Abstract
Background:
Leukotriene antagonists (LTAs) provide a potential strategy for the management of chronic rhinosinusitis with nasal polyposis (CRSwNP), which is often refractory to medical and surgical treatment. The purpose of this study is to determine the impact of LTA treatment alone and in conjunction with intranasal corticosteroids (INCSs) on nasal symptoms, objective clinical outcomes, and immune parameters in CRSwNP.
Methods:
A systematic review was performed including studies that assessed the effectiveness of LTAs on clinical outcome measures of CRSwNP. Exclusion criteria were trials assessing LTAs in CRS without nasal polyps or asthma symptoms only. Quantitative analysis was performed using a random effects model.
Results:
Twelve studies fulfilled eligibility: five randomized control trials and seven case series. LTAs showed significant improvements in CRSwNP symptoms over placebo; however, these randomized trials were unable to be combined via meta-analysis. The two studies used in meta-analysis showed a standardized mean difference of pooled overall symptom scores of 0.02 (95% confidence interval, −0.39–0.44) between LTA and INCS study arms, indicating no difference between the treatment modalities. Improvement was described by all studies in symptoms, clinical outcomes, and/or immune parameters after LTA treatment, with greater improvements in a subset of symptoms beyond that observed with INCSs. Concomitant asthma, aspirin-exacerbated respiratory disease, and atopy did not significantly or consistently affect these results.
Conclusion:
LTAs are an effective tool for treating CRSwNP, with limited benefit as an adjunctive therapy. Additional study is required to determine the most beneficial strategy and patient population for their use.
Keywords: Antagonist, chronic, leukotriene, montelukast, polyps, rhinosinusitis, sinusitis, symptoms, zafirlukast, zileuton
Chronic rhinosinusitis with nasal polyposis (CRSwNP) is one of the most difficult forms of CRS to treat and exhibits frequent recurrence regardless of therapeutic modality, with postoperative recurrence rates ranging from 11.8% at 3 years to 51.2% at a median of 6 (range, 0–60 years) years.1 Current consensus guidelines support long-term medical treatment with intranasal corticosteroid (INCS) sprays, supplemented with short, infrequent courses of oral corticosteroids. Several reviews have systematically examined local corticosteroid use in patients with CRSwNP, showing improvements in symptom scores and polyp size via meta-analysis.2,3 Similarly, high-level evidence supports the use of oral corticosteroids in CRSwNP patients to improve symptoms and polyp size4; however, the effects are short lived, and long-term use is limited because of the risk of severe side effects. Despite the routine use of corticosteroid medications, a large percentage of patients with CRSwNP will continue to have ongoing symptoms requiring additional treatment, usually in the form of surgery, which provides immediate improvement but is not curative.
There has been much study into the immunologic basis of CRSwNP in hopes of identifying more targeted pharmacologic therapies. Studies have shown increased levels of leukotrienes (LTs) and their receptors localized to nasal polyps.5,6 Cysteinyl-LTs, produced though arachidonic acid metabolism in inflammatory cells characteristic of CRS, viz., eosinophils and mast cells, bind to G-protein coupled receptors to promote localized inflammation, including eosinophil infiltration, mucous secretion, collagen deposition, and release of mast cell cytokines.7 This process can be inhibited either by blocking the receptor with an LT receptor antagonist, such as montelukast, or by preventing the formation of cysteinyl-LTs with a 5-lipooxygenase inhibitor, such as zileuton. LT antagonists (LTAs) have proven efficacious in chronic inflammatory conditions of the airways, including allergic rhinitis, asthma, and aspirin-exacerbated respiratory disease (AERD),8–11 all diseases that often coexist with CRSwNP. Several studies have shown positive effects of LTAs as a primary treatment for CRSwNP. A number of these were discussed in European Position Paper on Rhinosinusitis and Nasal Polyps 2012,12 which proposed a recommendation against their use. The recently published Canadian clinical guidelines for treating CRS propose a weak recommendation in favor of the use of LTAs with the caveat that the evidence behind this recommendation is weak.13 Neither review was done in a systematic fashion, and no formal meta-analysis was performed. With these limitations in mind, the aim of this study was to systematically review clinical studies that examine LTAs as a treatment for CRSwNP, pooling outcomes, where possible, via formal meta-analysis. Data from this review can be used to inform future guidelines with respect to the use of LTAs in CRSwNP.
METHODS
Search Method
Two reviewers (J.L.W. and K.D.) independently performed a literature search in PUBMED (1950 to April 2013) and MEDLINE (January 1966 to April 2013) for studies evaluating the effectiveness of LTA medications in patients with nasal polyposis. The keywords and MESH terms used were “leukotriene antagonist,” “montelukast,” or “zileuton” AND “sinusitis,” or “nasal polyps,” “rhinosinusitis,” “Samter's triad,” or “aspirin-exacerbated respiratory disease.” The only limits used in the search were “humans.” The reference lists of all identified articles were examined for additional relevant studies. All articles were considered regardless of language. This study was considered exempt by the Medical University of South Carolina's Institutional Review Board.
