Abstract
Background:
Chronic rhinosinusitis (CRS) is one of the most common chronic medical conditions, with a significant impact on patient quality of life. CRS is broadly classified into two groups: CRS with nasal polyposis (CRSwNP) and CRS without NP (CRSsNP). Clinically, the major subtypes of CRSwNP may be divided into eosinophilic chronic rhinosinusitis (e.g., allergic fungal rhinosinusitis and aspirin-exacerbated respiratory disease [AERD]) and nasal polyps associated with neutrophilic inflammation (e.g., cystic fibrosis [CF]). CF is characterized by mutation of the gene encoding the CF transmembrane conductance regulator. Functional endoscopic sinus surgery is usually required for most NP patients with increased frequency in patients with AERD. This study provides a review of the epidemiology and major classification of CRSwNP.
Methods:
A review was performed of the literature regarding different subtypes of CRSwNP.
Results:
Many definitions of CRSwNP exist and estimates of prevalence vary.
Conclusion:
CRSwNP is a clinical syndrome with a heterogeneous inflammatory profile. Of the subtypes associated with eosinophilic inflammation, AERD remains the most recalcitrant to medical and surgical therapeutic interventions.
Keywords: Allergic fungal rhinosinusitis, aspirin-exacerbated respiratory disease, CFTR, chronic sinusitis, cystic fibrosis, functional endoscopic sinus surgery, nasal polyps, rhinosinusitis, Samter's triad, sinus surgery
Chronic rhinosinusitis (CRS) is a clinical syndrome characterized by mucosal inflammation of the nose and paranasal sinuses. It has been suggested that CRS occurs as the result of an inappropriate or exaggerated response to external environmental triggers.1 The original criteria for diagnosis were first established in 1997 by the Task Force on Rhinosinusitis, which required the presence of either two major or one major and two minor criteria for a period of >12 weeks.2 Major criteria include facial pressure, nasal obstruction or blockage, hyposmia or anosmia, and purulent nasal drainage. Minor criteria include headache, fever, halitosis, cough, dental pain, fatigue, and ear pain or pressure. The diagnostic criteria for CRS were further updated by the American Academy of Otolaryngology in 20073 and again with the update of the European Position Statement in 2012,4,5 which required radiological or endoscopic documentation of inflammation in addition to two major criteria.
Clinically, CRS is generally divided into two broad categories—CRS with nasal polyposis (CRSwNP) and CRS without nasal NP (CRSsNP)—with the understanding that there is often significant overlap within a broad spectrum of inflammatory disease. CRSwNP is typically associated with other respiratory diseases such as asthma,6 aspirin sensitivity,7 and idiopathic bronchiectasis.8 In this article, the epidemiology of CRSwNP and its major subtypes are discussed.
EPIDEMIOLOGY
CRS is one of the most prevalent chronic diseases worldwide.2 Affecting one in seven American adults, it is the second most common chronic condition in the United States.9,10 In Europe, the Global Allergy and Asthma Network of Excellence study reported the prevalence of CRS, as defined by European Position Statement criteria, as 10.9%.11 CRS also has a significant impact on quality of life, with symptom severity comparable with classically debilitating diseases such as congestive heart failure, chronic back pain, and chronic obstructive pulmonary disease.3,4 Despite these data, the prevalence of CRS formally diagnosed by physicians is only 2%.12 Potential causes of this variability in estimated prevalence include the use of different diagnostic criteria, diagnosis and management by variously trained medical professionals, and inconsistent diagnostic definitions. NP, in particular, can be clinically silent,13 and epidemiological studies use a mix of subjective and objective metrics of polyposis. Objective evidence of NP includes evaluation by anterior rhinoscopy or nasal endoscopy, and subjective measures rely on self-report surveys—leading to concerns that these measures may overestimate the actual prevalence. Overall, the literature suggests that CRSwNP increases with age, with a mean onset across all ethnic groups of 42 years.14–16 In most studies, NP is uncommon under the age of 20 years and occurs more frequently in men than in women17–19; aspirin-sensitive patients, however, are more likely to be women.14,20
PATHOPHYSIOLOGY
Despite growing evidence that bacteria, fungi, allergens, and superantigens play a prominent role in the pathophysiology of CRSwNP, the exact cause of polyposis is still unknown. The etiology of CRS is considered multifactorial.4 CRS is a clinical diagnosis characterized by a heterogeneous pattern of inflammation that may be initiated by any number of factors, with the result being a dysregulated interaction between the sinus epithelium and the lymphoid system.
