Abstract
Coupling between bone formation and bone resorption refers to the process within basic multicellular units in which resorption by osteoclasts is met by the generation of osteoblasts from precursors, and their bone-forming activity, which needs to be sufficient to replace the bone lost. There are many sources of activities that contribute to coupling at remodeling sites, including growth factors released from the matrix, soluble and membrane products of osteoclasts and their precursors, signals from osteocytes and from immune cells and signaling taking place within the osteoblast lineage. Coupling is therefore a process that involves the interaction of a wide range of cell types and control mechanisms. As bone remodeling occurs at many sites asynchronously throughout the skeleton, locally generated activities comprise very important control mechanisms. In this review, we explore the potential roles of a number of these factors, including sphingosine-1-phosphate, semaphorins, ephrins, interleukin-6 (IL-6) family cytokines and marrow-derived factors. Their interactions achieve the essential tight control of coupling within individual remodeling units that is required for control of skeletal mass.
Introduction
Generation and maintenance of the shape of bone during skeletal growth depends on bone modeling, which lasts from the beginning of skeletal development in fetal life until the end of the second decade when longitudinal growth of the skeleton is completed. Modeling differs from remodeling, in that bone is formed at sites that have not undergone prior resorption, thus resulting in a change in the shape or macroarchitecture of the bone. The modeling effects on the size and shape of the bone dictate the simultaneous widening of long bones and development of the medullary cavity by bone formation at the periosteal surface and resorption at the endosteal surface, respectively.
In the bone remodeling process that occurs throughout life, on the other hand, small packets of bone are resorbed by osteoclasts, and this is followed by the recruitment of osteoblast precursors that differentiate and replace the amount of removed bone. The remodeling process takes place asynchronously throughout the skeleton at anatomically distinct sites termed basic multicellular units (BMUs).1 The resorption activity in a BMU in adult human bone takes approximately 3 weeks and the formation response 3 to 4 months. The process is such that remodeling replaces about 5–10% of the skeleton each year, with the entire adult human skeleton replaced in 10 years.2 The remodeling process is an integral part of the calcium homeostatic system and provides a crucial mechanism for adaptation to physical stress, the removal of old bone and the repair of damaged bone. It is thus central to the maintenance of the mechanical integrity of the skeleton and the repair of damaged bone.1,3,4,5
Bone remodeling
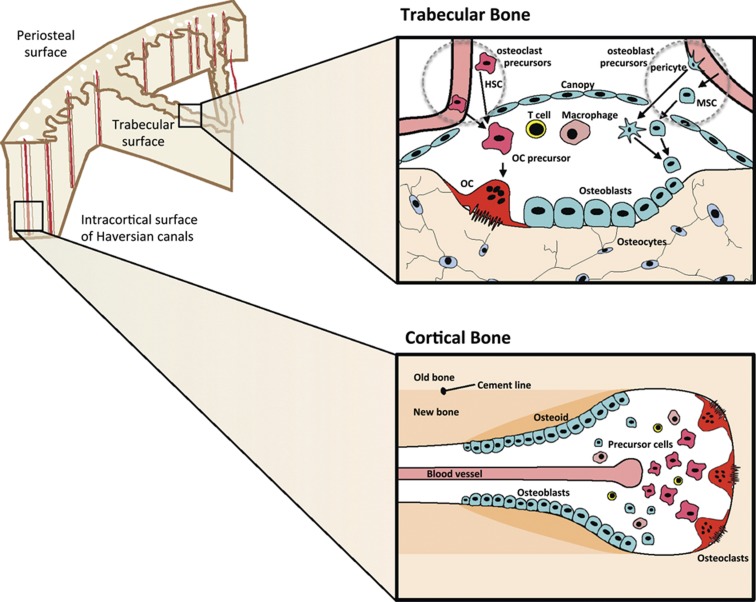
Tight control of bone remodeling at the level of the BMU throughout the skeleton is essential to maintain structural integrity. The development of concepts in this area owes much to the work of Harold Frost. In the 1960s Frost examined multiple sections through human cortical bone, identifying the scalloped contours of Howship's lacunae as sites of resorption by osteoclasts.6 The BMUs in cortical and trabecular bone differ greatly in their structures and the ways in which they remove and replace bone. In trabecular bone the BMU is located on the surface and becomes covered by a canopy predominantly of mesenchymal cell origin (v infra), with osteoclasts resorbing an amount of bone. The resorbed surface is cleaned up by lining cells and probably macrophages,7 and osteoblast precursors differentiate to fill the space that has been resorbed (Figure 1). The BMU in the cortex comprises a cutting zone led by osteoclasts that proceed through bone followed by differentiating osteoblasts, and with the space filled by blood vessels, nerves and connective tissue (Figure 1). Frost pointed out that, in remodeling, completed sites of bone formation can be recognized by cement lines that follow the scallop-shaped surfaces that were scalloped by osteoclasts. On the other hand, smooth cement lines indicated that formation had taken place on previously unresorbed surfaces—that is, modeling. Remodeling was also noted to be a surface event, with more than 90% of formation in trabecular bone taking place on previously resorbed surfaces. This work provided the basis for the development of current concepts of bone remodeling taking place at BMUs asynchronously throughout the skeleton.3,8
Figure 1.
Remodeling is initiated within BRCs at points beneath the canopy of cells lining trabecular bone (upper panels) and within cortical bone Haversian canals (lower panels). Osteoclasts (OCs) are formed from hemopoietic precursors (HSC) supplied by marrow and the bloodstream. Precursors of osteoblasts come from MSCs in the marrow, from blood and from pericytes, and differentiate within the BMU through the osteoblast precursor stage to fully functional synthesizing osteoblasts and to osteocytes; lining cells may also differentiate into active osteoblasts. T cells and macrophages can gain access to the BRC from the blood supply (see text for details).
Initiation of bone remodeling
Bone is maintained in a healthy state because remodeling takes place only where it is needed to replace old or damaged bone. For that reason an essential first step in the process is that sites that need to be remodeled must be selected from among the millions possible at any one time in the skeleton. To initiate a remodeling cycle the site of activity must be chosen, regardless of whether remodeling is initiated in response to damage, to change in loading or to remove old bone. Although the initiating event has usually been described as resorption by osteoclasts, and it is true that this is an early event, other mechanisms operate to ensure that this takes place.