Inclusion/Exclusion Criteria
Any study that assessed the effectiveness of LTAs on clinical outcome measures of CRSwNP in human subjects was considered for inclusion. Reviews and single case reports were excluded, as were studies assessing the effect of LTAs on asthma symptoms only. Studies that examined LTA efficacy on CRS without nasal polyps were also excluded. The data from these studies were extracted and analyzed independently by two authors (J.L.W. and K.D.). Level of evidence was determined through standard clinical guidelines as described previously.14
Statistical Analysis
The primary outcome of interest was symptom score. Secondary outcome measures included objective clinical measurements, such as polyp size and computed tomography score and immune parameters. Analysis began with placebo-controlled randomized controlled trials (RCTs), but also compared treatment using LTAs versus other pharmacotherapies, as well as LTAs as an adjunct to traditional therapy. Data from uncontrolled studies was summarized with respect to each outcome measure of interest.
Meta-analysis of outcomes with a continuous measure (comparison of means and standard deviations between control and treatment groups) was performed with Cochrane Review Manager (RevMan) Version 5.1.15 Given the likelihood of study variability, a random effects model was used and the standardized mean difference (SMD) and 95% confidence interval was calculated. The SMD represents a transformation of the study outcome data into standard deviation units by dividing the difference in mean outcome between two groups by the pooled standard deviation. Heterogeneity was assessed with the I2 statistic; the values of 25, 50, and 75% were considered to indicate low, medium, and high heterogeneity.
RESULTS
Included Studies
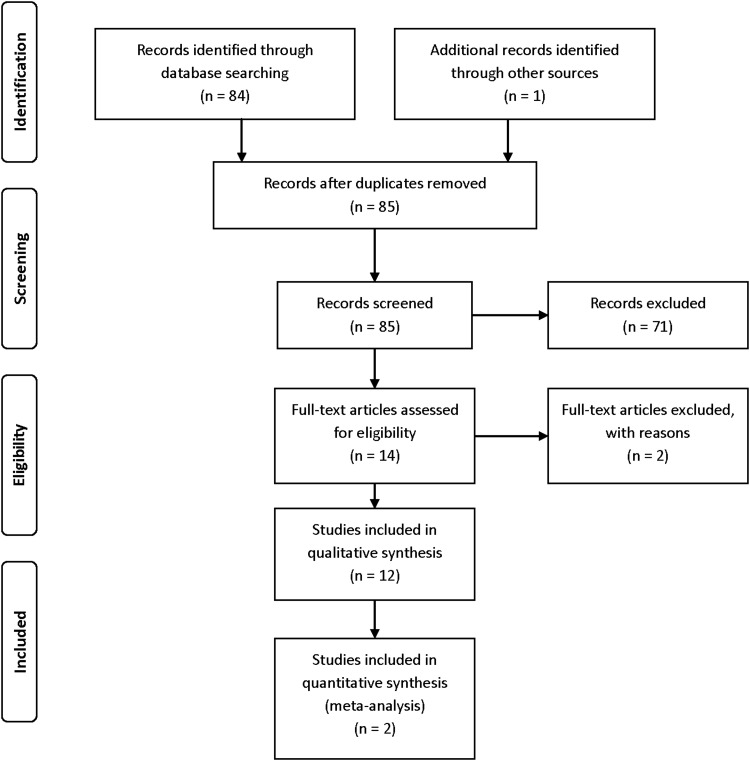
The literature search identified 84 unique abstracts and 1 additional study found through review of the article reference lists. These 85 records were screened and assessed for eligibility in this review. The search strategy with flow diagram is presented per PRISMA guidelines (Fig. 1).16 After review of the 14 prescreened full-length articles, 2 were excluded due to studying CRS without nasal polyposis. The agreement, κ-score, between reviewers at the abstract and full text level, was 0.82 and 0.79, respectively, and differences were discussed and resolved among all authors. The 12 remaining studies were selected for this review and consisted of 5 RCTs17–21 and 7 case series.22–28 Results of these studies are summarized in Tables 1–3.
Figure 1.
Literature search strategy. PRISMA flowchart detailing literature search and review.16
Table 1.
