Google Scholar]
- 25.Schenck CH, Hurwitz TD, Mahowald MW. Symposium: Normal and abnormal REM sleep regulation: REM sleep behaviour disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. 1993;2:224–231. doi: 10.1111/j.1365-2869.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 26.Sforza E, Krieger J, Petiau C. REM sleep behavior disorder: clinical and physiopathological findings. Sleep Med Rev. 1997;1:57–69. doi: 10.1016/s1087-0792(97)90006-x. [DOI] [PubMed] [Google Scholar]
- 27.Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333–337. doi: 10.1016/S0002-9343(97)89493-4. [DOI] [PubMed] [Google Scholar]
- 28.Anderson KN, Shneerson JM. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med. 2009;5:235–239. [PMC free article] [PubMed] [Google Scholar]
- 29.Aurora RN, Zak RS, Maganti RK, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD) J Clin Sleep Med. 2010;6:85. [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi N, Uchimura N, Hashizume Y, et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. 2001;55:267–269. doi: 10.1046/j.1440-1819.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 31.Kunz D, Bes F. Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov Disord. 1999;14:507–511. doi: 10.1002/1531-8257(199905)14:3<507::aid-mds1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4:281–284. doi: 10.1016/s1389-9457(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 33.Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res. 2010;19:591–596. doi: 10.1111/j.1365-2869.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 34.McCarter SJ, Boswell CL, St Louis EK, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14:237–242. doi: 10.1016/j.sleep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotagal V, Albin RL, Muller ML, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol. 2012;71:560–568. doi: 10.1002/ana.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Giacopo R, Fasano A, Quaranta D, Della Marca G, Bove F, Bentivoglio AR. Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson's disease. Mov Disord. 2012;27:559–561. doi: 10.1002/mds.24909. [DOI] [PubMed] [Google Scholar]
- 37.Ringman JM, Simmons JH. Treatment of REM sleep behavior disorder with donepezil: a report of three cases. Neurology. 2000;55:870–871. doi: 10.1212/wnl.55.6.870. [DOI] [PubMed] [Google Scholar]
- 38.Massironi G, Galluzzi S, Frisoni GB. Drug treatment of REM sleep behavior disorders in dementia with Lewy bodies. Int Psychogeriatr. 2003;15:377–383. doi: 10.1017/s1041610203009621. [DOI] [PubMed] [Google Scholar]
- 39.Larsson V, Aarsland D, Ballard C, Minthon L, Londos E. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson's disease dementia. Int J Geriatr Psychiatry. 2010;25:1030–1038. doi: 10.1002/gps.2506. [DOI] [PubMed] [Google Scholar]
- 40.Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61:1418–1420. doi: 10.1212/wnl.61.10.1418. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt MH, Koshal VB, Schmidt HS. Use of pramipexole in REM sleep behavior disorder: results from a case series. Sleep Med. 2006;7:418–423. doi: 10.1016/j.sleep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Sasai T, Inoue Y, Matsuura M. Effectiveness of pramipexole, a dopamine agonist, on rapid eye movement sleep behavior disorder. Tohoku J Exp Med. 2012;226:177–181. doi: 10.1620/tjem.226.177. [DOI] [PubMed] [Google Scholar]
- 43.Kumru H, Iranzo A, Carrasco E, et al. Lack of effects of pramipexole on REM sleep behavior disorder in Parkinson disease. Sleep. 2008;31:1418–1421. [PMC free article] [PubMed] [Google Scholar]
- 44.Sasai T, Matsuura M, Inoue Y. Factors associated with the effect of pramipexole on symptoms of idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2013;19:153–157. doi: 10.1016/j.parkreldis.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Tan A, Salgado M, Fahn S. Rapid eye movement sleep behavior disorder preceding Parkinson's disease with therapeutic response to levodopa. Mov Disord. 1996;11:214–216. doi: 10.1002/mds.870110216. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25:140–142. doi: 10.1016/s0163-8343(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 47.Sharf B, Moskovitz C, Lupton MD, Klawans HL. Dream phenomena induced by chronic levodopa therapy. J Neural Transm. 1978;43:143–151. doi: 10.1007/BF01579073. [DOI] [PubMed] [Google Scholar]
