Abstract
Rationale
The reinforcing effects of cocaine are mediated by the mesolimbic dopamine system. Behavioral and neurochemical studies have shown that the cholinergic muscarinic M4 receptor subtype plays an important role in regulation of dopaminergic neurotransmission.
Objectives
Here we investigated for the first time the involvement of M4 receptors in the reinforcing effects of cocaine using chronic intravenous cocaine self-administration in extensively backcrossed M4 receptor knockout (M4−/−) mice.
Methods
We evaluated acquisition of cocaine self-administration in experimentally naïve mice. Both cocaine self-administration and food-maintained operant behavior were evaluated under fixed ratio 1 (FR 1) and progressive ratio (PR) schedules of reinforcement. In addition, cocaine-induced dopamine release and cocaine-induced hyperactivity was evaluated.
Results
M4−/− mice earned significantly more cocaine reinforcers and reached higher breaking points than their wildtype littermates (M4+/+) at intermediate doses of cocaine under both FR 1 and PR schedules of reinforcement. Under the PR schedule, M4−/− mice exhibited significantly higher response rates at the lowest liquid food concentration. In accordance with these results, cocaine-induced dopamine efflux in the nucleus accumbens and hyperlocomotion were increased in M4−/− mice compared to M4+/+ mice.
Conclusions
Our data suggest that M4 receptors play an important role in regulation of the reward circuitry and may serve as a new target in the medical treatment of drug addiction.
Keywords: Acetylcholine, muscarinic, M4, knockout, self-administration, cocaine
Introduction
The activity of midbrain dopaminergic neurons projecting to the nucleus accumbens (NAc) is believed to mediate the reinforcing effects of drugs of abuse (Koob 1992; Koob et al. 1998; Wise 1996). The M4 muscarinic receptor, one of the five muscarinic acetylcholine receptor subtypes (M1-M5), is highly expressed in the central nervous system, particularly in the striatum and also, at lower levels, in cerebral cortex, hippocampus and midbrain (Levey 1993; Sugaya et al.1997; Vilaro et al. 1993; Yasuda et al. 1993). In the striatum, M4 receptors are co-expressed with D1 dopamine receptors on striatal projection neurons (Weiner et al. 1990). M4 receptors are structurally closely related to M2 receptors, both couple to G proteins of the Gi/Go family and inhibit adenylyl cyclase, thereby reducing cAMP formation (Olianas et al. 1996). The precise functional roles of the individual mAChR subtypes have remained elusive due to their overlapping expression pattern and the lack of subtype specific ligands. To overcome these obstacles, muscarinic receptor knockout mice have been developed (Wess et al. 2007). Gomeza et al. (1999) showed that M4 receptor knockout (M4−/−) mice display increased basal locomotor activity and increased hyperlocomotion induced by the nonselective dopamine agonist apomorphine and by the selective dopamine D1 receptor agonist SKF 38393. Furthermore, M4−/− mice displayed enhanced striatal dopamine efflux in response to amphetamine and phencyclidine (PCP) (Tzavara et al. 2004). However, it has not been investigated whether the M4 receptor plays a role in the reinforcing effects of drugs of abuse.
In the present study, we investigated chronic cocaine self-administration in M4−/− and control mice (M4+/+) under a broad range of conditions in experimentally naïve mice. The effects of cocaine at various doses were determined under a fixed ratio 1 (FR 1) and a progressive ratio (PR) schedule of reinforcement. Food-maintained behavior was also evaluated to assess general operant performance under FR 1 and PR schedules. Furthermore, we tested the effects of cocaine on extracellular levels of dopamine in NAc using in vivo microdialysis as well as on locomotor activity in M4−/− and M4+/+ mice. To ensure that the obtained results were not caused by basal behavioral phenotype differences or changes in dopamine D1 or D2 receptor expression, we performed the SHIRPA behavioral phenotype assessment as well as dopamine D1 and D2 receptor autoradiography.
Materials and Methods
Animals
M4−/− mice were generated as previously described (Gomeza et al. 1999). Founder mice of mixed genetic background (129SvEv/CF1) were backcrossed to the C57BL/6Ntac strain for 11 generations. M4−/− mice, heterozygotes (M4+/−) and wildtype littermates (M4+/+) were bred at the animal facilities at the Panum Institute, University of Copenhagen. Genotyping was performed on mouse-tail DNA using the Polymerase Chain Reaction (PCR). Mice were acclimatized to the animal facilities, where experiments were conducted, for at least one week prior to experiments. We used group-housed (macrolon type III cages) male mice (8-14 weeks, 22-32.5 g at the start of experiments) kept on a 12-h light/dark cycle in a temperature (22-24°C) and humidity (55%) controlled room. Food and water was available ad libitum, except in cocaine self-administration experiments, where food was available ad libitum until operant training started and was then delivered once daily after self-administration sessions (~3.7 g/d per mouse). Experiments were conducted during the light-phase (8.00 am – 6.00 pm). All procedures were conducted in accordance with guidelines from the Animal Experimentation Inspectorate, Denmark and the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Drugs used for behavioral studies
Cocaine hydrochloride was obtained from the Copenhagen University Hospital-Pharmacy. Cocaine hydrochloride was dissolved or diluted in saline, and solutions were adjusted to a pH range of 4.5–7.0 for subcutaneous or intraveneous injections (10 ml/kg).
Chronic self-administration
Equipment, training and evaluation procedures were previously described (Caine et al. 1999; Thomsen and Caine 2005; Thomsen et al. 2005).
Operant chambers
Operant chambers (Med Associates, USA) contained two nose-poke holes 10 mm above the grid floor, both equipped with photocells and a discriminative cue light, positioned on either side of a small dish-shaped plate into which liquid food could be delivered. Responding in the right hole resulted in delivery of a reinforcer and illumination of the cue light for 20 s, during which additional responses were counted but had no scheduled consequences (i.e., post-reinforcer timeout). Early, unpublished observations in rodents in our laboratory (US location) indicated that the left side (which is furthest from the chamber door) was the preferred side. Consequently, we used the right (expected least preferred) side as the reinforced manipulandum.