Over the past 10 years, research has revealed unique cytokine and cellular inflammatory profiles that contribute to CRS. Approximately 80% of CRSwNP in western countries are characterized by a robust T-helper 2 (Th2) response, eosinophilic infiltration15,21 decreased T-regulatory function,15 and an abundance of IL-5 cytokine.16,22 This pattern is in contrast to nasal polyps found in Chinese patients where the majority of nasal polyps present with Th1 and Th17 (Th1/Th17) inflammatory profiles.21 This pattern has also been found in other Asian countries such as Thailand and Korea. Eosinophilic polyps were identified in 33.3% of patients (n = 30) in Korea23 and only 11.7% of patients (n = 145) in Thailand.24 Allergic inflammation in the upper and lower airways is common in CRSwNP,7,8 with 60% of patients suffering from comorbid asthma.25 The pattern of inflammation shown in CRS is seen in asthma as well, and asthmatic patients have increased IL-5 and Th2 activity.26,27 Of note, a recent pan-European sinusitis cohort within the Global Allergy and Asthma Network of Excellence study found the relationship between asthma and sinusitis to be of even greater magnitude in polyposis patients.9 The relationship between upper and lower airway pathology is further corroborated by the improvement in lower airway disease that is observed when CRSwNP is effectively treated.28
DIFFERENTIAL DIAGNOSIS
Eosinophilic Chronic Rhinosinusitis
In western countries, eosinophilic CRS (ECRS) is the most common general category of CRSwNP. ECRS encompasses allergic fungal rhinosinusitis (AFRS), eosinophilic mucin rhinosinusitis (EMRS), eosinophilic fungal rhinosinusitis (EFRS), and even aspirin-exacerbated respiratory disease (AERD). The main distinction between the first three subtypes is with regard to the presence of allergy to molds and/or the presence of fungus in the sinuses. In AFRS, there is allergic mucin and local eosinophilia, as well as mold allergy. There is no specific IgE to mold allergens in EMRS and EFRS, but there is absence of fungus in EMRS. In both subtypes, polyps appear similar to AFRS but are typically bilateral without characteristic hyperdense secretions and less bony erosion into the orbit or skull base.29,30
Several studies point to fungus as a possible inflammatory trigger for CRS independent of a type I IgE allergic mechanism as seen in AFRS.26 Observations that eosinophils cluster around fungi intimate that eosinophils might target the fungi and trigger the inflammation in CRS patients. Furthermore, peripheral lymphocytes from CRS patients will produce large quantities of the cytokines IL-5 and IL-13 when they are exposed to certain fungal antigens.31 Thus, the fungi in the nasal and sinus mucus may activate and induce Th2-type cytokine production independent from allergy. This has added to the controversy regarding the diagnosis of EMRS because of the advent of more sensitive techniques such as the use of chitin staining.32
Polymerase chain reaction amplification33–35 used to detect fungi indicates EMRS may also be related to a fungal inflammatory response. Ponikau et al.36 in 1999 suggested that fungi are present in almost all patients with CRS (96%) with <25% of these exhibiting a type I hypersensitivity to fungal antigens. Subsequent studies indicated the presence of Th1 and Th2 cellular responses in peripheral blood mononuclear cells from patients with CRS when exposed to common airborne fungi,31 lending further support to the premise that fungi may elicit eosinophilic inflammation in the absence of a type I hypersensitivity.36,37