Summary of placebo-controlled RCTs (level of evidence = 1b)
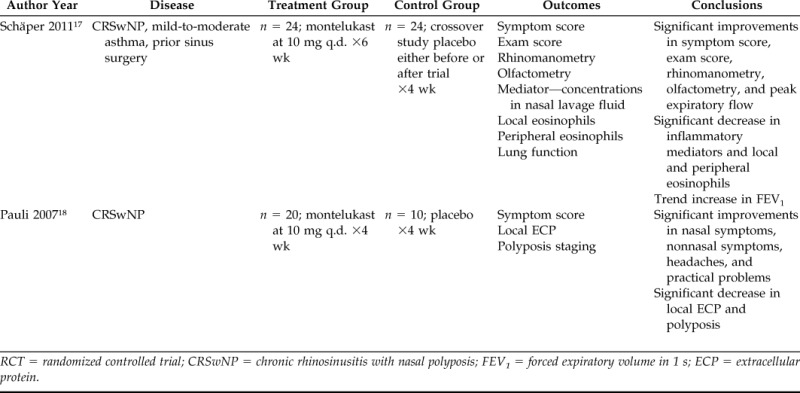
RCT = randomized controlled trial; CRSwNP = chronic rhinosinusitis with nasal polyposis; FEV1 = forced expiratory volume in 1 s; ECP = extracellular protein.
Table 3.
Summary of case series (level of evidence = 4)
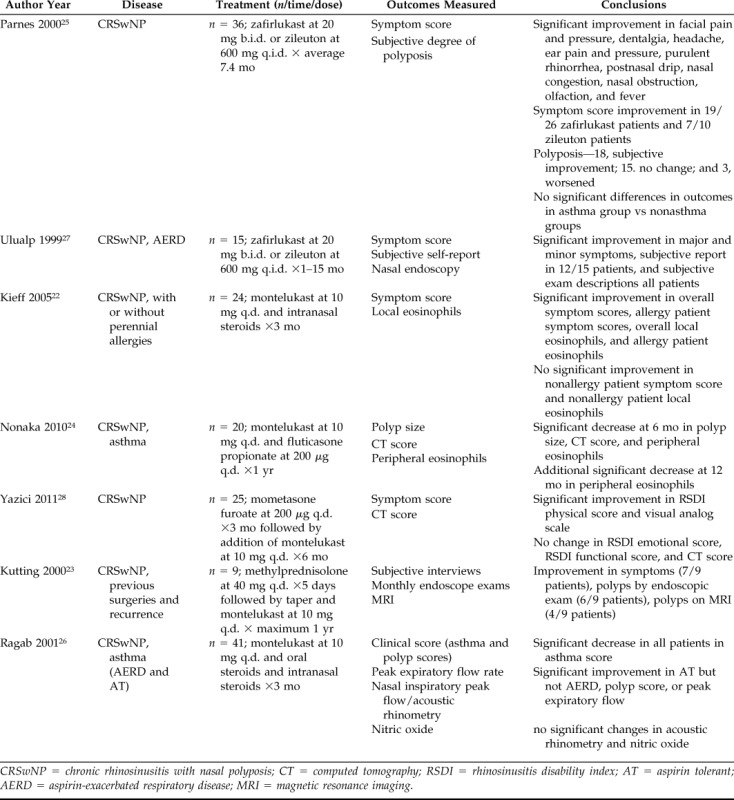
CRSwNP = chronic rhinosinusitis with nasal polyposis; CT = computed tomography; RSDI = rhinosinusitis disability index; AT = aspirin tolerant; AERD = aspirin-exacerbated respiratory disease; MRI = magnetic resonance imaging.
Table 2.
Summary of nonplacebo controlled RCTs (level of evidence = 1b)
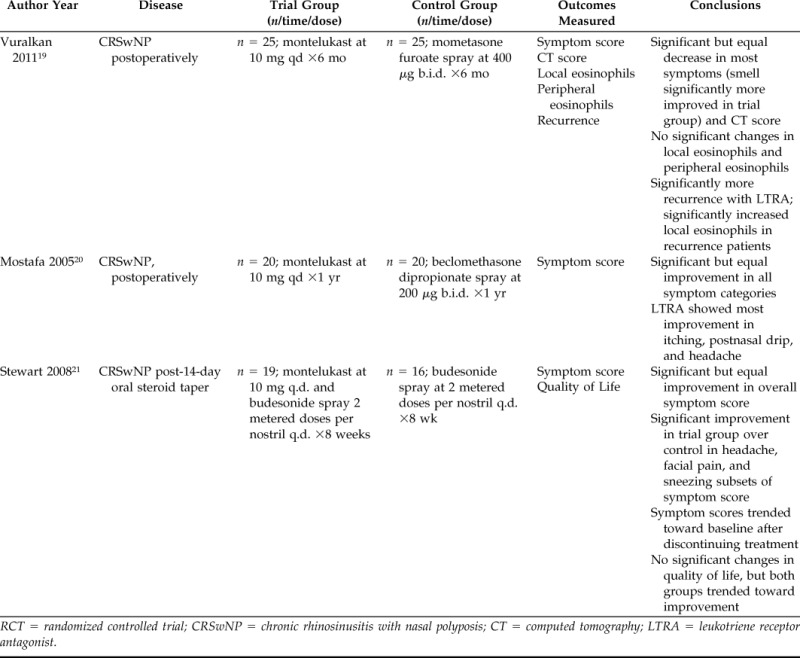
RCT = randomized controlled trial; CRSwNP = chronic rhinosinusitis with nasal polyposis; CT = computed tomography; LTRA = leukotriene receptor antagonist.