Catheter implantation surgery and maintenance
Under oxygen/isoflurane vapor anesthesia a catheter (SILASTIC tubing, 0.18 mm inner diameter, 0.41 mm outer diameter) was inserted 1.2 cm into the right or left jugular vein and anchored to the vein with sutures. The catheter ran subcutaneously to the base located above the midscapular region. During the subsequent 7 days of postsurgical recovery, 0.02 ml of 0.9% saline containing heparin (30 U/ml, SAD, Denmark) and antibiotic (cefazolin, 50 mg/ml, Hexal, Germany) was infused daily through the catheter to prevent clotting and infection. Catheter patency was confirmed 7 days after surgery and after completion of each experimental phase by the loss of muscle tone and clear signs of anesthesia within 3 s after infusion of 0.02-0.03 ml ketamine (15 mg/ml, Pfizer, Denmark) plus midazolam (0.75 mg/ml, Matrix Pharmaceuticals, Denmark) in saline.
Acquisition of cocaine self-administration behavior in experimentally naïve mice
After catheter implantation and recovery, experimentally naïve mice were introduced to the operant chamber with one active (right) and one inactive (left) nose-poke hole for daily 3 h sessions, 5-6 days/week and with one of three doses of intravenous cocaine (cocaine-HCl in 0.9% saline; Copenhagen University Hospital-Pharmacy, Denmark) as the reinforcer: 0.03, 0.32 or 1.0 mg/kg/infusion. Activation of the right nose-poke hole led to infusion of the reinforcer under a FR 1 schedule. Sessions started immediately before introducing the mice to the operant chambers and, as an exception to the other experiments, no non-contingent infusion was delivered. Mice were allowed to self-administer cocaine for 10 sessions or until acquisition criteria were met, whichever occurred first. These mice were never food-deprived. Criteria for acquisition of self-administration were: (1) ≥15 reinforcers earned per session for two of three consecutive sessions (“acquisition level”); (2) ≥70% responses in the active hole on the second day; (3) extinction of responding when saline was substituted for cocaine (<80% of the acquisition level); and (4) an increase in active responses to or above the acquisition level when cocaine was again made available after extinction. After acquisition, mice were allowed to self-administer 1.0 mg/kg/infusion of cocaine to stable levels before proceeding with the FR 1 or PR schedule.
Cocaine self-administration under a FR schedule
Cocaine (1.0 mg/kg/infusion) was available in daily 3 h sessions until baseline criteria were met (≥20 reinforcers earned, with ≤20% variation over two consecutive sessions and ≥70% responses in the active hole). In consecutive sessions, saline was substituted for cocaine until extinction criteria were met (<80% of the baseline responding for cocaine self-administration). Subsequently, dose-effect functions (saline, 0.03, 0.1, 0.3, 1.0, 3.2 mg/kg/infusion of cocaine) were determined for each mouse according to a Latin-square design. To prevent overdosing, total drug intake was limited to 30 mg/kg per session.
Liquid food self-administration under a FR schedule
Another set of experimentally naïve mice was used for self-administration of a non-drug reinforcer under FR and PR schedules. The mice were mildly food-deprived before the first presentation of liquid food (5 ml of Ensure Protein Drink, vanilla flavor, Abbott Laboratories, USA) in the operant chamber (i.e., ad libitum dry food in the home cage was removed 18-20 h before the session, water remained available). When ≥1.5 ml of the 5 ml available was consumed per 2 h session, mice were placed in the operant chamber with one active and one inactive nose-poke hole for daily 2 h sessions similar to cocaine FR 1 self-administration. Acquisition lasted for at least five consecutive sessions and until criteria were met (≥20 reinforcers earned, with ≤20% variation over two consecutive sessions and ≥70% responses in the active hole). Subsequently, water was substituted for at least three sessions and until responding was extinguished to <80% of food-maintained responding. Then a range of liquid food dilutions (water, 3, 10, 32 and 100%) was presented according to a Latin-square design, determined twice in each mouse.
Cocaine self-administration and liquid food maintained behavior under a PR schedule
After the FR 1 schedule, mice proceeded with cocaine self-administration under a PR schedule of reinforcement. Mice that had self-administered liquid food under a FR 1 schedule proceeded in a similar PR schedule with liquid food as the reinforcer. After stable responding under the FR 1 schedule maintained by cocaine (1.0 mg/kg/infusion) or undiluted food, an FR 3 schedule was used as a transition from the FR 1 schedule before introducing the PR schedule. For the PR schedule, the starting ratio was 3, and then increased by 0.115 log units after each reinforcer delivery (i.e., 3, 4, 6, 7, 10, 13, 16, 21, 28, 36…). The breaking point was defined as the step value associated with the last completed ratio (i.e., number of reinforcers earned) after a 60 min limited hold (i.e., period with no reinforcer earned). If a breaking point was not reached within 6 h, the session was terminated to prevent health hazard, and the last reached ratio was used. After stable baseline was achieved (two consecutive sessions with breaking points >5 and with <20% variation), saline or water was substituted until responding extinguished to ≤50% of the baseline breaking point. Cocaine dose-effect curves (0.03, 0.3, 1.0, 3.2 mg/kg/infusion cocaine) and liquid food concentration-effect curves (0, 3, 10, 32 and 100% food in water) were determined according to a Latin-square design, with each dose tested for two or three consecutive sessions (i.e., if the breaking points reached in the two first determinations varied by >20%, a third determination was made).