The presence of multiclonal IgE in nasal polyps associated with local IgE against Alternaria or Staphylococcus provides another potential role of fungi in NP.38–40 Importantly, local IgE hypersensitivity to Alternaria and Aspergillus were also noted to contribute to local tissue eosinophilia in the adenoids of both atopic as well as nonatopic children (more so in atopic) who did not show systemic sensitization.41 AFRS and AERD will be discussed in more detail later in this article.
Allergic Fungal Rhinosinusitis
The pathogenesis of AFRS is an area of active research and deserves further mention. Studies indicate that a type I and, to a lesser extent, type III hypersensitivity to fungal allergens is responsible for the pathological findings and clinical symptoms.42,43 AFRS has been described as the nasal analog of allergic bronchopulmonary aspergillosis, because both are associated with specific type II major histocompatibility complexes.44
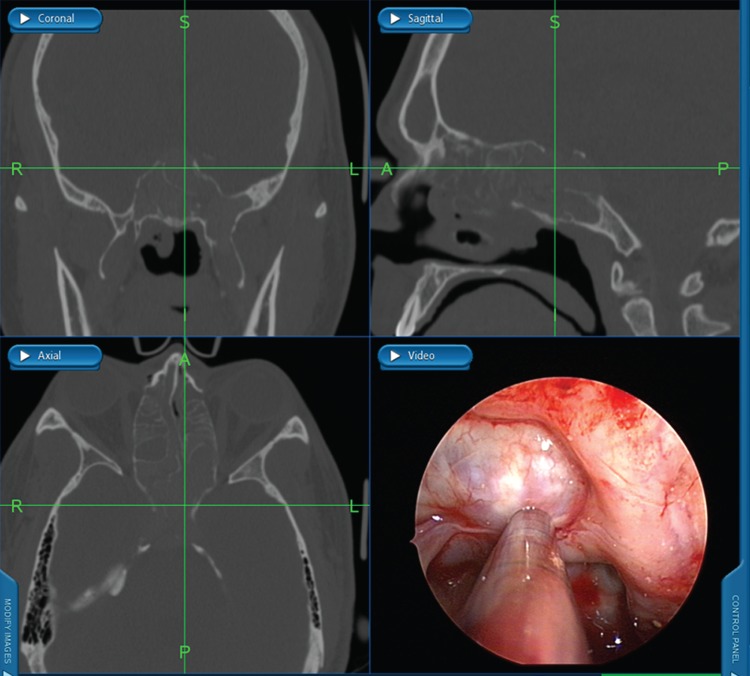
Historically, the diagnosis of AFRS was established by Kuhn's criteria,45 which include: (1) presence of nasal polyps, (2) type I hypersensitivity, (3) characteristic computed tomography (CT) findings, (4) presence of allergic mucin without tissue invasion (Fig. 1), and (5) microscopic evidence of fungi. Characteristic CT scan findings include unilateral (up to 50%) or bilateral opacification of the sinuses with areas of hyperattenuation representing allergic mucin (Fig. 2). Significant bony erosion of the skull base can be seen (Fig. 3). Because the diagnostic criteria require histopathology, AFRS is typically diagnosed only after endoscopic sinus surgery. Fungal cultures obtained in these patients often yield dematiaceous fungi such as Bipolaris spicifera, Curvularia lunata, and Aspergillus species including Aspergillus fumigatus, Aspergillus flavus, and Aspergillus niger.46,47 Elevated serum IgE suggests AFRS, and IgE levels tend to fluctuate with disease activity.48 Shubert in 199848 developed another set of criteria for AFRS that excluded the presence of type I allergy to the fungus. In these criteria, the surgically obtained mucin must be positive for fungus with no tissue invasion and exclusion of other causes of fungal rhinosinusitis. The diagnosis of AFRS has been further modified according to the guidelines for clinical research in CRS.49 Criteria according to this classification require the presence of more than one clinical symptom of CRS with endoscopic documentation of allergic mucin and inflammation that includes either edema or polyposis, radiological evidence of CRS, and histological documentation of allergic mucin as well as type I hypersensitivity to fungi. These criteria were put forth because of the problems in culturing fungus postoperatively and the difficulty in fulfilling all Bent and Kuhn criteria. The presence of fungus culture, which has a yield of 64–100%, does not establish or eliminate the diagnosis of AFRS.50
Figure 1.
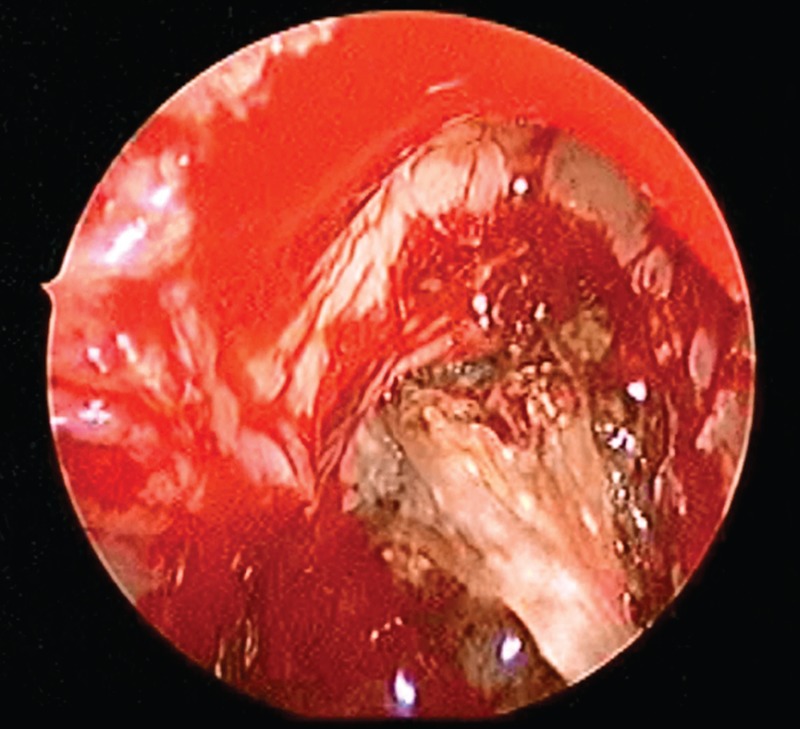
Endoscopic view of allergic fungal mucin.
Figure 2.