Valuable insights into these processes have come from the description of a particular structure around the bone remodeling sites.9,10 This structure, referred to as the bone remodeling compartment (BRC), is a canopy of bone lining cells/osteoblasts and a nearby capillary covering the BMU.11 Most of the recent interest in the canopy over remodeling sites arises from work with human bone, and at the time of writing it remains difficult to establish with certainity that the canopy exists in the same way in mouse bone. What has been shown in the mouse is that tissue-specific macrophages (‘osteomacs') form a canopy structure over mature osteoblasts at sites of bone formation, that is, in remodeling.12 A similar structure to the BRC has been predicted at remodeling sites in the mouse,13 but has yet to be shown. The possibility of the existence of the canopy had been raised some 30 years earlier by Rasmussen and Bordier,14 who indicated that bone lining cells persist over sites of remodeling, separating both osteoclasts and osteoblasts from the marrow. Parfitt15 drew attention to this when discussing the findings of Hauge et al.,10 who provided direct evidence that when the need for a remodeling event is recognized at a site, lining cells separate from the underlying bone and form a raised canopy over the site to be resorbed. Such an initiating event might come from osteocytes recognizing that a specific area of bone needs to be replaced,16 and signaling through their canaliculae to surface cells. Subsequent signals could arise from apoptosis of osteocytes, or even of lining cells themselves, resulting in the release of paracrine factors and chemokines that attract osteoblast and osteoclast precursors and vascular elements. The BRC concept is that bone remodeling takes place within the canopy, and in this compartment intercellular communication occurs among the component bone cells, from endothelial and vascular cells, and perhaps also from immune cells that could become accessible to remodeling sites through the blood supply. The need to take the latter into consideration comes particularly from the findings of Pacifici and co-workers17,18,19 that T cells can mediate the anabolic action of parathyroid hormone (PTH), the known effects of several T-cell cytokines as either promoters or inhibitors of osteoclast formation,20,21,22 and the recent identification of Wnt1 as a B-cell product in bone marrow that promotes bone formation.23
Hemopoietic precursors for osteoclast formation are provided through the capillary blood supply closely associated with and penetrating the BRC,11 as well as from nearby marrow precursors. In the case of cortical remodeling, the blood vessel is provided through Haversian canals (Figure 1b). The signals of macrophage-colony-stimulating factor (M-CSF), receptor activator of nuclear factor-κB ligand (RANKL), vascular endothelial growth factor (VEGF) and nitric oxide necessary for programming of osteoclast formation likely come from osteoblast lineage and endothelial cells, and also from within the BRC.11 Although there is intriguing evidence in the mouse for partially differentiated, vascular-derived quiescent osteoclast precursors that can be readily activated to resorb,24 these have not yet been demonstrated in human bone. The egress of osteoclast precursors from the vasculature is stimulated by chemotactic factors including sphingosine-1-phosphate (S1P)25 via a process that is stimulated by 1,25-dihydroxyvitamin-D3.26 The extensive evidence for promotion of osteoclast formation by active vitamin D will be discussed by Takahashi et al.27. There are nevertheless paradoxical actions of 1,25-dihydroxyvitamin-D3 on bone that continue to pose questions,28 and local events related to remodeling are providing clues. S1P is a lysophospholipid mediator in blood that facilitates the migration of osteoclast precursors from bone to blood through actions on one of its receptors, S1PR1.25 A second receptor, S1PR2, mediates the reverse effect of chemorepulsion, resulting in a change in direction of osteoclast precursors from blood to bone. Active vitamin D has been shown to inhibit production of the chemorepulsive S1PR2, thereby inhibiting osteoclast generation and bone resorption.26 These actions related to S1P are all the more intriguing because it is one of the several osteoclast-derived factors currently postulated as contributing to the coupling of bone formation to resorption (v infra). The mesenchymal precursors to generate osteoblasts can also be blood borne,29,30 but importantly also arise from adjacent marrow stromal precursors and from pericytes. The latter cells adhering to adjacent vessel walls and expressing smooth muscle actin are recognized to undergo osteogenic differentiation.31 They also express CXCL12 that equips them to contribute to the formation of the niche that houses hemopoietic stem cells (HSCs)32,33 and possibly also metastatic cancer cells.34
The final stage of osteoclast differentiation depends on factors produced by osteoblast lineage cells, in particular M-CSF and RANKL.35 Recent in vivo studies in genetically manipulated mice demonstrated osteopetrosis in those mice lacking RANKL throughout the osteoblast lineage, and less markedly so in mice with deletion in mature cells and osteocytes only.36,37 These data suggested that it is not only early osteoblast precursors but also fully differentiated and matrix-embedded osteocytes that provide RANKL to the osteoclast precursors, consistent with our early identification of RANKL in these cells.38 Furthermore, when genetic deletion of RANKL in the osteoblast lineage was delayed until adulthood, a variable 50% reduction of RANKL in the entire osteoblast lineage did not lead to osteopetrosis, leading the authors to suggest that it is only the osteocyte that provides RANKL for osteoclast formation, although osteocytic deletion would also have been achieved.37 This finding was not reproduced in a very recent manuscript from Fumoto et al.39 that achieved a similar level of delayed RANKL knockdown, albeit in younger mice, that resulted in osteopetrosis of the same severity as that in mice lacking RANKL in osteoblast lineage throughout life. Notable in the latter work, in direct contrast to the findings of Nakashima et al.36, was that RANKL mRNA levels were higher in osteoblast-rich cell preparations compared with osteocyte-rich preparations. Relevant to this question also is that mice with the vitamin D receptor (VDR) gene deleted in osteocytes did not develop osteopetrosis or even a reduced level of RANKL expression in the bone, and exhibited a robust response to 1,25-dihydroxy-vitamin-D3 administration with an increase in the number of osteoclasts.40 This suggests that, at least with regard to the VDR–RANKL axis, RANKL was provided by cells of the osteoblast lineage earlier than osteocytes. The concepts arising from all this recent work provide new questions about the control of bone remodeling, and clearly the relative contributions of different stages of the osteoblast lineage will need to be elucidated in the coming years.