In vivo microdialysis studies
Before surgery the mice received an analgesic (Metacam, Boehringer Ingelheim, 5 mg/kg s.c.). Mice were then anesthetized using sevoflurane (Baxter) and placed in a stereotaxic frame equipped with a mouse adapter (Kopf). Intracerebral guide cannulae (CMA/7, CMA) were stereotaxically implanted into the brain to allow the positioning of the dialysis probe tip in the NAc (AP: +1.0 mm; ML: +0.8 mm; DV: −3.0 mm; Franklin and Paxinos 1997) and fixed in place with two anchor screws and dental cement. Animals were left to recover for at least 2 days. The correct placement of the probe was verified histologically at the end of the experiment. For in vivo microdialysis studies, a Ringer’s solution (147 mM NaCl, 4 mM KCl, and 2.3 mM CaCl2) was perfused at a rate of 1.2 μl/min through the probe (CMA 7, 1 mm, cut-off 6000 Daltons, CMA) and the microdialysis probe was slowly inserted into the brain through the guide cannulae. Following a 1 hr acclimatization period, 20 min samples were collected into a fraction collector. Time points were corrected for lag time of the perfusate from the microdialysis site to the probe outlet. Three samples were collected to establish dopamine baseline levels. Subsequently, mice were injected with physiological saline or cocaine (10 or 30 mg/kg, s.c.) in a between-subjects design, and nine additional samples were collected. Using swivel and bowl, the animals were able to move freely during the dialysis. The samples were stored at −80 °C until analysis with HPLC coupled to electrochemical detection. For measurement of dopamine levels, the collected dialysates (20 μl) were injected onto a Prodigy C18 HPLC column (YMC Europe) by a refrigerated microsampler system (717plus, Waters). Dopamine was separated by reverse phase liquid chromatography using a mobile phase consisting of 97% of 94.2 mM NaH2PO4, 0.98 mM octanesulfonic acid, 0.06 mM Na2EDTA, adjusted to pH 3.7 with 1 M phosphoric acid and 7% acetonitrile (v/v) at a flow rate of 0.25 ml/min. Electrochemical detection of dopamine was accomplished using a amperimetic detector with a glassy carbon electrode operating at + 0.7 V vs. Ag/AgCl reference electrode. Data were collected and analyzed using Empower software (Waters). The limit of detection (at a signal to noise ratio of 1:2) was 3±0.2 fmol/15 μl.
Locomotor activity measurements
Mice were injected subcutaneously with saline or cocaine (5, 10, and 30 mg/kg) and immediately transferred to activity test cages with a scant lining of fresh bedding. The test cages were situated in frames emitting 8 infrared lines 1.5 cm above the floor. The total number of beam breaks during a 1 hr observation period was measured with Photobeam Activity System-Home cage (Ellegaard systems) in a between-subjects design.
Behavioral phenotype assessment, primary screen (SHIRPA)
Behavioral and physical characteristics of experimentally naïve mice were assessed using the SHIRPA primary screen procedure derived from Irwin (1968), which comprises several measures covering various reflexes and basic sensorimotor functions (Lalonde et al., 2005; Rogers et al., 1997; Rogers et al., 1999). In brief, body weight and body length were measured and assessment of the mice began with observation of undisturbed behavior in a cylindrical clear Perspex viewing jar (14 cm x 10 cm in diameter) for 5 minutes. Here the following was evaluated: body position, spontaneous activity, respiration and tremor. The mice were then transferred to an arena (55 × 35 × 18 cm) for observation of transfer arousal, palpebral closure, piloerection, startle response, gait, pelvic elevation, tail elevation, touch-escape and positional passivity. The mice were then grabbed by the tail and removed from the arena, the absence or presence of trunk curl and limb grasping was recorded. The animal was then lowered towards a grid (20 × 36 cm, 9 mm in diameter) where visual acuity was evaluated. The mouse was allowed to grip the grid, a gentle backwards pull was applied and grip strength, body tone and reflexes of the ears, eyes and limbs were scored. Subsequently, the mice were hung by their forelegs on a wire (15 cm long, 1 mm in diameter) and behavior was scored. The mice were then restrained in a supine position to record skin color, heart rate, limb tone, abdominal tone, lacrimation, salivation and provoked biting. The screen was completed with the measurement of the righting reflex, contact righting reflex and negative geotaxis. Throughout the procedure incidences of abnormal behavior, fear, irritability, aggression or vocalization were recorded. Viewing jar and arena were thoroughly cleaned between each mouse.
Basal locomotor activity was measured in monitoring frames equipped with seven horizontal infrared light beams along the long axis of the frame placed 4.3 cm apart and 3.3 cm above the surface. Standard cages (macrolon type III) with a scant lining of fresh wood-chip bedding were placed in the monitoring frames and covered with Perspex tops with ventilation holes. The set-up was situated in a ventilated soundproof room with dimmed light settings. A computer program (YMOT16) recorded interruptions of the light beams as counts of photo beam breaks in minimum intervals of one minute. Basal locomotor activity was measured for 150 minutes.
Dopamine D1 and D2 family receptor autoradiography
For receptor autoradiography, M4−/− and M4+/+ mice were sedated with CO2 and decapitated, the brains were dissected and immersed in –40°C isopentane for 1 min and stored at –80°C until sectioning. Using a cryostat, coronal sections (15 μm) were obtained from caudate-putamen (CPu) and NAc (1.54 to 0.74 mm relative to bregma; Franklin and Paxinos 1997), ventral tegmental area/substantia nigra (VTA/SN) (–3.08 to –3.64 mm), and the prefrontal cortex (PFC) (2.80 to 2.10 mm). The sections were thaw- mounted onto Superfrost Plus microscope slides (Menzel-Gläser, Braunschweig, Germany), and stored at –80°C until use.
Autoradiographic detection of D1-family receptors
The brain sections were preincubated in a buffer containing 50 mM TRIS base, 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 20 nM MDL 100.907 (H. Lundbeck, Valby, Denmark) at 0°C for 15 min before being incubated with 3[H]SCH23390 (1.0 nM, PerkinElmer Danmark A/S, Hvidovre, Denmark, 85 Ci/mmol) for 1 h at 4°C in the same buffer, followed by two 5-min washes at 0°C in buffer. Nonspecific binding was determined in the presence of the D1-, D2-, and 5-HT2 receptor antagonist cis(Z)-flupentixol (10 μM, H. Lundbeck, Valby, Denmark). After being dipped in demineralized water, slides were dried and exposed to Kodak Biomax MR-films (Amersham Biosciences, Hillerød, Denmark). The films were exposed for 40 days (CPu/NAc and VTA/SN) or 60 days (PFC) in autoradiography cassettes at –20°C before being developed.