Of the five RCTs identified, which included a total of 179 patients, two examined the efficacy of montelukast versus placebo,17,18 with one using the entire group of patients as crossover controls. Two additional RCTs studied the clinical outcomes of montelukast versus INCS after surgical treatment.19,20 A fifth RCT followed outcomes of combination treatment with montelukast plus INCS versus INCS alone after a course of oral steroids.21 These studies were published between 2005 and 2012 and had a treatment period that ranged from 4 weeks to 1 year.
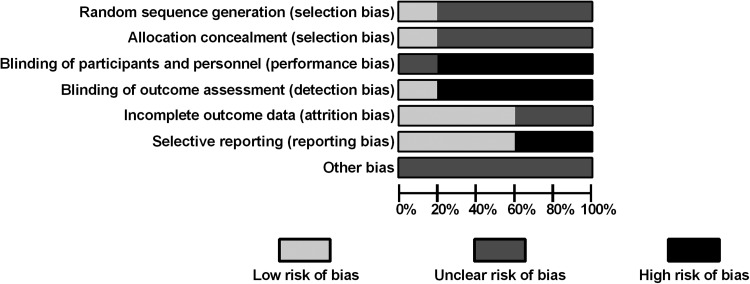
Risk of bias was evaluated for the RCTs using the Cochrane Collaboration assessment tool (Fig. 2).29 There was some evidence of bias mostly attributable to a lack of blinding in four of the five trials. One of these was single blinded17 and the remaining three were comparing an oral medication to an intranasal one without the use of placebo pills or sprays.19–21 A second source of bias resulted from selective reporting of data, with some outcomes containing detailed overview and statistics and others simply listed in terms of relative significance.17,18
Figure 2.
Randomized control trials (RCTs) risk of bias. Risk of bias was evaluated using the Cochrane Collaboration bias assessment tool29 and reported as percentages across all RCTs.
The seven case series, published between 1999 and 2011, included 170 patients with CRSwNP treated with LTAs. Treatment regimens included LTA alone (n = 2)25,27 and LTA as an adjunct to intranasal steroids (n = 3),22,24,28 oral steroids (n = 1),23 or a combination of oral and intranasal steroids (n = 1).26 Patients were followed between 1 and 15 months with a patient-weighted average follow-up of 6 months.
Quality assessment measures were evaluated for the case series as described by Chambers et al.30 Using these criteria two studies were given a rating of “good,”24,28 three studies were “satisfactory,”22,25,26 and two were given a rating of “poor,”23,27 either because of a retrospective methodology or a >10% patient dropout rate.
Symptom Scores
The primary outcome of interest was the impact of LTAs on symptom scores. Both placebo-controlled RCTs showed a significant improvement (p < 0.01) in nasal symptom scores over the 4- to 6-week course of treatment with no significant change seen from baseline scores in the placebo groups.17,18 Schäper et al. also noted that the order of the crossover, either placebo or LTA first, did not change the outcome or significance. We attempted to pool these results via meta-analysis; however, the necessary statistical data required for analysis were not available from the publications, and attempts to directly contact the authors to obtain this information were unsuccessful.
The two RCTs that examined montelukast versus INCSs in the postoperative period showed significant improvement in symptoms compared with baseline in the LTA arm.19,20 However, the degree of improvement with LTAs was not statistically different from that seen with INCSs. Vuralkan et al. reported a significant (p < 0.05) difference favoring LTAs over INCSs in subjective assessment of smell, and Mostafa et al. established the symptoms most improved by montelukast were headache, postnasal drip, and nasal pruritus. When these data were pooled, the SMD calculated was 0.02 (95% confidence interval, −0.39–0.44; I2 = 0%). With the currently accepted rubric of an SMD of 0.2 standard deviation units being considered a small, 0.5 a moderate, and 0.8 a large difference between groups,31 an SMD of 0.02 suggests no significant difference between the treatment groups with regard to overall symptom scores (Fig. 3).
Figure 3.