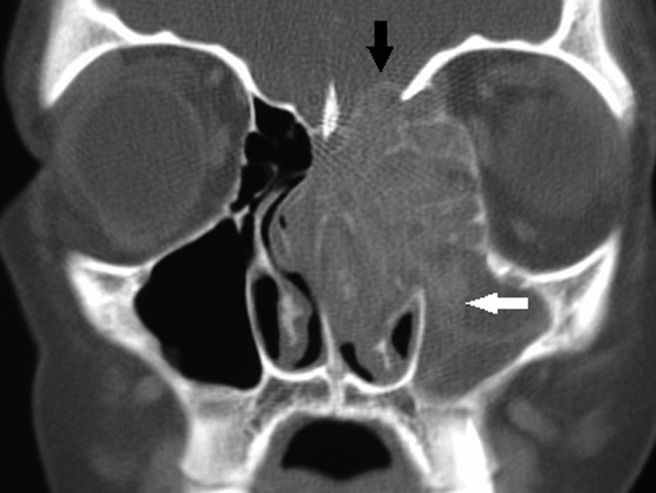
Coronal computed tomography (CT) scan of the paranasal sinuses with soft tissue windows showing unilateral opacification of the maxillary and ethmoid sinuses with bony erosion into the skull base (black arrow) as well as expansion into the left orbit. Note the characteristic hyperattenuated areas in the maxillary sinus suggestive of allergic fungal rhinosinusitis (AFRS; white arrow).
Figure 3.
Intraoperative triplanar imaging and endoscopic view after removal of fungal mucin shows widespread skull base erosion typical for allergic fungal rhinosinusitis (AFRS).
A strong association between Staphylococcus aureus superantigens and AFRS was found among patients with CRSwNP. S. aureus colonization was significantly more prevalent in AFRS when compared with other subtypes of CRSwNP.51 S. aureus superantigen–specific IgE levels correlate with total serum IgE levels in patients with AFRS, intimating a relationship between bacterial colonization and allergic hypersensitivity.52 It has been postulated that superantigens may have an activating role, promoting the immune dysregulation that contributes to AFRS.53,54 These concepts underscore the likelihood of related and overlapping mechanisms contributing to eosinophilia in ECRS.
Aspirin-Exacerbated Respiratory Disease
In 1922, Widal et al.55 were the first to describe the association between nasal polyps, asthma, and aspirin hypersensitivity. After further characterization by Samter, the association became known as “Samter's triad.”56 AERD is a clinical syndrome consisting of chronic sinusitis, NP, bronchoconstriction, and angioedema or urticaria associated with the ingestion of nonsteroidal anti-inflammatory drugs (NSAIDs). Estimates of prevalence vary; research suggests AERD affects between 0.3 and 2.5% of the general population.57–59 Some studies, however, report up to a 33% prevalence in patients undergoing endoscopic sinus surgery for CRS.60 The prevalence of AERD is likely underestimated in the literature, partly because of the difficulty in establishing the diagnosis when relying on patient history alone. One study reported a 23% prevalence of AERD when patients with NP with or without asthma were evaluated with the oral aspirin provocation test.61 Of these patients who tested positive, 25% had no history of aspirin hypersensitivity. In a large study from Poland that included 12,971 randomly selected adults, the prevalence of AERD was 0.6% in the general population, 4.3% in asthmatic patients, 0.7–2.6% in CRS, and as high as 14–22% in NP patients.62
AERD is typically seen in the third decade of life with a female/male ratio of 3:2.14,20 There are no clear ethnic associations,63–67 but individuals expressing the HLA A1/B8 phenotype have a higher incidence of AERD.68 The pathogenesis of AERD is related to dysregulation of eicosanoid synthesis. Eicosanoids are molecules that are derived from arachidonic acid through a series of enzymic modifications via the cyclooxygenase and lipoxygenase pathways. Aspirin competes with arachidonic acid by irreversibly binding to the cyclooxygenase active site, shifting substrates to the lipoxygenase pathway with a resultant increase in leukotrienes and reduction in prostaglandins.57,59,69,70
Why some patients are more sensitive to these biochemical shifts is poorly understood, and some have theorized that viral infection in genetically predisposed patients may be the inciting event.63 T cells cultured with respiratory viruses have increased release of cytokines that attract eosinophils.64 These cytokines, particularly IL-5, also contribute to the overproduction of eosinophils in patients with aspirin sensitivity.71 Other potential pathophysiological and molecular dysregulations have been reported to be linked with NP and particularly with AERD. In a study by Fruth et al.,72 an antiapoptotic cell regulator called surviving was overexpressed in patients with AERD polyposis. Thus, decreased apoptosis as well as cellular hyperplasia may contribute to the pathophysiology of nasal polyp formation.