Autoradiographic detection of D2-family receptors
The brain sections were preincubated in a buffer containing 50 mM TRIS base, 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2 at 0°C for 15 min before being incubated with 3[H]raclopride (4 nM, PerkinElmer Danmark A/S, Hvidovre, Denmark, 60.1 Ci/mmol) for 1 h at 4°C in the same buffer, followed by two 30-min washes at 0°C in buffer. Nonspecific binding was determined in the presence of 10 μM sertindole (H. Lundbeck, Valby, Denmark), a D2-, 5-HT2-, and alpha1 receptor antagonist. Slides were further processed as described above. Exposure time was 40 days for both CPu/NAc and VTA/SN. Developed films were analyzed with a computer-assisted video densitometer (Scion-Image; Scion Corporation, Frederick, MD) using the standard curve generated from 3H standards (Amersham Biosciences, Hillerød, Denmark).
Data analysis
Cocaine-induced locomotor activity and microdialysis results were analyzed by two-way ANOVA followed by Bonferroni-corrected pair wise comparisons. Extracellular dopamine levels measured by microdialysis are represented as percentage increase relative to baseline levels (−40-0 min). The 20-80 min microdialysis intervals were analysed by two-way ANOVA followed by Student’s t-test. For cocaine-self-administration latencies to acquisition of self-administration were compared between genotypes using log-rank test. The number of sessions before criteria for different experimental phases was compared between genotypes using Student’s t-test. Cocaine dose-effect and food concentration-effect functions determined under the FR schedule were analyzed using mixed-model ANOVA with genotype as between-subjects variable and cocaine dose or food concentration as within-subjects variables. PR schedule data were analyzed using two-way ANOVA with genotype and cocaine dose or food concentration as between-subjects variables. Significant effects were followed with post-hoc Student’s t-test. Animals with extreme values, defined as more than 3 interquartile ranges below or above the 25th and 75th percentile, respectively, were excluded from statistical analysis (SPSS Inc., 2004). This resulted in exclusion of one M4+/+ mouse from the FR 1 cocaine study. D1 and D2 receptor density was determined for each animal with a total of six measurements for each brain area (Each cerebral hemisphere in three sections). The mean of these six measurements was used for further statistical analysis for each animal and brain area. Two-tailed independent samples Student’s t-test was used to compare the M4−/− and M4+/+ mice. For the SHIRPA procedure, body weight, body length and spontaneous locomotor activity were analyzed using Student’s t-test. Scores of remaining parameters of the SHIRPA procedure were compared using Mann-Whitney U-test. Data are presented as mean ± SEM if not otherwise stated. A p value of < 0.05 was considered significant.
Results
Acquisition of cocaine self-administration behavior in experimentally naïve mice
Acquisition of cocaine self-administration behavior was examined in experimentally naïve mice that were introduced to the operant chamber with a dose of 0.03, 0.3 or 1.0 mg/kg/infusion of cocaine. No non-contingent infusion was delivered to these mice and they were tested until acquisition criteria were met or for 10 sessions, whichever occurred first. The mean number of sessions before criteria were met and the percentage of mice that met criteria within the 10 sessions are shown in figure 1 a and b. M4−/− and M4+/+ mice required similar number of sessions to meet acquisition criteria at all doses. Furthermore, comparison of acquisition with the log-rank test revealed no differences between the genotypes at any of the doses.
Fig 1.

Acquisition of cocaine self-administration behavior in experimentally naïve M4−/− mice (white) and M4+/+ mice (black). a: Number of sessions before acquisition criteria were met. b: Percentage of mice that met criteria for cocaine self-administration. Abscissas: Unit dose of cocaine (mg/kg/infusion). Ordinates: Sessions before criteria were met (a) and percentage of mice that acquired self-administration behavior (b). Group sizes: n = 8-9. The M4−/− mice did not differ from their wildtype littermates in acquisition of cocaine self-administration behavior.
Cocaine self-administration under FR 1 and PR schedules of reinforcement
Table 1 summarizes the number of sessions necessary before criteria were met for the different experimental phases of cocaine- self-administration and liquid food-maintained operant behavior under FR and PR schedules of reinforcement. Student’s t-test showed a significant difference between the number of sessions to extinction in M4+/+ and M4−/− mice under the FR 1 schedule (p < 0.01). M4−/− mice needed an average of 2.8 sessions more than M4+/+ mice before extinction criteria were met. No significant differences between the genotypes were found in any of the remaining measures. Figure 2 shows the cocaine dose-effect functions in M4+/+ and M4−/− mice under the FR 1 (a) and PR (b) schedule of reinforcement. Mixed model ANOVA of the dose-effect function for mice evaluated under the FR 1 schedule showed a significant effect of cocaine dose (F105,5 = 16.45; p < 0.0001) and a significant effect of genotype (F105,1 = 7.88; p < 0.05) with no genotype by dose interaction. Post-hoc analysis indicated that M4−/− mice responded with significantly more nose-poke activations at 0.3 mg/kg/infusion (p < 0.05) and 1.0 mg/kg/infusion (p < 0.001) of cocaine compared to M4+/+ mice. For both M4+/+ and M4−/− mice doses of 0.03-1.0 mg/kg/infusion of cocaine increased the response rate significantly compared to saline with a peak around 0.1 mg/kg/infusion of cocaine. The dose-effect curve was therefore shifted upward in the M4−/− mice relative to wildtype mice.
Table 1.
Mean number of sessions to criteria in M4+/+ and M4−/− mice. Data are group means ± SEM, with n in parentheses. Mice were trained with either cocaine or liquid food under an FR 1 schedule of reinforcement and subsequently under a PR schedule. M4−/− mice extinguished responding significantly slower than wildtype mice after meeting FR 1 acquisition criteria.
| Criteria | M4+/+ | M4−/− |
|---|---|---|
| Cocaine FR 1 baseline | 2.8 ± 0.5 (13) | 3.2 ± 0.6 (10) |
| Cocaine FR 1 extinction | 2.3 ± 0.3 (13) | 5.1 ± 0.8 (10)** |
| Cocaine PR baseline | 3.3 ± 0.6 (8) | 2.4 ± 0.3 (8) |
| Cocaine PR extinction | 3.9 ± 0.8 (8) | 2.9 ± 0.7 (8) |
| Liquid food FR 1 acquisition | 4.1 ± 0.5 (10) | 3.3 ± 0.4 (13) |
| Liquid food FR 1 extinction | 3.1 ± 0.5 (10) | 2.3 ± 0.3 (13) |
| Liquid food PR baseline | 2.6 ± 0.6 (10) | 2.2 ± 0.3 (9) |
| Liquid food PR extinction | 1.9 ± 0.5 (10) | 3.7 ± 0.8 (9) |
p<0.01 versus wildtype, Student’s t test.