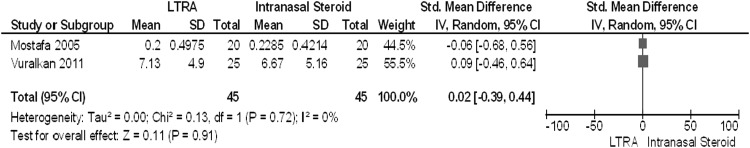
Meta-analysis of leukotriene antagonist (LTA) versus intranasal corticosteroid (INCS) treatments in chronic rhinosinusitis with nasal polyposis (CRSwNP). Forest plot of pooled analyses of symptom scores in randomized control trials (RCTs) of CRSwNP patients treated with montelukast versus intranasal steroids.
The remaining clinical trial randomized patients to either INCS or INCS plus montelukast after 2 weeks of oral prednisolone.21 After randomization there were significantly more patients in the montelukast arm with atopy (p = 0.04) as well as a trend toward more patients who had prior surgery (p = 0.07). Both groups showed improvements in all symptom score categories over the 8-week course of treatment, but the group receiving montelukast plus INCS showed a significantly greater reduction in symptom scores at 8 weeks with respect to headache (p = 0.013), facial pain (p = 0.048), and sneezing (p = 0.03). The overall symptom score was not significantly different between the two treatment groups at 8 or 12 weeks, although in all categories the symptoms scores were markedly higher at baseline in the montelukast plus INCS arm, and trended back toward these baseline scores after discontinuing treatment.
Of the seven case series, four reported symptom scores as outcome measures.22,25,27,28 Overall, these series report improvement in some symptom domains with variable responses among patients. Two series25,27 with a total of 51 patients taking either zafirlukast or zileuton over an average of 7.2 months showed significant improvement (p < 0.05) in overall symptom scores, with Parnes et al. attributing the majority of the contribution to improvements in facial pain, dentalgia, headache, ear pain, purulent rhinorrhea, postnasal drip, nasal congestion, nasal obstruction, olfaction, and fever. Subjective improvement was noted in 38 of the 51 patients. The remaining two series22,28 focused on 49 patients treated with montelukast plus INCS for 3–6 months. Overall symptom score was significantly (p < 0.001) improved in the study by Kieff et al., whereas Yazici et al. analyzed individual symptom domains to show a significant improvement in the Rhinosinusitis Disability Index physical (p = 0.003) and visual analog (p = 0.043) scores only. One case series23 that interviewed patients 1 year after starting montelukast after a course of oral methylprednisolone reported a subjective improvement in symptoms in seven of nine patients.
Objective Clinical Outcomes
Of the five RCTs, three reported objective clinical outcome measures for sinus disease.17–19 Significant improvements were seen in exam score (p < 0.05), rhinomanometry (p < 0.01), and olfactometry (p < 0.001) in a placebo-controlled study.17 Although endoscopic staging failed to significantly improve (p > 0.05) in a placebo-controlled trial,18 a separate RCT observed a substantial decrease in computed tomography scores after montelukast treatment (p < 0.05) that was equivalent to the improvement shown after INCS treatment (p > 0.05).19
Case series using a variety of LTAs alone or in combination with INCSs have generally shown improvement in radiographic and endoscopic outcomes in 50–66% of patients.23,24,27,28 The combination of oral and INCSs with montelukast showed a significant improvement (p < 0.01) in polyp score only in aspirin-tolerant asthma (ATA) patients, as opposed to those with AERD, with no significant changes in acoustic rhinometry or nasal nitric oxide in either group.26
Immune Parameters
One placebo-controlled RCT found significantly (p < 0.05) decreased inflammatory mediators in nasal lavage fluid, including substance P, neurokinin A, circulating intercellular adhesion molecule 1 eosinophil cationic protein, and cysteinyl-LTs, as well as a reduction in local and peripheral eosinophil levels.17 Case series using the combination of montelukast and INCSs have shown decreased eosinophils both peripherally24 and in nasal polyp biopsy specimens.22
Recurrence
Two RCTs reported information on recurrence in patients treated with either montelukast or INCSs.19,20 Vuralkan et al. showed significantly (p < 0.05) higher recurrence (48%) at 6 months in LTA alone versus INCS treatment (20%). It was also noted that there were significantly (p < 0.05) higher levels of polyp eosinophils in patients who experienced recurrence. Mostafa et al. did not report a rate, but stated that there was no difference in recurrence between the montelukast and INCS groups after 1 year.
Stewart et al. stated that two patients, who had been improving with montelukast treatment after a 12-day course of oral methylprednisolone, experienced a recurrence of nasal polyps 4–8 weeks after discontinuing treatment.
Allergy, Asthma, and Aspirin Tolerance
Although two RCTs17,21 and four case series22,24,26,28 commented on allergy testing in the patient population, only one case series randomized treatment groups by atopic status.22 In this study, patients with positive allergy testing showed significant improvement in symptom scores (p < 0.001) and local eosinophil levels (p < 0.02) after a 3-month trial of montelukast plus INCS. Symptom scores in patients without allergies did not reach significant levels (p = 0.07).