NP in AERD is clinically very aggressive when compared with other forms of ECRS. Overall, between 36 and 96% of AERD patients are afflicted with NP.57,70 The early symptoms of AERD are rhinorrhea and nasal congestion.57,65,70 Patients typically present with nasal congestion, rhinitis, hyposmia, and chronic rhinorrhea, ultimately progressing to pansinusitis and, eventually, polyposis. They frequently undergo multiple sinus surgeries before diagnosis. The disease may evolve over time into full-blown AERD with NSAID intolerance and symptoms of asthma and bronchoconstriction. Individuals may also develop facial deformation due to the increased pressure of polyps on the sinonasal bones.66,67
The gold standard for diagnosing AERD is an aspirin challenge13,58,60,66,68,73–76; however, clinical diagnosis is important in identifying individuals with the constellation of symptoms and signs that are typical for AERD. Some patients will present before their first reaction to NSAIDs, but aspirin challenge will be diagnostic.68
Cystic Fibrosis
Neutrophilic CRSwNP in western countries is unusual, but the genetic disease cystic fibrosis (CF) merits discussion. CF is an autosomal recessive disorder affecting around 30,000 children in the United States.77 CF patients have thick, tenacious mucus secondary to a mutation in the CF transmembrane conductance regulator (CFTR) chloride channel that affects mucociliary transport.78 Absence or lack of functional channels has deleterious effects on other organ systems, most notably the lungs and gastrointestinal tract. The diagnosis of CF consists of identifying one of two manifestations as well as the presence of either two CF mutations, an abnormal transepithelial nasal potential difference, or two abnormal sweat chloride tests.79 Although the majority of CF patients do not report sinonasal symptoms, CRS is ubiquitous.80 Nasal polyps are seen in up to 86% of patients with CF.81 In CF CRS, inflammation is neutrophil driven where IL-8 is the primary cytokine.82 Sinus cultures typically grow Pseudomonas aeruginosa or S. aureus.83
Examination of CF patients with NP usually reveals bilateral polyposis, thick anterior and posterior nasal discharge, and facial deformities such as hypertelorism due to widening of the nasal bridge with relative proptosis.84 In addition to NP, CT scan findings include hypoplasia of the frontal or sphenoid sinuses as well as demineralization and medial displacement of the uncinate process.82,85–88 Mucocele formation is not uncommon in CF, and the presence of mucoceles in a child should raise suspicion for CF.89,90 Pediatric patients with CRS that meet criteria for CF are more likely to be heterozygous for a CFTR mutation when compared with the general population (12% versus 4%).91 Furthermore, acquired defects in CFTR may be present in individuals without CF secondary to environmental local perturbations indicating defects in mucus clearance are present in individuals with sinus inflammation and NP.92–95 Further characterization of the link between acquired defects in CFTR and NP is ongoing.96–99
CONCLUSIONS
The majority of polyps in patients with CRSwNP in western countries are secondary to eosinophil-driven inflammation; CF is a prominent exception. The exact etiology of NP is still unknown. Multiple factors, including allergy, superantigens, and fungal infection, contribute to the pathophysiology of CRSwNP. Among the various eosinophilic subtypes of CRSwNPs, AERD is the most treatment resistant.
Footnotes
Presented at the North American Rhinology and Allergy Conference, Puerto Rico, February 2, 2013
Funded, in part, by the Flight Attendant's Medical Research Institute Young Clinical Scientist Award (072218) and NIH/NHLBI (1K08HL107142-01) to BAW
BA Woodworth is a consultant for ArthroCare ENT, Gyrus ENT, and Cook Medical and he is an inventor on a patent submitted regarding the use of chloride secretagogues for therapy of sinus disease (35 U.S.C. n111(b) and 37 C.F.R n.53 (c)) in the United States Patent and Trademark Office. The remaining authors have no conflicts of interest to declare pertaining to this article
Presented at the North American Rhinology and Allergy Conference, Puerto Rico, February 1, 2013
REFERENCES
- 1. Kern RC, Conley DB, Walsh W, et al. Perspectives on the etiology of chronic rhinosinusitis: An immune barrier hypothesis. Am J Rhinol 22:549–559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 117:S1–-S7, 1997. [DOI] [PubMed] [Google Scholar]
- 3. Rosenfeld R. Clinical practice guideline on adult sinusitis. Otolaryngol Head Neck Surg 137:365–377, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl 23:1–298, 2012. [PubMed] [Google Scholar]
- 6. Alobid I, Cardelus S, Benitez P, et al. Persistent asthma has an accumulative impact on the loss of smell in patients with nasal polyposis. Rhinology 49:519–524, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Alobid I, Benitez P, Bernal-Sprekelsen M, et al. The impact of asthma and aspirin sensitivity on quality of life of patients with nasal polyposis. Qual Life Res 14:789–793, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Guilemany JM, Angrill J, Alobid I, et al. United airways: The impact of chronic rhinosinusitis and nasal polyps in bronchiectasic patient's quality of life. Allergy 64:1524–1529, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Collins JG. Prevalence of selected chronic conditions: United States, 1990–1992. Vital Health Stat 10 194:1–89, 1997. [PubMed] [Google Scholar]