Fig 2.
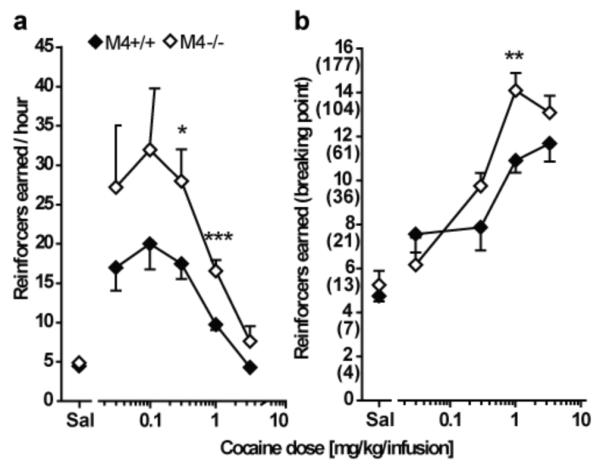
Intravenous cocaine self-administration under the FR 1 (a) and the PR (b) schedule of reinforcement in M4−/− mice (white) and wildtype littermates (black). Abscissas: Unit dose of cocaine in saline (mg/kg/infusion). Ordinates: Reinforcers earned under the FR 1 (reinforcers per hour, a) and under the PR (breaking point, b) schedule of reinforcement. The final ratio corresponding to each breaking point is indicated in parentheses for the PR schedule. Group means were calculated from the average for each mouse of two determinations per dose evaluated according to a Latin square design. Group sizes: FR 1 schedule: n = 10-13; PR schedule: n = 8. A significant main effect of genotype was found under both FR 1 and PR schedules of reinforcement. M4−/− mice exhibited higher response rates than wildtype mice at doses of 0.3 and 1.0 mg/kg/infusion under the FR 1 schedule. Under the PR schedule of reinforcement, M4−/− mice reached higher breaking points than wildtype mice at the 1.0 mg/kg per infusion dose. *p < 0.05, **p < 0.01, ***p < 0.001 vs. wildtype; post-hoc t-test following overall significant genotype effect in two-way ANOVA.
Under the PR schedule of reinforcement two-way ANOVA similarly showed significant effects of cocaine dose (F67,4 = 37.83; p < 0.0001) and of genotype (F67,1 = 5.16; p < 0.05) and no genotype by dose interaction. Post-hoc analysis revealed that M4−/− mice reached significantly higher breaking points than wildtype mice at the 1.0 mg/kg/infusion cocaine dose (p < 0.01). For M4+/+ mice doses of 0.03-3.2 mg/kg/infusion of cocaine and for M4−/− mice doses of 0.03-3.2 mg/kg/infusion of cocaine increased breaking points significantly compared to saline. Similar to self-administration under the FR 1 schedule, the dose-effect curve appeared shifted upward in the M4−/− mice relative to wildtype under the PR schedule of reinforcement.
Liquid food self-administration under FR 1 and PR schedules of reinforcement
Another set of experimentally naïve mice was used to evaluate self-administration behavior maintained with liquid food. Most M4+/+ and M4−/− mice met acquisition criteria within five sessions (M4+/+: 83.3%, M4−/−: 92.8%) and log-rank test revealed no effect of genotype. Furthermore, the number of sessions necessary to meet criteria for the different experimental phases (table 1) did not differ between M4+/+ and M4−/− mice in any of the measures.
Figure 3 shows the concentration-effect functions for M4+/+ and M4−/− mice under the FR 1 (a) and PR (b) schedules of reinforcement. Under the FR 1 schedule, mixed-model ANOVA revealed a significant effect of food concentration (F84,4 = 32.37; p < 0.0001), but no effect of genotype and no interaction. Post-hoc analysis of the food concentration effect revealed that response rates increased significantly with concentrations of 10-100% of liquid food compared to water. Under the PR schedule, two-way ANOVA showed significant effect of food concentration (F83,4 = 17.17; p < 0.0001) and significant effect of genotype (F83,1 = 16.94; p < 0.0001) with no genotype by food concentration interaction. Post-hoc analysis showed that M4−/− mice reached significantly higher breaking points at the 3% liquid food concentration compared to M4+/+ mice. Post-hoc analysis of the concentration effect revealed that all concentrations maintained higher breaking points than water in M4−/− mice while liquid food concentrations of 10-100% increased the breaking points above water in M4+/+ mice.
Fig 3.
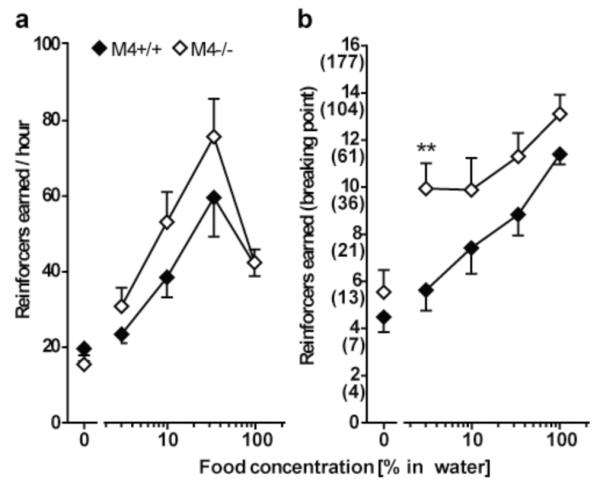
Food-maintained operant behavior under the FR 1 (a) and the PR (b) schedule of reinforcement in M4−/− mice (white) and wildtype littermates (black). Abscissas: Concentration of liquid food in water. Ordinates: Reinforcers earned under the FR 1 (reinforcers per hour, a) and under the PR (breaking point, b) schedule of reinforcement. The final ratio corresponding to each breaking point is indicated in parentheses for the PR schedule. Group means were calculated from the average for each mouse of two determinations per concentration evaluated according to a Latin square design. Group sizes: FR 1 schedule: n = 10-13; PR schedule: n = 9-10. The M4−/− mice did not differ from their wildtype littermates in food-maintained responding under the FR 1 schedule. Under the PR schedule of reinforcement M4−/− mice reached higher breaking points at a concentration of 3% liquid food. **p < 0.01 vs. wildtype; post-hoc t-test following overall significant genotype effect in two-way ANOVA.