The balance in production of RANKL and its decoy receptor osteoprotegerin (OPG) by the osteoblast lineage, and their interaction with RANK on the cell membrane of osteoclast precursors is perhaps the most completely described intercellular interaction within the BMU. This is for the simple reason that the formation of osteoclasts in response to most hormones and cytokines, including 1,25-dihydroxyvitamin-D3, depends on the presence of the osteoblast lineage and the stimulation of their production of RANKL.35 A number of local factors, produced by osteoblasts that stimulate RANKL production, have been identified that are also required for 1,25-dihydroxy-vitamin-D3-induced osteoclast formation, such as semaphorin 3B,41,42 oncostatin M (OSM)42 and the interleukin-6 (IL-6) family coreceptor subunit, glycoprotein 130.43
Coupling of bone formation to resorption
The need to match bone formation with the size of the resorption pit
An essential requirement for balanced remodeling is that the formation component of remodeling needs to replace in the BMU the exact amount removed by resorption. It is the latter strict co-ordination of the two processes, with generation of the appropriate number of osteoblasts in each BMU, that is referred to as ‘coupling'. This term is confined to events taking place within individual BMUs throughout the body; at each BMU the volume of bone removed by resorption is approximately equaled by that replaced by formation. On the other hand, the overall ‘balance' of bone resorption and formation—the close matching of the whole-body rates of these two pointed out by Harris and Heaney8—represents the summation of the contributions from BMUs throughout the body, where very many BMUs are at different stages of maturation. Activation frequency, remodeling rates and resorption depths can vary, and all contribute to balance.
Although circulating hormones, including PTH and 1,25-dihydroxyvitamin-D3, were considered to be the prime regulators of remodeling, it has been clear for some years that locally generated cytokines are the key influences, influencing bone cell communication and subsequent function in complex ways, and often themselves regulated by the hormones and the nervous system. The very nature of the remodeling process, occurring as it does in different parts of the skeleton asynchronously and at different times, highlights the importance of locally generated and regulated factors in ensuring appropriate communication mechanisms among the participating cells. Appreciation of the fact that the cells of bone engage in complex communication processes has come upon us only relatively recently. The separate origins of the osteoblast from mesenchymal and osteoclast from hemopoietic precursors was not accepted until the late 1970s, which was also the first time that bone cells could be cultured and studied in vitro. When it was suggested that the osteoblast lineage might control osteoclast formation and activation,44,45,46 this was greeted with skepticism. This theory was, and still is, often misinterpreted as suggesting that osteoclastogenesis is stimulated by the same cells that produce the bone matrix, but this was not the case.44,47 It nevertheless led to the discovery of the physiological control of osteoclast formation and activity by osteoblast lineage-derived RANKL, its signaling through its receptor, RANK, on hemopoietic cells, and inhibition of this by the decoy receptor, OPG, derived from osteoblasts.48,49 The importance of this control mechanism in bone remodeling is well established. With such a powerful, specific, finely regulated signaling system from osteoblast to osteoclast lineage beyond doubt, it would seem almost self-evident that messages and signaling would take place in the reverse direction, from osteoclasts to osteoblasts within the BMU. Arguments began to be presented in favor of this,50,51 and in the past few years a number of candidate ‘coupling factors' of osteoclast origin have been proposed. What is clear from this accumulating data is that a network of cell communication exists at each BMU, with signals both ways, between the osteoblast and osteoclast lineage, and involving contributions from cells of the vascular and immune systems. The coupling between bone resorption and formation might best be regarded as a multifaceted process, with many contributing regulator molecules, and perhaps one of the prime functions of the BRC is to ensure maintenance of local concentrations of coupling activities and of the positioning of cells that require contact for their communication mechanisms.
Matrix-derived signals
One of the earliest suggestions of a coupling mechanism came from Howard et al.,52 who showed that short-term treatment of organ cultures with either PTH or 1,25-dihydroxyvitamin-D3 induced a rapid increase in bone resorption, followed by an increase in bone formation that could also be stimulated by providing conditioned medium collected during the phase of resorption. Their proposal was that resorption was accompanied by the release of growth factors from their ample stores in the bone matrix itself, with transforming growth factor-β (TGFβ) and the insulin-like growth factors as the favored candidates. This mechanism has met with favor over the years. Although it has always been difficult to accept that active growth factor release in this way could be sufficiently regulated to ensure that the precise amount of bone was replaced at the level of the BMU, it does indicate how specific resorption products could contribute to the overall process of coupling. Strength has been added to this concept by more recent work in which mouse genetic experiments have been used to show that active TGFβ1 released during bone resorption might couple bone formation to resorption by inducing the migration of bone mesenchymal stem cells (MSCs) to sites that have been resorbed, thus making them available within the BMU for differentiation and bone formation in remodeling.53 Complementing that, osteoblast- and matrix-derived insulin growth factor-1 (IGF-1) was found to promote osteoblast differentiation by favoring recruitment of MSCs by activation of mammalian target of rapamycin.54 The question remains about quantitative control through recruitment of a pool of stem cells, but sufficient regulation could be provided at the next stage with these cells, with their proliferation and differentiation under the influence of locally generated factors. The growth factor mechanism needs to be regarded as a significant contributor to the complex process of coupling.
The availability of TGFβ and IGF-1 might not be exclusively dependent on release from matrix through resorption. Both are produced by osteoblastic lineage cells and are released in latent complex forms that are activated by plasmin generated by plasminogen activators. Activation of both TGFβ55 and IGF-156 has been shown in vitro by this means. As plasminogen activator activity in osteoblasts is enhanced specifically by PTH and 1,25-dihydroxyvitamin-D3,57,58 the growth factors could be released from latent complexes at appropriate sites by plasmin generated from plasminogen activators.
Secreted contributors to coupling
On the basis of experiments in mice with inactivating mutations of each of the two alternative signaling pathways of gp130, it was concluded that resorption alone was insufficient to promote coupled bone formation, but that active osteoclasts are the likely source, and that the coupling pathway is IL-6/gp130-dependent.50,59 Another proposed pathway of gp130 involvement was through the gp130 signaling cytokine, cardiotrophin-1 (CT-1). In mice with global deletion of CT-1, although osteoclast numbers are high, their activity is low, and so too is the activity of their osteoblasts, indicating a lack of coupling factor production.60 CT-1 was detected in resorbing osteoclasts by immunohistochemistry, and was shown to stimulate osteoblast differentiation in vitro and bone formation in vivo.60
Osteoclasts, compared with macrophages, express high levels of the known anabolic agents BMP-6 and Wnt10b,61,62,63 suggesting their identity as coupling factors. However, whether these agents are produced at high enough levels by the osteoclast to be effective has been questioned.64 Osteoclasts also express high levels of S1P,62 which has both inhibitory and stimulatory effects on osteoblasts depending on the stage of cell differentiation and on the source of osteoblast precursors, such as human MSCs, immortalized MSCs and mouse calvarial osteoblasts.62,65,66 S1P is also expressed by cells in the vasculature, and acts on its receptor, expressed in osteoclast precursors, to stimulate osteoclastic recruitment in vitro.67 Furthermore, in vivo and in vitro studies indicated that S1P can limit bone resorption by enhancing the chemotaxis and regulating migration of osteoclast precursors, essentially resulting in increased recirculation from bone to blood.25 In that same work, knockout of S1P receptor (S1PR1) yielded mice with excessive bone loss and enhanced osteoclast attachment to bone surfaces, and treatment with FTY720, a drug agonist of four of the five S1PRs, including S1PR1, was effective in preventing bone loss in ovariectomized mice. Data that might be more suggestive of some role for osteoclast-derived S1P in the coupling mechanism comes from a study in which cathepsin K was rendered null in osteoclasts, resulting in impaired resorption while osteoclast numbers and bone formation were maintained.68 Ex vivo cultures showed that the mutated osteoclasts had enhanced capability of promoting aspects of osteoblast differentiation in coculture, an effect inhibited by S1PR antagonist. Although the role of S1P in the coupling process in the BMU is suggestive, it needs to be explored further and put into the context of other actions of S1P, which has been invoked as a signaling mechanism in the actions of a number of cytokines, growth factors and hormones (reviewed in Alvarez et al.69). Among these an interaction with vitamin D has been reported, in that 1,25-dihydroxyvitamin-D3 inhibition of apoptosis in HL60 cells70 and keratinocytes71 has been found to be mediated by S1P; perhaps a similar antiapoptotic role for S1P exists in osteoblasts.