- 10. Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat 10 242:1–157, 2009. [PubMed] [Google Scholar]
- 11. Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: The GA2LEN survey in Europe. Allergy 67:91–98, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Shashy RG, Moore EJ, Weaver A. Prevalence of the chronic sinusitis diagnosis in Olmsted County, Minnesota. Arch Otolaryngol Head Neck Surg 130:320–323, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Johansson L, Akerlund A, Holmberg K, et al. Prevalence of nasal polyps in adults: The Skövdepopulation-based study. Ann Otol Rhinol Laryngol 112:625–629, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope 113:1199–1205, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61:1280–1289, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Jiang XD, Li GY, Li L, et al. The characterization of IL-17A expression in patients with chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy 25:e171–e175, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Hedman J, Kaprio J, Poussa T, Nieminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol 28:717–722, 1999. [DOI] [PubMed] [Google Scholar]
- 18. Hosemann W, Gode U, Wagner W. Epidemiology, pathophysiology of nasal polyposis, and spectrum of endonasal sinus surgery. Am J Otolaryngol 15:85–98, 1994. [DOI] [PubMed] [Google Scholar]
- 19. Larsen K, Tos M. The estimated incidence of symptomatic nasal polyps. Acta Otolaryngol 122:179–182, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 129(suppl):S1–S32, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Zhang N, Van Zele T, Perez-Novo C, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 122:961–968, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Wen W, Liu W, Zhang L, et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol 129:1522–1528, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Kim JW, Hong SL, Kim YK, et al. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg 137:925–930, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Jareonchasri P, Bunnag C, Muangsomboon S, et al. Clinical and histopathological classification of nasal polyps in Thias. Siriraj Hosp Gaz 54:689–697, 2002. [Google Scholar]
- 25. Ragab A, Clement P, Vincken W. Objective assessment of lower airway involvement in chronic rhinosinusitis. Am J Rhinol 18:15–21, 2004. [PubMed] [Google Scholar]
- 26. Bachert C, Zhang N, Holtappels G, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol 126:962–968, 2010. [DOI] [PubMed] [Google Scholar]
- 27. Chanez P, Vignola AM, Vic P, et al. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. Am J Respir Crit Care Med 159:588–595, 1999. [DOI] [PubMed] [Google Scholar]
- 28. Mattos JL, Woodard CR, Payne SC. Trends in common rhinologic illnesses: Analysis of U.S. healthcare surveys 1995–2007. Int Forum Allergy Rhinol 1:3–12, 2011. [DOI] [PubMed] [Google Scholar]
- 29. Ferguson BJ. Eosinophilic mucin rhinosinusitis: A distinct clinicopathological entity. Laryngoscope 110:799–813, 2000. [DOI] [PubMed] [Google Scholar]
- 30. Saravanan K, Panda NK, Chakrabarti A, et al. Allergic fungal rhinosinusitis: An attempt to resolve the diagnostic dilemma. Arch Otolaryngol Head Neck Surg 132:173–178, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Shin SH, Ponikau JU, Sherris DA, et al. Chronic rhinosinusitis: An enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol 114:1369–1375, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Taylor MJ, Ponikau JU, Sherris DA, et al. Detection of fungal organisms in eosinophilic mucin using a fluorescein-labeled chitin-specific binding protein. Otolaryngol Head Neck Surg 127:377–383, 2002. [DOI] [PubMed] [Google Scholar]
- 33. Kim ST, Choi JH, Jeon HG, et al. Comparison between polymerase chain reaction and fungal culture for the detection of fungi in patients with chronic sinusitis and normal controls. Acta Otolaryngol 125:72–75, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Polzehl D, Weschta M, Podbielski A, et al. Fungus culture and PCR in nasal lavage samples of patients with chronic rhinosinusitis. J Med Microbiol 54:31–37, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Rao AK, Mathers PH, Ramadan HH. Detection of fungi in the sinus mucosa using polymerase chain reaction. Otolaryngol Head Neck Surg 134:581–585, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc 74:877–884, 1999. [DOI] [PubMed] [Google Scholar]
- 37. Panikau JU, Sherris DA, Kephart GM, et al. Striking deposition of toxic eosinophil major protein in mucus: Implications for chronic rhinosinusitis. J Allergy Clin Immunol 116:362–369, 2005. [DOI] [PubMed] [Google Scholar]
- 38. Bachert C, Gevaert P, Holtappels G, et al. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol 107:607–614, 2001. [DOI] [PubMed] [Google Scholar]