- 48.Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Age, drugs, or disease: what alters the macrostructure of sleep in Parkinson's disease? Sleep Med. 2012;13:1178–1183. doi: 10.1016/j.sleep.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Bliwise DL, Trotti LM, Greer SA, Juncos JJ, Rye DB. Phasic muscle activity in sleep and clinical features of Parkinson disease. Ann Neurol. 2010;68:353–359. doi: 10.1002/ana.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shneerson JM. Successful treatment of REM sleep behavior disorder with sodium oxybate. Clin Neuropharmacol. 2009;32:158–159. doi: 10.1097/WNF.0b013e318193e394. [DOI] [PubMed] [Google Scholar]
- 51.Bamford CR. Carbamazepine in REM sleep behavior disorder. Sleep. 1993;16:33–34. [PubMed] [Google Scholar]
- 52.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 53.Nash JR, Wilson SJ, Potokar JP, Nutt DJ. Mirtazapine induces REM sleep behavior disorder (RBD) in parkinsonism. Neurology. 2003;61:1161. doi: 10.1212/wnl.61.8.1161. [DOI] [PubMed] [Google Scholar]
- 54.Vaughan CP, Juncos JL, Trotti LM, Johnson TM, 2nd, Bliwise DL. Nocturia and overnight polysomnography in Parkinson disease. Neurourol Urodynam 2013 Jan 28. [DOI] [PMC free article] [PubMed]
- 55.Dobkin RD, Menza M, Bienfait KL, et al. Depression in Parkinson's disease: symptom improvement and residual symptoms after acute pharmacologic management. Am J Geriatr Psychiatry. 2011;19:222–229. doi: 10.1097/JGP.0b013e3181e448f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An american academy of sleep medicine report. Sleep. 2006;29:1415–1419. [PubMed] [Google Scholar]
- 57.Rios Romenets S, Creti L, Fichten C, et al. Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson's disease - A randomized study. Parkinsonism Relat Disord. 2013;19:670–675. doi: 10.1016/j.parkreldis.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Menza M, Dobkin RD, Marin H, et al. Treatment of insomnia in Parkinson's disease: a controlled trial of eszopiclone and placebo. Mov Disord. 2010;25:1708–1714. doi: 10.1002/mds.23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daniele A, Albanese A, Gainotti G, Gregori B, Bartolomeo P. Zolpidem in Parkinson's disease. Lancet. 1997;349:1222–1223. doi: 10.1016/S0140-6736(05)62416-6. [DOI] [PubMed] [Google Scholar]
- 60.Roth AJ, McCall WV, Liguori A. Cognitive, psychomotor, and polysomnographic effects of trazodone in primary insomniacs. J Sleep Res. 2011;20:552–558. doi: 10.1111/j.1365-2869.2011.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Werneck ALS, Rosso AL, Vincent MB. The use of an antagonist 5-HT2A/C for depression and motor function in Parkinson's disease. Arq Neuropsiquiatr. 2009;67:407–412. doi: 10.1590/s0004-282x2009000300007. [DOI] [PubMed] [Google Scholar]
- 62.Oderda LH, Young JR, Asche CV, Pepper GA. Psychotropic-related hip fractures: Meta-analysis of first generation and second-generation antidepressant and antipsychotic drugs. Ann Pharmacother. 2012;46:917–928. doi: 10.1345/aph.1Q589. [DOI] [PubMed] [Google Scholar]
- 63.Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ip EJ, Bui QV, Barnett MJ, et al. The effect of trazodone on standardized field sobriety tests. Pharmacotherapy. 2013;33:369–374. doi: 10.1002/phar.1210. [DOI] [PubMed] [Google Scholar]
- 65.Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhaes MC, de Lourdes Seabra M, de Bruin VM. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson's disease. A randomized, double blind, placebo-controlled study. J Neurol. 2007;254:459–464. doi: 10.1007/s00415-006-0390-x. [DOI] [PubMed] [Google Scholar]
- 66.Buscemi N, Vandermeer B, Hooton N, et al. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J Gen Intern Med. 2005;20:1151–1158. doi: 10.1111/j.1525-1497.2005.0243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brzezinski A, Vangel MG, Wurtman RJ, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Juri C, Chana P, Tapia J, Kunstmann C, Parrao T. Quetiapine for insomnia in Parkinson disease: results from an open-label trial. Clin Neuropharmacol. 2005;28:185–187. doi: 10.1097/01.wnf.0000174932.82134.e2. [DOI] [PubMed] [Google Scholar]
- 69.Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord. 2009;24:1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 70.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 71.Rye DB, Bliwise DL, Dihenia B, Gurecki P. FAST TRACK: daytime sleepiness in Parkinson's disease. J Sleep Res. 2000;9:63–69. doi: 10.1046/j.1365-2869.2000.00201.x. [DOI] [PubMed] [Google Scholar]