Cocaine-induced dopamine release in NAc is enhanced in M4−/− mice
We used in vivo microdialysis to investigate whether extracellular dopamine levels were altered in the NAc of M4− /− mice, as compared to control littermates. Two-way mixed model ANOVA showed a significant effect of treatment (F5,290=29.6, p<0.001), time (F10,290=46.4, p<0.001) as well as interaction (F50,290=12.4, p<0.001) on extracellular dopamine level. Post hoc analysis showed that cocaine, 10 and 30 mg/kg induced a significant increase in extracellular dopamine in both genotypes compared to saline (F2,9=12.6; p=0.0047, F2,9=2,037; p<0.0001) (figure 4 insert). Cocaine 30 mg/kg resulted in a dramatic increase in extracellular dopamine in M4−/− mice as compared to M4+/+ mice (20-120 min, p<0.001-0.05) (figure 4). Following saline injections, no differences in extracellular dopamine levels were observed between the two genotypes. Microdialysate concentrations in saline treated M4−/− and M4+/+ mice were not significantly different (9.2±0.8 and 8.9±1.4 fmol/15 μL, respectively).
Fig 4.
Time course of extracellular NAc dopamine efflux in response to cocaine in M4−/− (white) and M4+/+ (black) mice, n=4-7. Cocaine 30 mg/kg (circle) significantly increased extracellular dopamine in M4−/− mice (samples 20 min to 140 min, p<0.001). This response was significantly enhanced in M4−/− mice compared to M4+/+ mice (***p<0.001, **p<0.01 and *p<0.05). Figure 4. Insert shows cocaine induced dopamine efflux as AUC from 20-80 min. Both genotypes; M4−/− and M4+/+ mice show both a significant dose- and genotype-dependent increase in dopamine efflux (***p<0.001 and **p<0.01).
Cocaine-induced hyperlocomotor responses are increased in M4−/− mice
We performed locomotor activity measurements following administration of cocaine (5-30 mg/kg, s.c.), a psychostimulant that increases synaptic dopamine levels by blocking the dopamine transporter. In both M4−/− mice and their wildtype littermates (M4+/+ mice), cocaine caused dose-dependent increases in locomotor activity (figure 5). Cocaine, 30 mg/kg induced a significantly greater locomotor response in M4−/− than in M4+/+ mice (p<0.05).
Fig 5.
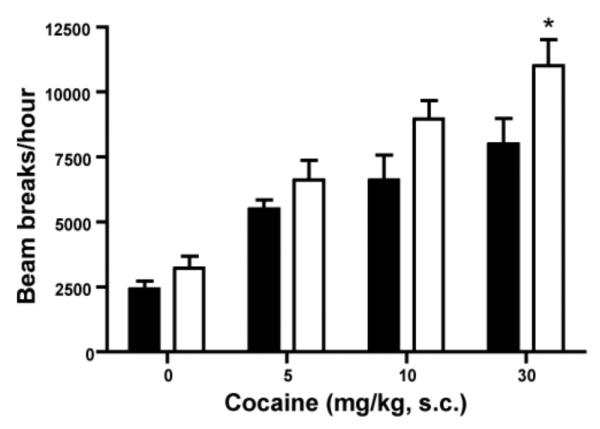
Effects of cocaine on locomotor activity in naive M4−/− mice (white) and M4+/+ mice (black). M4−/− mice and control littermates (n=5-10) were injected subcutaneously with the indicated doses of cocaine. Locomotor activity was assessed during a 1 h test period. *p<0.05 vs. control; post-hoc t-test following overall significant genotype effect in two-way ANOVA.
Basal behavioral testing (primary screen)
We subjected naïve M4−/− mice and control littermates to a series of basic tests examining reflexes and sensorimotor functions, using the SHIRPA primary screen procedure (Rogers et al. 1997, 1999). We found no significant differences between the two genotypes in any of the measures investigated (supplemental table 1).
D1 and D2 Receptor Autoradiography
Receptor autoradiography on D1 and D2 receptors was performed to investigate potential compensatory changes in the density of dopamine receptors induced by the knockout of the M4 receptor gene. The investigated brain areas were selected because of their involvement in dopaminergic projections of importance for locomotion and reward. These included the PFC, CPu, NAc, and VTA/SN. In addition, the olfactory tubercle was studied because of the high density of dopamine receptors in this area. However, D2 receptor binding was not measured in the PFC because this receptor is not detectable in this structure of the mouse brain (Camps et al. 1990). D2 receptor binding was also below detection limit in the VTA/SN region in the present study. No differences in D1 or D2 receptor binding were found in any of the analyzed brain regions between M4−/− and M4+/+ mice (Table 2 and figure 6).
Table 2.
D1 and D2 receptor autoradiography. Density of D1 and D2 receptors were assessed by autoradiography with 3[H]SCH23390 and 3[H]raclopride, respectively. D1 and D2 receptor densities were examined in the nucleus accumbens (NAc), caudate-putamen (CPu), olfactory tubercle (TO), ventral tegmental area/substantia nigra (VTA/SN) and prefrontal cortex (PFC). No significant differences were found between M4+/+ and M4−/− mice in any of the investigated brain areas. ND = Not determined. Data are group means ± SEM. N=6-7 animals per group.
| D1-like binding | D2-like binding | |||
|---|---|---|---|---|
| M4+/+ | M4−/− | M4+/+ | M4−/− | |
| NAc | 796.5 Bq/mg ± 51.0 |
867.6 Bq/mg ± 31.2 |
151.7 Bq/mg ± 10.6 |
139.9 Bq/mg ± 6.4 |
| CPu | 881.1 Bq/mg ± 33.1 |
954.5 Bq/mg ± 39.7 |
202.5 Bq/mg ± 17.9 |
186.3 Bq/mg ± 7.7 |
| TO | 1065.5 Bq/mg ± 77.7 |
1027.9 Bq/mg ± 48.7 |
124.5 Bq/mg ± 6.3 |
120.1 Bq/mg ± 4.6 |
| VTA/SN | 820.4 Bq/mg ± 125.0 |
739.0 Bq/mg ± 96.5 |
ND | ND |
| PFC | 179.6 Bq/mg ± 10.6 |
163.3 Bq/mg ± 8.1 |
ND | ND |
No significant differences were found between M4+/+ and M4−/−. ND = Not determined.