In a very recent report, collagen triple helix repeat containing 1 (CTHRC1) was suggested as a coupling factor by virtue of its production by actively resorbing osteoclasts and the findings that it stimulated bone formation in vitro and in vivo.72 The latter was a confirmation of previous findings,73 but a key difference between the two studies is the identity of the key CTHRC1-producing cells. Kimura et al.73 found CTHRC1 to be a product of the osteoblast lineage, including mesenchymal precursors. In contrast, Takeshita et al.72 concluded, using in situ hybridization, that CTHRC1 was not produced by osteoblasts, but in the adult skeleton was produced only by osteoclasts, with expression by chondrocytes also noted in the embryo and in the active growth plate until 3 months' age. Reconciliation of these discrepant findings will require careful cellular localization studies, but whether as an osteoclast product or a signal within the osteoblast lineage, CTHRC1 might be a further participant in local events that contribute to the overall remodeling process.
Other candidate secreted ‘coupling factors' emerge from time to time from in vitro and ex vivo studies. Recent examples include afamin, a member of the albumin/vitamin D-binding protein family,74 that is produced by osteoclasts and caused the recruitment of a mouse preosteoblastic cell line in vitro, in a manner that was lost by in vitro knockdown of afamin production.75 Another is PDGF-BB produced by non-resorbing osteoclasts that induces migration of bone marrow-derived human MSCs76 and mouse preosteoblasts,77 whereas another study indicated that PDGF-BB inhibited osteoblastogenesis.78 The contradictory reports, variability of experimental systems that have been used and limited nature of in vitro studies have made these reports difficult to interpret. Furthermore, none of the in vitro studies have set out to determine whether osteoclast products influence different stages of osteoblast differentiation. It would seem likely that the coupling process within the BMU would require actions at different stages.
A combination of genetic and pharmacological approaches has drawn attention to a new class of molecule involved in remodeling and the coupling process—the semaphorins. Semaphorins include both secreted and membrane-associated molecules that use plexins and neuropilins as their primary receptors. Plexins are the usual receptors for membrane-associated semaphorins, and neuropilins are obligate coreceptors in the case of most soluble class III receptors.79,80,81 Transcriptional arrays carried out on osteoclasts revealed substantial expression of Sema4D,82 with none detectable in osteoblasts. Targeted genetic ablation of Sema4D in osteoclasts resulted in increased trabecular bone mass, due to increased number and activity of osteoblasts, whereas osteoclast formation was normal. Furthermore, marrow transfer to wild-type mice from Sema4D-null mice resulted in increased bone formation and trabecular bone mass, and treatment of osteoblasts in vitro with recombinant soluble Sema4D-Fc decreased the formation of mineralized nodules. The data point to Sema4D as an osteoclast-derived inhibitor of osteoblast differentiation and bone formation, properties that would equip it to be a ‘fine-tuning' mediator of remodeling in the BMU, acting as an inhibitor of the process. Just as OPG is a powerful negative influence on osteoclast formation and function, it comes as no surprise that some coupling factors would be inhibitory, and raises the possibility that more such activities might exist.
In contrast to the osteoclast origin of Sema4D, Sema3B is another soluble semaphorin that is produced by osteoblasts, where its production is substantially increased by 1,25-dihydroxyvitamin-D3. It enhances the action of RANKL to promote osteoclast formation,41 as do TGFβ83 and Wnt5a,24 although different mechanisms operate in each case. Overexpression of Sema3B in the osteoblast lineage in mice resulted in low bone mass owing to increased osteoclast formation.41 On the other hand, Sema3A was found to act upon the hemopoietic lineage to inhibit osteoclast formation and upon the stromal lineage to promote osteoblast differentiation and activity.84 Nrp2 is a receptor used by the Sema3A–G family, and is expressed both by osteoblasts and osteoclasts; global deletion of this receptor was associated with low trabecular bone mass in the presence of high osteoclast and low osteoblast numbers.85 These results contrast with those of others who found no effect of Sema3A on osteoclast formation.86,87 Notably also, Fukuda et al.87 report that although global knockout of Sema3A resulted in decreased bone mass and bone formation, osteoblast-specific deletion had no such effect. What they show is that Sema3A produced in neurons regulates bone remodeling by modulating sensory nerve development but not by a direct action on osteoblasts.
The complexity of semaphorin involvement in bone cell function is such that isolated actions of members of the semaphorin family cannot readily be put into perspective of the overall remodeling process. In introducing them briefly at this stage, we diverge from the central theme, but the molecular controls of coupling in the remodeling process are such that it is unrealistic to consider any pathway in isolation.
Membrane-bound contributors to coupling
Much of the recent emphasis in investigation of coupling mechanisms has been on soluble products of the osteoclast lineage, but contact-dependent mechanisms have been proposed as potential contributors also. Another class of axon guidance molecule that has been invoked is the Eph family of receptor tyrosine kinases and their membrane-bound ligands, ephrins.