Five case series24–28 and all but one RCT17–19,21 had some patients with asthma enrolled in the study. In the RCTs, asthma patients were divided evenly between the treatment and placebo or INCS alone arms, and no difference in outcomes based on asthma status was mentioned. The RCT that specifically omitted asthma patients still indicated significant improvement in symptom scores with LTA treatment.20 Two case series only included patients with CRSwNP and asthma,24,26 and another noted that there were no significant differences in symptom score or polyposis between patients with or without asthma.25
AERD was also accounted for and randomized between LTA, placebo, and INCS alone groups in three RCTs.17,19,21 Of the five case series with asthma patients, four also had a subset of patients with AERD.24–26,28 Ulualp et al. solely studied CRSwNP in AERD patients with significant (p < 0.01) improvement seen in symptom scores, subjective assessment of disease, and endoscopic exams. Conversely, Ragab et al. examined the differences in outcomes between AERD and ATA patients treated for 3 months with montelukast and intranasal and oral steroids. In this study, patients with ATA showed a significant (p < 0.01) improvement in polyp score that was not observed in those with AERD.
DISCUSSION
This systematic review has identified two randomized trials providing evidence that montelukast improves symptoms, objective clinical measurements, and immune profiles of patients with CRSwNP when compared with placebo. Meta-analysis of pooled RCT data comparing montelukast treatment to INCS failed to show a difference in CRS symptoms. Although the use of SMD is highly prone to interobserver variation and potential errors regarding data selection,32 this analysis was limited to only two studies and the overall results were consistent with the individual conclusions. When focusing on specific symptoms montelukast provided an additional improvement over INCS in nasal pruritus, postnasal drip, headache, facial pain, sneezing, and smell disturbance. However, combination treatment with LTA plus INCS showed little overall symptom improvement compared with either treatment alone. There is some evidence to suggest that recurrence rates may be higher with montelukast treatment alone compared with INCS, but this result was not duplicated by other studies and may have been a consequence of randomization error with regard to severity of polyp eosinophilia. Although a daily dose of 10 mg of montelukast provides a consistent benefit over long periods, the therapeutic gains are lost shortly after discontinuation, as suggested by studies of its use on other disease states.33–36
Allergic rhinitis and asthma, diseases that are known to be improved by LTAs,8–10 often coexist with CRSwNP, and thus must be considered when assessing the efficacy of treatment. In most trials not specifically addressing the effects of LTAs on atopy or asthma these conditions were randomized between the treatment groups or sufficiently addressed as a patient subset so as to balance the effects of these conditions on patient outcomes. Patients with CRSwNP and concomitant asthma or atopy are theoretically more responsive to treatment with LTAs. This is supported by the decrease in local polyp and systemic eosinophils observed after montelukast treatment, because eosinophil levels are closely linked to disease severity in asthma, allergies, and CRSwNP.37–41 Improvement in atopic CRSwNP in contrast to nonatopic CRSwNP is suggested by Kieff, who reported that atopic patients experienced significant improvements in symptoms and polyp eosinophils after combination montelukast plus INCS, whereas their nonatopic counterparts did not. However, the nonatopic subset of patients was smaller and did experience noticeable improvement in symptoms and eosinophil levels (p = 0.07). It is possible that there was simply insufficient power to reach significance in this group. The presence or absence of asthma seems to have no bearing on the efficacy of LTA treatment for CRSwNP-specific outcomes, because improvement was seen regardless of whether or not asthma patients were included in the study. However, it is still likely that LTAs improve asthma and allergic rhinitis–specific symptoms, and thus could prove useful in improving the health and quality of life in populations with comorbid atopy and/or asthma. One series limited to patients with AERD documented significant improvements in symptoms and exam scores, whereas another noted improvement in only 50% of AERD patients that was not statistically significant. Those with ATA were also documented to have significant improvements in polyp size and symptoms, suggesting that aspirin sensitivity may not be a central factor when considering LTA therapy.
Several LTAs exist that could theoretically have different efficacies in CRSwNP. Unfortunately, the question as to which type of LTA would achieve maximal effectiveness in CRSwNP has received little attention. All but two of the studies reviewed used montelukast. Two case series included a substitute LTA, zafirlukast, as well as the 5-lipooxygenase inhibitor zileuton. One of these studies did not distinguish between their use with respect to outcomes and reported similar improvements in symptom and clinical exam measures as studies that focused on montelukast use. The other compared symptom scores after zafirlukast or zileuton treatment and reported significant improvement in ∼70% of patients regardless of the LTA used. It is also worth noting that in this series no attempts were made to regulate the prescription or use of additional medications such as local or systemic steroids and antihistamines, thus limiting the effectiveness of this comparison.