Fig 6.
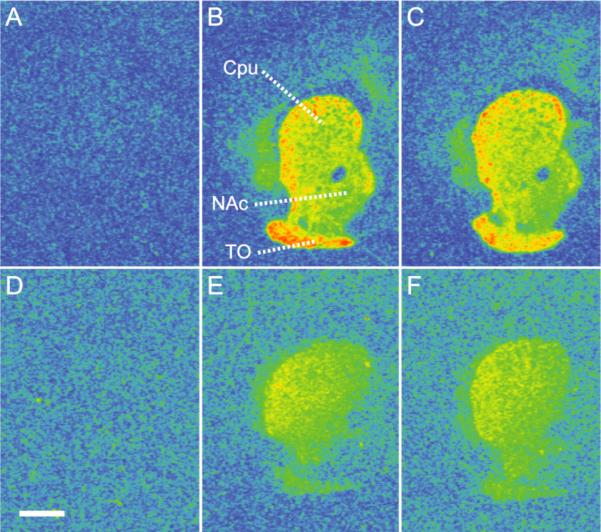
Pseudocolored autoradiograms showing 3[H]SCH23390 binding to D1 receptors (A-C) and 3[H]raclopride binding to D2 receptors (D-F) at the level of the caudate-putamen (CPu), nucleus accumbens (NAc) and olfactory tubercle (TO) (color order blue, green, yellow, and red indicate progressively increased binding levels, respectively). A: Non-specific binding in M4+/+ mouse brain. B: Total D1 binding in M4+/+ mouse brain. C: Total D1 binding in M4−/− mouse brain. D: Non-specific binding in M4+/+ mouse brain. E: Total D2 binding in M4+/+ mouse brain. F: Total D2 binding in M4−/− mouse brain. The density of D1 and D2 receptors did not differ between M4−/− and M4+/+ mice (see Table 2 for quantitative data). Scale bar = 1 mm.
Discussion
In the present study, we show for the first time that cocaine self-administration is increased in M4− /− mice compared to M4+/+ mice. This effect was observed at intermediate doses of cocaine under both a FR and a PR schedule of reinforcement. To support these findings we measured extracellular concentrations of dopamine in the NAc of M4−/− and M4+/+ mice using in vivo microdialysis . We found that the cocaine-induced increase in NAc dopamine levels was strongly augmented in M4−/− mice compared to M4+/+ mice. In agreement with these results we found that cocaine-induced hyperactivity was increased in M4−/− mice compared to M4+/+ mice. These differences could not be explained by changes in sensorimotor or behavioral phenotype or differences in D1 or D2 receptor density between the genotypes.
Cocaine self-administration dose-effect functions were investigated under a FR 1 schedule of reinforcement, and M4−/− mice showed increased response rates compared to M4+/+ mice at 0.3 and 1.0 mg/kg/infusion of cocaine and no difference in responses to lower and higher doses, indicating an upward shift of the dose-effect curve. Similarly, M4−/− mice reached higher breaking points at 1.0 mg/kg/perfusion of cocaine compared to M4+/+ mice and an upward shift in the dose-effect curve when investigated under the PR schedule. One concern could be that perseveration may have contributing to the increased responding in the M4−/− mice. However, we find this unlikely because responding, when saline was available, did not differ between genotypes. No differences in the ability to acquire self-administration were observed, as similar percentages of experimentally naïve M4−/− and M4+/+ mice met acquisition criteria and similar numbers of sessions were required for both genotypes to meet criteria at three doses of cocaine.
Upward shifts of the dose-effect function have been related to a phenotype with increased propensity to develop drug-addiction (Piazza et al. 2000) and taken together with the microdialysis data presented here, we propose increased reinforcing efficacy of cocaine in M4−/− mice.
The M4−/− mice required more sessions to extinguish self-administration behavior than M4+/+ mice after stable baseline had been achieved under the FR 1 schedule. This protracted extinction may further reflect increased reinforcing effects of cocaine in M4−/− mice. However, this difference in extinction rates was not observed throughout the other experimental phases, and the number of sessions to extinguish in the M4−/− mice was similar to that reported for wildtype C57BL/6J mice (Thomsen et al. 2005; Thomsen and Caine 2006). Thus it is equally possible that wildtype mice, in this particular phase, showed uncharacteristically fast extinction. Further studies aimed at investigating extinction of reinforced behaviors in M4−/− mice may be warranted.
With respect to food consumption M4−/− mice under the PR schedule exhibited significantly higher response rates at the lowest liquid food concentration and, food-maintained operant responding under a FR 1 schedule was higher, although not significant, in the M4−/− mice compared to the M4+/+ mice. No differences in acquisition and extinction of food-maintained behavior under either FR 1 or PR schedules were observed. Consequently, M4 receptor deletion may also change operant responding for food. However, these effects did not appear to affect body weight that was similar in the two genotypes. We observed no differences in sensorimotor and behavioral parameters between M4+/+ and M4−/− mice and we also found no significant differences between basal dopamine efflux in NAc in the two groups of mice.
M4 receptors are co-localized with D1 receptors on GABAergic projections to the midbrain of intact mice (Di Chiara et al. 1994), and the lack of M4 receptors co-localized with D1 receptors on GABAergic projections may indirectly activate dopamine firing (Tzavara et al. 2004). We have recently reported increased behavioral sensitization following treatment with cocaine in mice exclusively lacking M4 receptors co-localized with D1 receptors (Jeon et al. 2010). Taken together, we suggest that the M4 receptor inhibits the reinforcing properties of cocaine through actions on GABAergic medium spiny projection neurons, decreasing the input to dopaminergic projections to the NAc. Selective M4 receptor ligands are therefore an interesting target for the treatment of drug abuse. To this end, highly selective positive allosteric modulators of the M4 receptor have recently been developed (Brady et al. 2008; Chan et al. 2008; Shirey et al. 2008) and these were able to reverse amphetamine-induced hyperlocomotion in rats (Brady et al. 2008). Thus investigation of the effects these new allosteric modulators on cocaine self-administration are warranted.