Ephrin/Eph family members are recognized as local mediators of cell function through contact-dependent processes in many tissues in development and in maturity.88,89 A particular feature is their capacity for bi-directional signaling, in that when an ephrin acts upon its corresponding Eph receptor tyrosine kinase, the latter can signal in the reverse direction, acting on ligand by promoting rapid phosphorylation on highly conserved tyrosine residues.90 EphrinB2 has been shown to be expressed by osteoclasts both in vitro and in vivo.21,91 Studies in vivo and ex vivo of genetically manipulated mice suggested that that osteoclast-derived ephrinB2 can act through its receptor, EphB4, in osteoblasts, to promote osteoblast differentiation, and that reverse signaling by direct contact with osteoblast-derived EphB4 can suppress the formation of osteoclast precursors.91 This contact-dependent action of osteoclast-derived ephrinB2 on osteoblastic EphB4 quickly came to be regarded as an explanation for the coupling mechanism,92,93 but it left many questions unanswered. First, it requires ephrinB2 in active, bone-resorbing osteoclasts to be in contact with cells of the osteoblast lineage capable of progressing through differentiation to bone formation. Although such contact is readily accommodated in vitro, this is likely a rare interaction to achieve in vivo, especially within the BMU. Second, myeloid lineage-specific knockout of ephrinB2 yielded mice with no osteoclast defect.91 Further, transgenic overexpression of EphB4 in osteoblasts provided unconvincing evidence of increased bone formation.91 Inhibition of osteoclast formation by ephrinB2 reverse signaling in vivo is likely to require contact between osteoblasts and osteoclast progenitors, rather than being restricted contact between osteoblasts and mature, active osteoclasts. On the basis of the present information, we thus do not consider that osteoclast-derived ephrinB2 contributes significantly to the coupling process.
More importantly though, ephrinB2 is produced by the osteoblast lineage, including osteocytes,21,91,94 and treatment of osteoblasts in vitro or mouse and rat bone in vivo with PTH resulted rapidly in an up to 10-fold increase in mRNA for ephrinB2, and persistent increase in protein; notably for this review, ephrinB2 was not upregulated by 1,25-dihydroxyvitamin-D3.21 The upregulation of ephrinB2 by PTH led to a study in which ephrinB2/EphB4 receptor blockade was carried out together with PTH treatment in an anabolic regimen, with the cotreatment resulting in blockade of the PTH anabolic response, including accumulation of partially differentiated osteoblasts.95 This work suggested that ephrinB2/EphB4 signaling within the osteoblast lineage is important in the process of osteoblast differentiation. Interestingly, in that same work, ephrinB2/EphB4 receptor blockade resulted in enhanced osteoclast formation in vivo and in vitro, most likely at least partly due to interruption of the ephrinB2 reverse signaling in osteoclast progenitors, which inhibits osteoclast differentiation as proposed by Zhao et al.91
Another membrane mechanism coming with a novel idea put forward recently is that reverse signaling through RANKL in the osteoblast might contribute to coupling.96 A peptide antagonist of TNFα–receptor interaction that also blocks RANKL binding and inhibits osteoclast formation (W9)97 was found to promote bone formation in vivo. The compound enhanced osteoblast differentiation in vitro synergistically with BMP-2, with its signaling pathway through activation of p38 mitogen-activated protein kinase. The latter is required for osteoblast differentiation,98 and contributes to reverse signaling through RANKL in T cells. The authors hypothesize that RANK drives reverse signaling through RANKL in the osteoblast lineage, perhaps in co-operation with other paracrine factors, and thus providing another possible coupling pathway.96 This is another mechanism that would require contact between the appropriate cells to be effective, and further work will be of interest.
Signals to and from other marrow components
In addition to regulating bone structure, osteoblasts and osteoclasts have been shown to regulate HSC development. Osteoclasts may regulate HSC development by the high levels of calcium they release during the process of bone resorption, which influences stem cell differentiation through the calcium receptor.99 Osteoblast lineage cells, likely at early stages of differentiation, are required for normal HSC development into multiple lineages, including B-lymphocytes100,101 (reviewed in Panaroni and Wu102).
As HSCs maintain their own niche (the anatomical location in which they reside) and as the HSC niche includes an osteoblast progenitor population, it follows that HSC should also act to maintain osteoblast progenitors. There is some strong evidence for that, particularly suggesting that macrophages maintain osteoblast differentiation; initial studies were based on in vitro work where the addition of monocyte–macrophages to osteoblast cultures enhanced osteoblast differentiation.103,104 More recent in vivo studies using mice with macrophage ablation and detailed immunohistochemical studies indicate that the key cells responsible for this may be resident tissue macrophages termed ‘osteal macrophages', a population distinct from inflammatory macrophages;21 the relative contributions of these populations are yet to be identified, or described in human bone. One piece of evidence that reinforces a role for macrophages in the process of bone formation is that in HSC mobilization where G-CSF treatment induces the egress of macrophages into the circulation, there is a complete loss of osteoblasts and cessation of bone formation, but only on those bone surfaces in contact with the marrow,105,106 a phenonemon that also involves the loss of osteal macrophages.
One factor secreted by macrophages that may be important for maintaining osteoblast differentiation is OSM. OSM is produced by activated macrophages, and the stimulatory effect of macrophages on osteoblast differentiation has been shown to be blocked by an OSM-neutralizing antibody in two independent studies, using both human and murine cells.107,108 Whether the influence of macrophage-derived OSM on osteoblasts is contact-dependent or -independent, and the type of macrophage activation required for osteoblastic support remains controversial. This cytokine is also produced at all stages of osteoblast differentiation, including osteocytes,42 and stimulates both osteoblast and osteoclast activity through direct actions on the osteoblast and osteocyte.42 Whether macrophage-derived OSM contributes to physiological coupling, or if it is only a consideration in bone formation associated with macrophage activation remains to be established.
Marrow cells, particularly T and natural killer cells, also support osteoclast formation through their production of RANKL and M-CSF, particularly in the context of the pathology of inflammatory arthritis, where T-cell activation has occurred109,110 (for an extensive review see Wythe et al.111). Very recent work utilizing a mouse with CD4+ T-cell-restricted deletion of RANKL suggests that, in addition to their role in pathology, T cells provide a major source of RANKL for physiological bone remodeling, but the phenotype described is very mild indeed.39 As VDR controls activation of T cells,112 vitamin D metabolites may also regulate osteoclastogenesis by actions on the activated T cell. Inactive T cells do not express VDR,112 but they produce a number of factors that inhibit osteoclastogenesis, including IL-12, IL-18, IL-4 and IL-23.20,21,113 Notably, T cells also produce Sema4D,114 indicating multiple local sources of this factor that inhibits osteoblast differentiation. Dendritic cells are also found in the proximity of the BMU, and stimulate the production of RANKL by T cells.115 The dendritic cells also have the ability to also differentiate into osteoclasts, although the situations in which this occurs in vivo have not been identified.116,117 The role of T cells might not be limited to actions upon osteoclastogenesis, but could have a role in bone formation, where it has been shown to participate in the anabolic action of PTH through production of Wnt10b.17,19
There is also some evidence that B-lymphocytes regulate osteoblast and osteoclast differentiation. For example, Wnt1, which is expressed at high levels in B-lymphocytes, promotes osteoblast mineralization in vitro; notably, lineage tracing studies also suggested that Wnt1 is expressed in some osteocyte populations.23 B-lymphocytes have also been shown to inhibit osteoclast formation in vitro in a manner inhibited by a TGFβ-neutralizing antibody.118 In contrast to these studies, intramembranous bone formation in the process of fracture healing does not appear to be impaired in B-lymphocyte-deficient mice.119 In experiments in which mice lacking RANKL in B cells were partly protected against bone loss following ovariectomy,120 deletion of RANKL from T cells was reported to have no impact. Much remains to elucidate the physiological importance of B-cell and T-cell communication with osteoblasts and osteoclast precursors.