The question remains as to where LTAs fit into the management strategy for patients with CRSwNP. LTAs could be considered in patients with CRSwNP unable to tolerate topical steroids as they are shown to be superior to placebo in two different RCTs. Four of the five studies that evaluated LTAs as an adjunct to the current standard of care, INCS, were case series that lacked an examination of INCS treatment alone, thus limiting the strength of conclusions drawn on this subject. Future RCTs focused on the use of LTAs as add-on therapy to topical steroids would provide the most potentially relevant clinical information and could contribute to future modifications of treatment guidelines. Although atopic and asthmatic status may not affect CRSwNP-specific outcomes, LTAs may be indicated in these patients for treatment of allergic rhinitis and asthma specifically. Future clinical trials should focus on whether LTAs provide additional benefit above and beyond topical steroids alone and whether specific LTAs provide improved efficacy compared with others.
CONCLUSIONS
LTAs are superior to placebo in improving patient symptoms of CRS, although generally noncontributory, except in a small subset of sinus symptoms, as an adjunctive measure. LTA treatment in patients with CRSwNP results in measurable improvements in severity of symptoms, polyp size, and various immunologic parameters including eosinophil levels when compared with placebo. Effectiveness has been measured during the postoperative period, after a short course of oral steroids, in the chronic disease state, and in combination with INCSs. The greatest symptom improvements were observed in headache, facial pain, sneezing, nasal pruritus, postnasal drip, and smell disturbance. LTAs may provide additional benefit to INCS treatment with regard to these symptoms, but not in overall disease severity. Treatment outcomes appear unaffected by generalized asthma status, AERD, and atopy. Additional studies, particularly randomized trials, are necessary to elucidate the ideal combination of treatments and parameters for selecting patients to achieve the greatest benefit from LTAs in CRSwNP.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Hopkins C, Browne JP, Slack R, et al. The national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Clin Otolaryngol 31:390–398, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Joe S, Thambi R, Huang J. A systematic review of the use of intranasal steroids in the treatment of chronic rhinosinusitis. Otolaryngol Head Neck Surg 139:340–347, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Rudmik L, Schlosser RJ, Smith TL, Soler ZM. Impact of topical nasal steroid therapy on symptoms of nasal polyposis: A meta-analysis. Laryngoscope 122:1431–1437, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Howard B, Lal D. Oral steroid therapy in chronic rhinosinusitis with and without nasal polyposis. Curr Allergy Asthma Rep 13:236–243, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Baenkler H, Schafer D, Hosemann W. Eicosanoids from biopsy of normal and polypous nasal mucosa. Rhinology 34:166–170, 1996. [PubMed] [Google Scholar]
- 6. Chao S, Graham SM, Brown CL, et al. Cysteinyl leukotriene 1 receptor expression in nasal polyps. Ann Otol 115:394–397, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Holgate S, Peters-Golden M, Panettieri RA, et al. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J Allergy Clin Immunol 111:18–35, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Cao Y, Wang J, Bunjhoo H, et al. Comparison of leukotriene receptor antagonists in addition to inhaled corticosteroid and inhaled corticosteroid alone in the treatment of adolescents and adults with bronchial asthma: A meta-analysis. Asian Pac J Allergy Immunol 30:130–137, 2012. [PubMed] [Google Scholar]
- 9. Ciebiada M, Gorska-Ciebiada M, Barylski M, et al. Use of montelukast alone or in combination with desloratadine or levocetirizine in patients with persistent allergic rhinitis. Am J Rhinol Allergy 25:6, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Cingi C, Ozlugedik S. Effects of montelukast on quality of life in patients with persistent allergic rhinitis. Otolaryngol Head Neck Surg 142:654–658, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Moebus RG, Han JK. Immunomodulatory treatments for aspirin exacerbated respiratory disease. Am J Rhinol Allergy 26:134–140, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fokkens W, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps. Rhinology 50:1–329, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Desrosiers M, Evans GA, Keith PK, et al. Canadian clinical practice guidelines for acute and chronic rhinosinusitis. J Otolaryngol Head Neck Surg 40:S99–S193, 2011. [PubMed] [Google Scholar]
- 14. Shekelle P, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med 170:348–351, 1999. [PMC free article] [PubMed] [Google Scholar]