Previous studies reported that M4−/− mice exhibit increased basal locomotor activity (Gomeza et al. 1999) and increased levels of extracellular dopamine in NAc (Tzavara et al. 2004). In the present study, no significant difference in basal locomotor activity between M4−/− and M4−/− mice was measured (figure 5). These apparent discrepancies could be due to differences in mouse genetic background. Gomeza et al. (1999) and Tzavara et al. (2004) used mice maintained on a mixed genetic background whereas the mice used in the present study were extensively backcrossed to the C57BL/6Tac strain in order to limit the contribution of genetic background (Gerlai 1996).
While it cannot be excluded that some compensatory changes occurred in the constitutive M4−/− mice that could have contributed to the observed effects, no such changes have been observed, to the extend that they have been investigated. Generally, knockout mice lacking either one of the 5 muscarinic receptor subtypes have not displayed measurable changes in the levels of the other subtypes (see Wess, 2004 for review). Specifically for M4−/− mice, M2 receptor brain and spinal cord expression levels were evaluated, and found unaltered (Gomeza et al. 1999; Chen et al, 2005). In addition, M1 and M2 receptor levels were normal in mice lacking M4 receptors in D1-expressing cells, in which total M4 expression was almost abolished (Jeon et al., 2010).
The observed difference in cocaine self-administration between M4−/− and control mice is most likely not due to changes in dopamine D1 or D2 receptor expression, since no significant differences in dopamine D1 and D2 receptor densities were observed in PFC, Cpu, NAc or VTA/SN and Cpu and NAc, respectively (dopamine D2 receptor levels could not be quantified in PFC and VTA/SN).
In conclusion, M4−/− mice exhibited upward shifts in cocaine self-administration dose-effect functions and increased responding for a food reinforcer. Exaggerated increases in extracellular dopamine in response to cocaine as well as increased cocaine-induced locomotor activation were also observed, suggesting increased abuse-related effects of cocaine. Our data suggest that the M4 receptor among other effects is involved in modulating the reward circuitry and may represent an interesting new target for the pharmacological treatment of cocaine addiction.
Supplementary Material
Acknowledgements
The Ivan Nielsen Foundation and the Lundbeck Foundation supported the present work. We thank Pernille Clausen for expert technical assistance.
Footnotes
Disclosure/Conflict of interest:
All the authors declare that they have nothing to disclose and no conflicts of interest.
References
- Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ, Lindsley CW. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008;327(3):941–53. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology (Berl) 1999;147:22–24. doi: 10.1007/s002130051134. [DOI] [PubMed] [Google Scholar]
- Camps M, Kelly PH, Palacios JM. Autoradiographic localization of dopamine D1 and D2 receptors in the brain of several mammalian species. J Neuronal Transm Gen Sect. 1990;80:105–127. doi: 10.1007/BF01257077. [DOI] [PubMed] [Google Scholar]
- Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos Lazareno S, Birdsall NJM, Bymaster FP, Felder CC. Allosteric modulation of the muscarinic M4 receptor as an approach to treating Schizophrenia. PNAS. 2008;105:10978–83. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Franklin KJB, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; New York: 1997. [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: Is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng CX, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wörtwein G, Woldbye DPD, Cui Y, Davis AA, Levey AI, Schütz G, Sager TN, Mørk A, Li C, Deng C, Fink-Jensen A, Wess J. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulation dopamine-dependent behaviours. J Neurosci. 2010;30(6):2396–2405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drugs of Abuse - Anatomy, Pharmacology and Function of Reward Pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Dumont M, Staufenbiel M, Strazielle C. Neurobehavioral characterization of APP23 transgenic mice with the SHIRPA primary screen. Behav Brain Res. 2005;157:91–98. doi: 10.1016/j.bbr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Levey AI. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- Olianas MC, Adem A, Karlsson E, Onali P. Rat striatal muscarinic receptors coupled to the inhibition of adenylyl cyclase activity: potent block by the selective m4 ligand muscarinic toxin 3 (MT3) Br J Pharmacol. 1996;118:283–288. doi: 10.1111/j.1476-5381.1996.tb15400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaaza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20(11):4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8:711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Jones DN, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Shirey JK, Xiang Z, Orton D, Brady AE, Johnson KA, Williams R, Ayala JE, Rodriguez AL, Wess J, Weaver D, Niswender CM, Conn PJ. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat Chem Biol. 2008;4:42–50. doi: 10.1038/nchembio.2007.55. [DOI] [PubMed] [Google Scholar]
- SPSS Inc . SPSS Base version 13.0 User Manual. SPSS Inc; Chicago: 2004. [Google Scholar]
- Sugaya K, Clamp C, Bryan D, McKinney M. mRNA for the m4 muscarinic receptor subtype is expressed in adult rat brain cholinergic neurons. Brain Res Mol Brain Res. 1997;50:305–313. doi: 10.1016/s0169-328x(97)00199-x. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci. 2005 doi: 10.1002/0471142301.ns0920s32. Chapter 9: Unit 9.20. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Cocaine self-administration under fixed and progressive ratio schedules of reinforcement: comparison of C57BL/6J, 129X1/SvJ, and 129S6/SvEvTac inbred mice. Psychopharmacology (Berl) 2006;184:145–154. doi: 10.1007/s00213-005-0207-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–8149. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, McKinzie DL, Felder C, Nomikos GG. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- Vilaro MT, Mengod G, Palacios JM. Advances and limitations of the molecular neuroanatomy of cholinergic receptors: the example of multiple muscarinic receptors. Prog Brain Res. 1993;98:95–101. doi: 10.1016/s0079-6123(08)62385-7. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of Muscarinic Acetylcholine and Dopamine Receptor Messenger-RNAs in Rat Basal Ganglia. Proc Natl Acad Sci U S A. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB. Development of Antisera Selective for M4 and M5 Muscarinic Cholinergic Receptors - Distribution of M4 and M5 Receptors in Rat-Brain. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