Conclusion
It was a formidable insight of Harold Frost that led him in the 1960s to propose that bone formation and resorption were coupled in the BMU to preserve bone while ensuring its repair. With the advent of new technologies, recent work has led to new understandings of how coupling might be achieved at the level of the BMU. Although it was thought at first that there might be a single ‘coupling factor' analogous to the RANKL role in programming bone resorption, it has now become clear that there are very many cell and molecular contributors to coupling, and these are derived from multiple cellular sources. They include not only factors produced at different stages of osteoblast and osteoclast differentiation but also factors originating from cells of the immune system, including T and B cells—all of these need to be recognized as participants. Further unveiling of the anatomical basis of the BMU has also revealed that vascular supply, together with the canopy that houses the BMU, may provide a means of focus of the cells and their generated cytokines, at least as so far illustrated in human bone.
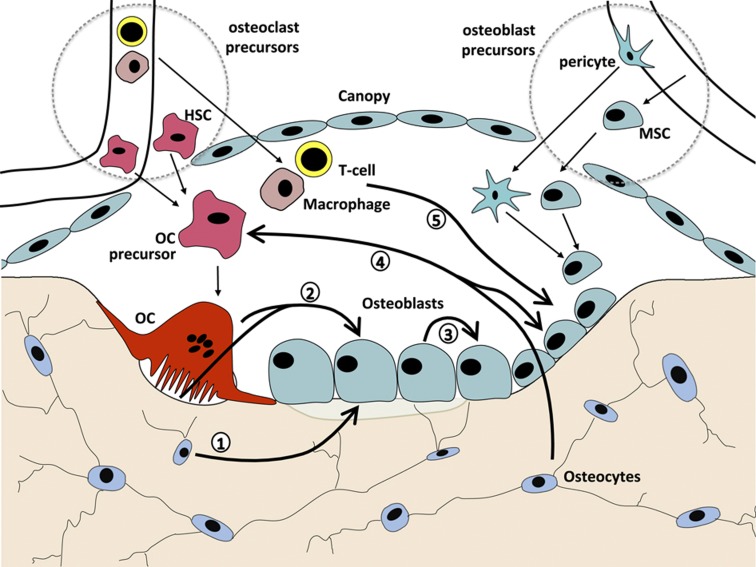
It would be misleading to continue to think of coupling between osteoclasts and osteoblasts as simply one key signal from resorbing osteoclasts to mature osteoblasts. The osteoblast lineage includes mesenchymal precursors, preosteoblasts that undergo progressive stages of differentiation that include changes in responsiveness to hormones, cytokines and growth factors, mature bone-forming osteoblasts, bone lining cells and osteocytes.121,122 Within the BMU during a remodeling cycle, paracrine contacts between the cell lineages are likely predominant, with both positive and negative signals arising from osteoclasts, their precursors and macrophages, as well as from T and possibly B cells. Contact-dependent mechanisms might take place readily within the mesenchymal/osteoblast lineage within the BMU. Mature osteoclast to osteoblast lineage contact is less likely, although osteoclast precursors could be more readily available. Figure 2 provides a schematic representation of some of the many pathways of regulation within the BMU for which there is evidence, and that have been discussed in this paper.
Figure 2.
Intercellular communication pathways within the BMU that comprise the remodeling process (see text for details). (1) Stimulatory and inhibitory signals from osteocytes to osteoblasts (e.g. OSM, PTHrP and sclerostin). (2) Stimulatory and inhibitory signals from osteoclasts to osteoblasts (e.g. matrix-derived TGFβ and IGF-1, secreted CT-1, Sema4D and S1P). (3) Signaling within the osteoblast lineage (e.g. ephrinB2 and EphB4, Sema3a, PTHrP, OSM). (4) Stimulatory and inhibitory signals between the osteoblast and osteoclast lineages (e.g. RANKL, Sema3B, Wnt5a and OPG). (5) Marrow cell signals to osteoblasts (e.g. macrophage-derived OSM, T-cell-derived interleukins and RANKL).
Importantly, signaling within lineages cannot be ignored. This is especially evident within the osteoblast lineage, where there are several examples of events that change the state of osteoblast differentiation and thus most likely influence how the cells participate in the coupling process in remodeling. These include (i) regulated production of ephrinB2, blockade of which impairs bone formation,95 (ii) production of Wnt5a that favors bone formation by inducing runx2123 and decreasing PPARγ,124 (iii) enhanced osteoblast commitment of early mesenchymal cells through Wnt signals from more mature cells,125 (iv) production of parathyroid hormone-related protein (PTHrP) within the osteoblast lineage, and action upon receptor-positive cells,126 (v) production of OSM by the osteoblast lineage that promotes osteoblast commitment at the expense of adipogenesis and suppresses osteocytic sclerostin production,42 and (vi) promotion of osteoblast differentiation by Sema3A produced within the lineage.84
With so many contributors to the coupling process in remodeling, it is remarkable that genetic deletion of even a single participant leads to changes in bone remodeling, when it might reasonably be expected that compensation from other factors would normalize the balance at the BMU. Some compensatory mechanisms must occur in vivo (e.g. the upregulation of osteoclast numbers in some osteopetrotic mice, see above), and contribute to the phenotypes observed in the whole animal. What we see reflects not only of the immediate effect of a lack of the gene of interest but also shows the way the body compensates for the lack of that factor. There is still much to be learned about cross-talk between the wide range of coupling factors, stage- and dose-specific effects of each factor and their regulation during bone growth and pathology. This will require further investigation with genetic mouse models, combined with pharmacological experiments in vivo and in human and murine and human cells in vitro, as well as protein localization studies. We will need all the tools at our disposal, and yet to be developed, to fully understand the relative contributions of local factors in the regulation of remodeling. To do so will provide us with a comprehensive ‘map' of the BMU, far more complex and intriguing than our current understanding, and with it, new insights into how manipulation of these pathways could successfully improve skeletal health.