- 72.Razmy A, Lang AE, Shapiro CM. Predictors of impaired daytime sleep and wakefulness in patients with Parkinson disease treated with older (ergot) vs newer (nonergot) dopamine agonists. Arch Neurol. 2004;61:97–102. doi: 10.1001/archneur.61.1.97. [DOI] [PubMed] [Google Scholar]
- 73.Roth T, Rye DB, Borchert LD, et al. Assessment of sleepiness and unintended sleep in Parkinson's disease patients taking dopamine agonists. Sleep Med. 2003;4:275–280. doi: 10.1016/s1389-9457(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 74.Bliwise DL, Trotti LM, Wilson AG, et al. Daytime alertness in Parkinson's disease: potentially dose-dependent, divergent effects by drug class. Mov Disord. 2012;27:1118–1124. doi: 10.1002/mds.25082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wienecke M, Werth E, Poryazova R, et al. Progressive dopamine and hypocretin deficiencies in Parkinson's disease: is there an impact on sleep and wakefulness? J Sleep Res. 2012;21:710–717. doi: 10.1111/j.1365-2869.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 76.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lou JS, Dimitrova DM, Park BS, et al. Using modafinil to treat fatigue in Parkinson disease: a double-blind, placebo-controlled pilot study. Clin Neuropharmacol. 2009;32:305–310. doi: 10.1097/WNF.0b013e3181aa916a. [DOI] [PubMed] [Google Scholar]
- 78.Adler CH, Caviness JN, Hentz JG, Lind M, Tiede J. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson's disease. Mov Disord. 2003;18:287–293. doi: 10.1002/mds.10390. [DOI] [PubMed] [Google Scholar]
- 79.Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry. 2005;76:1636–1639. doi: 10.1136/jnnp.2005.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hogl B, Saletu M, Brandauer E, et al. Modafinil for the treatment of daytime sleepiness in Parkinson's disease: a double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep. 2002;25:905–909. [PubMed] [Google Scholar]
- 81.Tyne HL, Taylor J, Baker GA, Steiger MJ. Modafinil for Parkinson's disease fatigue. J Neurol. 2010;257:452–456. doi: 10.1007/s00415-009-5351-8. [DOI] [PubMed] [Google Scholar]
- 82.Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice Parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74:924–931. doi: 10.1212/WNL.0b013e3181d55f24. [DOI] [PubMed] [Google Scholar]
- 83.Postuma RB, Lang AE, Munhoz RP, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79:651–658. doi: 10.1212/WNL.0b013e318263570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meter V., Jr Therapy of Parkinson's disease. Calif Med. 1950;73:322–324. [PMC free article] [PubMed] [Google Scholar]
- 85.Mendonca DA, Menezes K, Jog MS. Methylphenidate improves fatigue scores in Parkinson disease: a randomized controlled trial. Mov Disord. 2007;22:2070–2076. doi: 10.1002/mds.21656. [DOI] [PubMed] [Google Scholar]
- 86.Ondo WG, Shinawi L, Davidson A, Lai D. Memantine for non-motor features of Parkinson's disease: a double-blind placebo controlled exploratory pilot trial. Parkinsonism Relat Disord. 2011;17:156–159. doi: 10.1016/j.parkreldis.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 87.Ondo WG, Perkins T, Swick T, et al. Sodium oxybate for excessive daytime sleepiness in Parkinson disease: an open-label polysomnographic study. Arch Neurol. 2008;65:1337–1340. doi: 10.1001/archneur.65.10.1337. [DOI] [PubMed] [Google Scholar]
- 88.Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults-an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35:1039–1062. doi: 10.5665/sleep.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garcia-Borreguero D, Ferini-Strambi L, Kohnen R, et al. European guidelines on management of restless legs syndrome: report of a joint task force by the European Federation of Neurological Societies, the European Neurological Society and the European Sleep Research Society. Eur J Neurol. 2012;19:1385–1396. doi: 10.1111/j.1468-1331.2012.03853.x. [DOI] [PubMed] [Google Scholar]
- 90.Kedia S, Moro E, Tagliati M, Lang AE, Kumar R. Emergence of restless legs syndrome during subthalamic stimulation for Parkinson disease. Neurology. 2004;63:2410–2412. doi: 10.1212/01.wnl.0000147288.26029.b8. [DOI] [PubMed] [Google Scholar]