- 15. Review Manager (RevMan) [computer program]. Version 5.1 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011. [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. The PRISMA group preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 151:65–94, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Schaper C, Noga O, Koch B, et al. Anti-inflammatory properties of montelukast, a leukotriene receptor antagonist in patients with asthma and nasal polyposis. J Investig Allergol Clin Immunol 21:51–58, 2011. [PubMed] [Google Scholar]
- 18. Pauli C, Fintelmann R, Klemens C, et al. Polyposis nasi—Improvement in quality of life by the influence of leukotriene receptor antagonists. Laryngo-Rhino-Otol 86:282–286, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Vuralkan E, Saka C, Akin I, et al. Comparison of montelukast and mometasone furoate in the prevention of recurrent nasal polyps. Ther Adv Respir Dis 6:5–9, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Mostafa B, Hossam HA, Mohammed HE, et al. Role of leukotriene inhibitors in the postoperative management of nasal polyps. ORL J Otorhinolaryngol Relat Spec 67:148–153, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Stewart R, Ram B, Hamilton G, et al. Montelukast as an adjunct to oral and inhaled steroid therapy in chronic nasal polyposis. Otolaryngol Head Neck Surg 139:682–687, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Kieff D, Busaba NY. Efficacy of montelukast in the treatment of nasal polyposis. Ann Otol Rhinol Laryngol 114:941–945, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Kutting B, Nieschalk M, Brehler R. A new concept for treatment of sinonasal polyposis. Allergy 55:1091–1092, 2000. [DOI] [PubMed] [Google Scholar]
- 24. Nonaka M, Sakanushi A, Kusama K, et al. One-year evaluation of combined treatment with an intranasal corticosteroid and montelukast for chronic rhinosinusitis associated with asthma. J Nippon Med Sch 77:21–27, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Parnes S, Chuma AV. Acute effects of antileukotrienes on sinonasal polyposis and sinusitis. Ear Nose Throat J 79:18–21, 2000. [PubMed] [Google Scholar]
- 26. Ragab S, Parikh A, Darby C, et al. An open audit of montelukast, a leukotriene receptor antagonist, in nasal polyposis associated with asthma. Clin Exp Allergy 31:1385–1391, 2001. [DOI] [PubMed] [Google Scholar]
- 27. Ulualp S, Sterman BM, Toohill RJ. Antileukotriene therapy for the relief of sinus symptoms in aspirin triad disease. Ear Nose Throat J 78:604–610, 1999. [PubMed] [Google Scholar]
- 28. Yazici Z, Sayin I, Bozkurt E, et al. Effect of montelukast on quality of life in subjects with nasal polyposis accompanying bronchial asthma. Turkish J Ear Nose Throat 21:210–214, 2011. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:1–9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chambers DRM, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol 62:1253–1260, 2009. [DOI] [PubMed] [Google Scholar]
- 31. Cohen J. The use of tables for significance testing. In Statisical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum, 66–74, 1988. [Google Scholar]
- 32. Gotzsche PC, Hrobjartsson A, Maric K, Tendal B. Data extraction errors in meta-analyses that use standardized mean differences. JAMA 298:430–437, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Leff J, Busse WW, Pearlman D, et al. Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med 339:147–152, 1998. [DOI] [PubMed] [Google Scholar]
- 34. Altman L, Munk Z, Seltzer J, et al. A placebo-controlled, dose-ranging study of montelukast, a cysteinyl leukotrine-receptor antagonist. Montelukast Asthma Study Group. J Allergy Clin Immunol 102:50–55, 1998. [DOI] [PubMed] [Google Scholar]
- 35. Reiss T, Chervinsky P, Dockhorn RJ, et al. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma; a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch Intern Med 158:1213–1220, 1998. [DOI] [PubMed] [Google Scholar]
- 36. Noonan M, Chervinsky P, Brandon M, et al. Montelukast, a potent leukotriene receptor antagonist, causes dose-related improvements in chronic asthma. Montelukast Asthma Study Group. Eur Respir J 11:1232–1239, 1998. [DOI] [PubMed] [Google Scholar]
- 37. Ulrik C. Peripheral eosinophil counts as a marker of disease activity in intrinsic and extrinsic asthma. Clin Exp Allergy 25:820–826, 1995. [DOI] [PubMed] [Google Scholar]
- 38. Petsky H, Cates CJ, Lasserson TJ, et al. A systematic review and meta-analysis: Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils). Thorax 67:199–207, 2012. [DOI] [PubMed] [Google Scholar]
- 39. Varney V, Jacobson MR, Sudderick RM, et al. Immunohisotology of the nasal mucosa following allergen-induced rhinitis. Identification of activated T lymphocytes, eosinophils, and neutrophils. Am Rev Respir Dis 146:170–175, 1992. [DOI] [PubMed] [Google Scholar]
- 40. Ahmadiafshar A, Taghiloo D, Esmailzadeh A, et al. Nasal eosinophilia as a marker for allergic rhinitis: A controlled study of 50 patients. Ear Nose Throat J 91:122–124, 2012. [DOI] [PubMed] [Google Scholar]
- 41. Schmid C, Habermann W, Braun H, et al. Released intranasal eosinophilic major basic protein as a diagnostic marker for polypoid chronic rhinosinusitis. Otolaryngol Head Neck Surg 143:386–391, 2010. [DOI] [PubMed] [Google Scholar]