Acknowledgments
We thank Holly Brennan for preparation of Figures. Work from the authors' laboratories is supported by project grants from the National Health and Medical Research Council of Australia, and the Victorian Government OIS Program.
Footnotes
The authors declare no conflict of interest.
References
- Frost HM. Dynamics of bone remodeling. Bone Biodyn 1964;315–333 (book chapter). [Google Scholar]
- Parfitt A. Morphological basis of bone mineral measurements: transient and steady state effects of treatment in osteoporosis. Miner Elecrolyte Metab 1980;4:273–287. [Google Scholar]
- Parfitt AM. The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res 1982;4:1–6. [DOI] [PubMed] [Google Scholar]
- Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev 1986;7:379–408. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Gooi JH, Sims NA. Molecular mechanisms in coupling of bone formation to resorption. Crit Rev Eukaryot Gene Expr 2009;19:73–88. [DOI] [PubMed] [Google Scholar]
- Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature 1965;206:489–490. [DOI] [PubMed] [Google Scholar]
- Everts V, Delaisse JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P et al. The bone lining cell: its role in cleaning Howship's lacunae and initiating bone formation. J Bone Miner Res 2002;17:77–90. [DOI] [PubMed] [Google Scholar]
- Harris WH, Heaney RP. Skeletal renewal and metabolic bone disease. N Engl J Med 1969;280:193–202. [DOI] [PubMed] [Google Scholar]
- Andersen TL, Sondergaard TE, Skorzynska KE, Dagnaes-Hansen F, Plesner TL, Hauge EM et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol 2009;174:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res 2001;16:1575–1582. [DOI] [PubMed] [Google Scholar]
- Kristensen HB, Andersen TL, Marcussen N, Rolighed L, Delaisse JM. Increased presence of capillaries next to remodeling sites in adult human cancellous bone. J Bone Miner Res 2013;28:574–585. [DOI] [PubMed] [Google Scholar]
- Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 2008;181:1232–1244. [DOI] [PubMed] [Google Scholar]
- Pettit AR, Chang MK, Hume DA, Raggatt LJ. Osteal macrophages: a new twist on coupling during bone dynamics. Bone 2008;43:976–982. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Bordier P. The Physiological Basis of Metabolic Bone Disease Williams and Wilkins, Waverley Press: Baltimore, MD, USA, 1974;. [Google Scholar]
- Parfitt AM. The bone remodeling compartment: a circulatory function for bone lining cells. J Bone Miner Res 2001;16:1583–1585. [DOI] [PubMed] [Google Scholar]
- Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 2000;15:60–67. [DOI] [PubMed] [Google Scholar]
- Pacifici R. T cells: critical bone regulators in health and disease. Bone 2010;47:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfeek H, Bedi B, Li JY, Adams J, Kobayashi T, Weitzmann MN et al. Disruption of PTH receptor 1 in T cells protects against PTH-induced bone loss. PLoS One 2010;5:e12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi B, Li JY, Tawfeek H, Baek KH, Adams J, Vangara SS et al. Silencing of parathyroid hormone (PTH) receptor 1 in T cells blunts the bone anabolic activity of PTH. Proc Natl Acad Sci USA 2012;109:E725–E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirosavljevic D, Quinn JM, Elliott J, Horwood NJ, Martin TJ, Gillespie MT. T-cells mediate an inhibitory effect of interleukin-4 on osteoclastogenesis. J Bone Miner Res 2003;18:984–993. [DOI] [PubMed] [Google Scholar]
- Allan EH, Hausler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res 2008;23:1170–1181. [DOI] [PubMed] [Google Scholar]
- Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther 2007;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine CM, Joeng KS, Campeau PM, Kiviranta R, Tarkkonen K, Grover M et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med 2013;368:1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med 2012;18:405–412. [DOI] [PubMed] [Google Scholar]
- Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med 2010;207:2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta J, Kawamura S, Okiji F, Shirazaki M, Sakai S, Saito H et al. Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc Natl Acad Sci USA 2013;110:7009–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Udagawa N, Suda T. Vitamin D endocrine system and osteoclasts. BoneKEy Reports (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takahashi F, Takahashi N. Bone effects of vitamin D—discrepancies between in vivo and in vitro studies. Arch Biochem Biophys 2012;523:22–29. [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi GZ, Modder UI, Charatcharoenwitthaya N, Sanyal A, Undale AH, Clowes JA et al. Characterization of circulating osteoblast lineage cells in humans. Bone 2007;40:1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med 2005;352:1959–1966. [DOI] [PubMed] [Google Scholar]
- Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res 1998;13:828–838. [DOI] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013;495:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013;495:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Taichman RS. Cancer stem cells and the bone marrow microenvironment. Bonekey Reports 1: Article number: 48 (2012); 10.1038/bonekey.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 1999;20:345–357. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011;17:1231–1234. [DOI] [PubMed] [Google Scholar]
- Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med 2011;17:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM et al. Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone 1999;25:525–534. [DOI] [PubMed] [Google Scholar]
- Fumoto T, Takeshita S, Ito M, Ikeda K. Physiological functions of osteoblast lineage and T cell-derived RANKL in bone homeostasis. J Bone Miner Res (e-pub ahead of print 7 September 2013; 10.1002/jbmr.2096). [DOI] [PubMed] [Google Scholar]
- Lieben L, Masuyama R, Torrekens S, Van Looveren R, Schrooten J, Baatsen P et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D-induced inhibition of bone mineralization. J Clin Invest 2012;122:1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AL, Zhang X, Dowd DR, Kharode YP, Komm BS, Macdonald PN. Semaphorin 3B is a 1,25-dihydroxyvitamin D3-induced gene in osteoblasts that promotes osteoclastogenesis and induces osteopenia in mice. Mol Endocrinol 2008;22:1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest 2010;120:582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romas E, Udagawa N, Zhou H, Tamura T, Saito M, Taga T et al. The role of gp130-mediated signals in osteoclast development: regulation of interleukin 11 production by osteoblasts and distribution of its receptor in bone marrow cultures. J Exp Med 1996;183:2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption—a hypothesis. Calcif Tissue Int 1981;33:349–351. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Partridge NC, Greaves M, Atkins D, Ibbotson KJ. Prostaglandin effects on bone and role in cancer hypercalcaemia. In: MacIntyre I, Szelke M (ed).Molecular Endocrinology Elsevier: Amsterdam, The Netherlands, 1979; pp 251–264. [Google Scholar]