- 91.Chahine LM, Ahmed A, Sun Z. Effects of STN DBS for Parkinson's disease on restless legs syndrome and other sleep-related measures. Parkinsonism Relat Disord. 2011;17:208–211. doi: 10.1016/j.parkreldis.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 92.Driver-Dunckley E, Evidente VG, Adler CH, et al. Restless legs syndrome in Parkinson's disease patients may improve with subthalamic stimulation. Mov Disord. 2006;21:1287–1289. doi: 10.1002/mds.20911. [DOI] [PubMed] [Google Scholar]
- 93.Trotti LM, Bliwise DL. No increased risk of obstructive sleep apnea in Parkinson's disease. Mov Disord. 2010;25:2246–2249. doi: 10.1002/mds.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cochen De Cock V, Abouda M, Leu S, et al. Is obstructive sleep apnea a problem in Parkinson's disease? Sleep Med. 2010;11:247–252. doi: 10.1016/j.sleep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 95.Diederich NJ, Vaillant M, Leischen M, et al. Sleep apnea syndrome in Parkinson's disease. A case-control study in 49 patients. Mov Disord. 2005;20:1413–1418. doi: 10.1002/mds.20624. [DOI] [PubMed] [Google Scholar]
- 96.Yong MH, Fook-Chong S, Pavanni R, Lim LL, Tan EK. Case control polysomnographic studies of sleep disorders in Parkinson's disease. PLoS One. 2011;6:e22511. doi: 10.1371/journal.pone.0022511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Norlinah MI, Afidah KN, Noradina AT, et al. Sleep disturbances in Malaysian patients with Parkinson's disease using polysomnography and PDSS. Parkinsonism Relat Disord. 2009;15:670–674. doi: 10.1016/j.parkreldis.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 98.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 99.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006:CD001106. [DOI] [PubMed]
- 100.Ancoli-Israel S. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00492115?term=Parkinson%27s+disease+AND+sleep&rank=6 (accessed 3 July 2013).
- 101.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 102.Ferreira JJ, Galitzky M, Thalamas C, et al. Effect of ropinirole on sleep onset: a randomized, placebo-controlled study in healthy volunteers. Neurology. 2002;58:460–462. doi: 10.1212/wnl.58.3.460. [DOI] [PubMed] [Google Scholar]
- 103.Stowe RL, Ives NJ, Clarke C, et al. Dopamine agonist therapy in early Parkinson's disease. Cochrane Database Syst Rev 2008:CD006564. [DOI] [PubMed]
- 104.Nausieda P. Sleep in Parkinson disease. In: Thorpy N, editor. Handbook of sleep disorders. New York: Marcel Dekker; 1990. pp. 719–733. [Google Scholar]
- 105.Leeman AL, O'Neill CJ, Nicholson PW, et al. Parkinson's disease in the elderly: response to and optimal spacing of night time dosing with levodopa. Br J Clin Pharmacol. 1987;24:637–643. doi: 10.1111/j.1365-2125.1987.tb03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wolters EC, Horstink MW, Roos RA, Jansen EN. Clinical efficacy of Sinemet CR 50/200 versus Sinemet 25/100 in patients with fluctuating Parkinson's disease. An open, and a double-blind, double-dummy, multicenter treatment evaluation. The Dutch Sinemet CR Study Group. Clin Neurol Neurosurg. 1992;94:205–211. doi: 10.1016/0303-8467(92)90090-p. [DOI] [PubMed] [Google Scholar]
- 107.Wolters EC, Tesselaar HJ. International (NL-UK) double-blind study of Sinemet CR and standard Sinemet (25/100) in 170 patients with fluctuating Parkinson's disease. J Neurol. 1996;243:235–240. doi: 10.1007/BF00868520. [DOI] [PubMed] [Google Scholar]
- 108.Wailke S, Herzog J, Witt K, Deuschl G, Volkmann J. Effect of controlled-release levodopa on the microstructure of sleep in Parkinson's disease. Eur J Neurol. 2011;18:590–596. doi: 10.1111/j.1468-1331.2010.03213.x. [DOI] [PubMed] [Google Scholar]
- 109.Pahwa R, Stacy MA, Factor SA, et al. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology. 2007;68:1108–1115. doi: 10.1212/01.wnl.0000258660.74391.c1. [DOI] [PubMed] [Google Scholar]
- 110.Ray Chaudhuri K, Martinez-Martin P, Rolfe KA, et al. Improvements in nocturnal symptoms with ropinirole prolonged release in patients with advanced Parkinson's disease. Eur J Neurol. 2012;19:105–113. doi: 10.1111/j.1468-1331.2011.03442.x. [DOI] [PubMed] [Google Scholar]
- 111.Trenkwalder C, Kies B, Rudzinska M, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER) Mov Disord. 2011;26:90–99. doi: 10.1002/mds.23441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)