- 39. Gevaert P, Holtappels G, Johansson SG, et al. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy 60:71–79, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Sabirov A, Hamilton RG, Jacobs JB, et al. Role of local immunoglobulin E specific for Alternaria alternata in the pathogenesis of nasal polyposis. Laryngoscope 118:4–9, 2008. [DOI] [PubMed] [Google Scholar]
- 41. Shin SY, Ye YM, Eun YG, et al. Local IgE-mediated hypersensitivity to Alternaria in pediatric adenoid tissue. Int J Pediatr Otorhinolaryngol 76:1423–1428, 2001. [DOI] [PubMed] [Google Scholar]
- 42. Marple BF. Allergic fungal rhinosinusitis: Current theories and management strategies. Laryngoscope 111:1006–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 43. Horst M, Hejjaoui A, Horst V, et al. Double-blind, placebo-controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol 85:460–472, 1990. [DOI] [PubMed] [Google Scholar]
- 44. Chauhan B, Santiago L, Hutcheson PS, et al. Evidence for the involvement of two different MHC class II regions in susceptibility or protection in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 106:723–729, 2000. [DOI] [PubMed] [Google Scholar]
- 45. Bent JP, III, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg 111:580–588, 1994. [DOI] [PubMed] [Google Scholar]
- 46. Manning SC, Vuitch F, Weinberg AG, Brown OE. Allergic aspergillosis: A newly recognized form of sinusitis in the pediatric population. Laryngoscope 99:681–685, 1989. [DOI] [PubMed] [Google Scholar]
- 47. Schubert MS, Goetz DW. Evaluation and treatment of allergic fungal sinusitis. I. Demographics and diagnosis. J Allergy Clin Immunol 102:387–394, 1998. [DOI] [PubMed] [Google Scholar]
- 48. Schubert MS, Goetz DW. Evaluation and treatment of allergic fungal sinusitis. II. Treatment and follow-up. J Allergy Clin Immunol 102:395–402, 1998. [DOI] [PubMed] [Google Scholar]
- 49. Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 114(suppl):155–212, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Manning SC, Holman M. Further evidence for allergic pathophysiology in allergic fungal sinusitis. Laryngoscope 108:1485–1496, 1998. [DOI] [PubMed] [Google Scholar]
- 51. Clark DW, Wenaas A, Luong A, et al. Staphylococcus aureus prevalence in allergic fungal rhinosinusitis vs other subsets of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol 3:89–93, 2013. [DOI] [PubMed] [Google Scholar]
- 52. Hutcheson PS, Oliver DA, Schubert MS, Slavin RG. The association of total IgE and specific IgE anti-staphylococcal enterotoxin with chronic hyperplastic rhinosinusitis. J Allergy Clin Immunol 117:S71, 2006. (Ab 278). [Google Scholar]
- 53. Ferguson BJ, Seethala R, Wood WA. Eosinophilic bacterial chronic rhinosinusitis. Laryngoscope 117:2036–2040, 2007. [DOI] [PubMed] [Google Scholar]
- 54. Schubert MS. A superantigen hypothesis for the pathogenesis of chronic hypertrophic rhinosinusitis, allergic fungal sinusitis, and related disorders. Ann Allergy Asthma Immunol 87:181–188, 2001. [DOI] [PubMed] [Google Scholar]
- 55. Widal F, Abrami P, Lermoyez J. Anaphylaxie et idiosyncrasie. 1992 [Anaphylaxis and idiosyncrasy. 1992]. Allergy Proc 14:373–376, 1993. [PubMed] [Google Scholar]
- 56. Samter M, Beers RF., Jr Concerning the nature of intolerance to aspirin. J Allergy 40:281–293, 1967. [DOI] [PubMed] [Google Scholar]
- 57. Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol 89:474–478, 2002. [DOI] [PubMed] [Google Scholar]
- 58. Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ 328:434, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stevenson DD, Sanchez-Borges M, Szczeklik A. Classification of allergic and pseudoallergic reactions to drugs that inhibit cyclooxygenase enzymes. Ann Allergy Asthma Immunol 87:177–180, 2001. [DOI] [PubMed] [Google Scholar]
- 60. Stevenson DD. Aspirin desensitization in patients with AERD. Clin Rev Allergy Immunol 24:159–168, 2003. [DOI] [PubMed] [Google Scholar]
- 61. Bavbek S, Dursun B, Dursun E, et al. The prevalence of aspirin hypersensitivity in patients with nasal polyposis and contributing factors. Am J Rhinol Allergy 25:411–415, 2011. [DOI] [PubMed] [Google Scholar]
- 62. Kowalski ML, Grzegorczyk J, Pawliczak R, et al. Decreased apoptosis and distinct profile of infiltrating cells in the nasal polyps of patients with aspirin hypersensitivity. Allergy 57:493–500, 2002. [DOI] [PubMed] [Google Scholar]
- 63. Szczeklik A. Aspirin-induced asthma as a viral disease. Clin Allergy 18:15–20, 1988. [DOI] [PubMed] [Google Scholar]
- 64. Davoine F, Cao M, Wu Y, et al. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol 122:69–77, 2008. [DOI] [PubMed] [Google Scholar]
- 65. Douglas GC, Karkos PD, Swift AC. Aspirin sensitivity and the nose. Br J Hosp Med (Lond) 71:442–445, 2010. [DOI] [PubMed] [Google Scholar]
- 66. Kowalski ML. Rhinosinusitis and nasal polyposis in aspirin sensitive and aspirin tolerant patients: Are they different? Thorax 55(suppl 2):S84–-S86, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Picado C. Aspirin intolerance and nasal polyposis. Curr Allergy Asthma Rep 2:488–493, 2002. [DOI] [PubMed] [Google Scholar]
- 68. Palikhe NS, Kim JH, Park HS. Update on recent advances in the management of aspirin exacerbated respiratory disease. Yonsei Med J 50:744–750, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J 16:432–436, 2000. [DOI] [PubMed] [Google Scholar]
- 70. Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol 111:913–921, 2003. [DOI] [PubMed] [Google Scholar]
- 71. Bachert C, Gevaert P, van Cauwenberge P. Nasal polyposis—A new concept on the formation of polyps. Allergy Clin Immunol Int. 11:130–135, 1999. [Google Scholar]
- 72. Fruth K, Schramek E, Docter D, et al. Dysregulated survivin expression in nasal polyps of individuals with aspirin exacerbated respiratory disease. Am J Rhinol Allergy 26:380–384, 2012. [DOI] [PubMed] [Google Scholar]
- 73. Delaney JC. The diagnosis of aspirin idiosyncrasy by analgesic challenge. Clin Allergy 6:177–181, 1976. [DOI] [PubMed] [Google Scholar]
- 74. Stevenson DD, Zuraw BL. Pathogenesis of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol 24:169–188, 2003. [DOI] [PubMed] [Google Scholar]
- 75. Hope AP, Woessner KA, Simon RA, Stevenson DD. Rational approach to aspirin dosing during oral challenges and desensitization of patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 123:406–410, 2009. [DOI] [PubMed] [Google Scholar]
- 76. Baenkler HW. Salicylate intolerance: Pathophysiology, clinical spectrum, diagnosis and treatment. Dtsch Arztebl Int 105:137–142, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352:1992–2001, 2005. [DOI] [PubMed] [Google Scholar]
- 78. Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073, 1989. [DOI] [PubMed] [Google Scholar]
- 79. Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: A consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr 132:589–595, 1998. [DOI] [PubMed] [Google Scholar]
- 80. Tandon R, Derkay C. Contemporary management of rhinosinusitis and cystic fibrosis. Curr Opin Otolaryngol Head Neck Surg 11:41–44, 2003. [DOI] [PubMed] [Google Scholar]
- 81. Ryan MW. Diseases associated with chronic rhinosinusitis: What is the significance? Curr Opin Otolaryngol Head Neck Surg 16:231–236, 2008. [DOI] [PubMed] [Google Scholar]
- 82. Gentile VG, Isaacson G. Patterns of sinusitis in cystic fibrosis. Laryngoscope 106:1005–1009, 1996. [DOI] [PubMed] [Google Scholar]
- 83. Shapiro ED, Milmoe GJ, Wald ER, et al. Bacteriology of the maxillary sinuses in patients with cystic fibrosis. J Infect Dis 146:589–593, 1982. [DOI] [PubMed] [Google Scholar]
- 84. Hui Y, Gaffney R, Crysdale WS. Sinusitis in patients with cystic fibrosis. Eur Arch Otorhinolaryngol 252:191–196, 1995. [DOI] [PubMed] [Google Scholar]
- 85. Ledesma-Medina J, Osman MZ, Girdany BR. Abnormal paranasal sinuses in patients with cystic fibrosis of the pancreas. Radiological findings. Pediatr Radiol 9:61–64, 1980. [DOI] [PubMed] [Google Scholar]
- 86. Neely JG, Harrison GM, Jerger JF, et al. The otolaryngologic aspects of cystic fibrosis. Trans Am Acad Ophthalmol Otolaryngol 76:313–324, 1972. [PubMed] [Google Scholar]
- 87. Woodworth BA, Ahn C, Flume PA, Schlosser RJ. The delta F508 mutation in cystic fibrosis and impact on sinus development. Am J Rhinol 21:122–127, 2007. [DOI] [PubMed] [Google Scholar]
- 88. Seifert CM, Harvey RJ, Mathews JW, et al. Temporal bone pneumatization and its relationship to paranasal sinus development in cystic fibrosis. Rhinology 48:233–238, 2010. [DOI] [PubMed] [Google Scholar]
- 89. Guttenplan MD, Wetmore RF. Paranasal sinus mucocele in cystic fibrosis. Clin Pediatr (Phila) 28:429–430, 1989. [DOI] [PubMed] [Google Scholar]
- 90. Tunkel DE, Naclerio RM, Baroody FM, Rosenstein BJ. Bilateral maxillary sinus mucoceles in an infant with cystic fibrosis. Otolaryngol Head Neck Surg 111:116–120, 1994. [DOI] [PubMed] [Google Scholar]
- 91. Raman V, Clary R, Siegrist KL, et al. Increased prevalence of mutations in the cystic fibrosis transmembrane conductance regulator in children with chronic rhinosinusitis. Pediatrics 109:E13, 2002. [DOI] [PubMed] [Google Scholar]
- 92. Cohen NA, Zhang S, Sharp DB, et al. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope 6:177–181, 1976. [Google Scholar]
- 93. Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope 120:1465–1469, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope 121:1929–1934, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Alexander NS, Blount A, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope 122:1193–1197, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Azbell C, Zhang S, Skinner D, et al. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg 143:397–404, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. Laryngoscope 120:1051–1056, 2010. [DOI] [PubMed] [Google Scholar]
- 98. Alexander NS, Hatch N, Zhang S, et al. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. Laryngoscope 121:1313–1319, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang S, Smith N, Schuster D, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: Therapeutic implications for chronic rhinosinusitis. Am J Rhinol Allergy 25:307–312, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]