- Chambers TJ. The cellular basis of bone resorption. Clin Orthop Relat Res 1980;151:283–293. [PubMed] [Google Scholar]
- Rodan GA, Martin TJ. Calcif Tissue Int 1982;34: 311 (letter to editor). [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Udagawa N, Takahashi N, Yamaguchi K, Yasuda H, Shima N et al. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun 1998;246:199–204. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93:165–176. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 2005;11:76–81. [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K. Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res 2007;22:487–494. [DOI] [PubMed] [Google Scholar]
- Howard GA, Bottemiller BL, Turner RT, Rader JI, Baylink DJ. Parathyroid hormone stimulates bone formation and resorption in organ culture: evidence for a coupling mechanism. Proc Natl Acad Sci USA 1981;78:3204–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 2009;15:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med 2012;18:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JA, Yan L, Dominguez JC, Allan EH, Martin TJ. Plasminogen-dependent activation of latent transforming growth factor beta (TGF beta) by growing cultures of osteoblast-like cells. J Cell Physiol 1993;157:528–534. [DOI] [PubMed] [Google Scholar]
- Campbell PG, Novak JF, Yanosick TB, McMaster JH. Involvement of the plasmin system in dissociation of the insulin-like growth factor-binding protein complex. Endocrinology 1992;130:1401–1412. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Allan EH, Yee JA, Gelehrter TD, Martin TJ. Plasminogen activator regulation in osteoblasts: parathyroid hormone inhibition of type-1 plasminogen activator inhibitor and its mRNA. J Cell Physiol 1992;152:346–355. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Allan EH, Martin TJ. Regulation of plasminogen activator inhibitor-1 (PAI-1) expression by 1,25-dihydroxyvitamin D-3 in normal and malignant rat osteoblasts. Biochim Biophys Acta 1994;1201:223–228. [DOI] [PubMed] [Google Scholar]
- Sims NA, Jenkins BJ, Quinn JM, Nakamura A, Glatt M, Gillespie MT et al. Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J Clin Invest 2004;113:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, McGregor N, Poulton I, Pompolo S, Allan E, Quinn J et al. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res 2008;23:2025–2032. [DOI] [PubMed] [Google Scholar]
- Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 2007;148:2635–2643. [DOI] [PubMed] [Google Scholar]
- Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 2008;105:20764–20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev 2009;20:441–448. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Andreassen KV, Thudium CS, Gudmann KN, Moscatelli I, Cruger-Hansen CE et al. A specific subtype of osteoclasts secretes factors inducing nodule formation by osteoblasts. Bone 2012;51:353–361. [DOI] [PubMed] [Google Scholar]
- Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, Kim HH. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J 2006;25:5840–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint P, Ruan M, Pederson L, Kassem M, Westendorf JJ, Khosla S et al. Sphingosine 1-phosphate (S1P) receptors 1 and 2 coordinately induce mesenchymal cell migration through S1P activation of complementary kinase pathways. J Biol Chem 2013;288:5398–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 2009;458:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotinun S, Kiviranta R, Matsubara T, Alzate JA, Neff L, Luth A et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest 2013;123:666–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab 2007;18:300–307. [DOI] [PubMed] [Google Scholar]
- Kleuser B, Cuvillier O, Spiegel S. 1Alpha,25-dihydroxyvitamin D3 inhibits programmed cell death in HL-60 cells by activation of sphingosine kinase. Cancer Res 1998;58:1817–1824. [PubMed] [Google Scholar]
- Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schafer-Korting M et al. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol 2001;117:1241–1249. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Fumoto T, Matsuoka K, Park KA, Aburatani H, Kato S et al. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest 2013;123:3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Kwan KM, Zhang Z, Deng JM, Darnay BG, Behringer RR et al. Cthrc1 is a positive regulator of osteoblastic bone formation. PLoS One 2008;3:e3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichenstein HS, Lyons DE, Wurfel MM, Johnson DA, McGinley MD, Leidli JC et al. Afamin is a new member of the albumin, alpha-fetoprotein, and vitamin D-binding protein gene family. J Biol Chem 1994;269:18149–18154. [PubMed] [Google Scholar]
- Kim BJ, Lee YS, Lee SY, Park SY, Dieplinger H, Ryu SH et al. Afamin secreted from nonresorbing osteoclasts acts as a chemokine for preosteoblasts via the Akt-signaling pathway. Bone 2012;51:431–440. [DOI] [PubMed] [Google Scholar]
- Kreja L, Brenner RE, Tautzenberger A, Liedert A, Friemert B, Ehrnthaller C et al. Non-resorbing osteoclasts induce migration and osteogenic differentiation of mesenchymal stem cells. J Cell Biochem 2010;109:347–355. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS One 2008;3:e3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Sakikawa C, Katsumata M, Nakamura T, Wakabayashi K. Platelet-derived growth factor BB secreted from osteoclasts acts as an osteoblastogenesis inhibitory factor. J Bone Miner Res 2002;17:257–265. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 1999;99:59–69. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 1999;99:71–80. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M et al. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell 1998;95:903–916. [DOI] [PubMed] [Google Scholar]
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med 2011;17:1473–1480. [DOI] [PubMed] [Google Scholar]
- Quinn JM, Itoh K, Udagawa N, Hausler K, Yasuda H, Shima N et al. Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. J Bone Miner Res 2001;16:1787–1794. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature 2012;485:69–74. [DOI] [PubMed] [Google Scholar]
- Verlinden L, Kriebitzsch C, Beullens I, Tan BK, Carmeliet G, Verstuyf A. Nrp2 deficiency leads to trabecular bone loss and is accompanied by enhanced osteoclast and reduced osteoblast numbers. Bone 2013;55:465–475. [DOI] [PubMed] [Google Scholar]
- Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol 2006;8:615–622. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature 2013;497:490–493. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 1996;17:9–19. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 2005;6:462–475. [DOI] [PubMed] [Google Scholar]
- Murai KK, Pasquale EB. 'Eph'ective signaling: forward, reverse and crosstalk. J Cell Sci 2003;116:(Part 14): 2823–2832. [DOI] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 2006;4:111–121. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Zuscik MJ, Xing L. Biology of bone and cartilage. In: RV T, MP W, JA E, T I (eds)Genetics of Bone Biology and Skeletal Disease Elsevier: London, 2013; pp 3–24. [Google Scholar]
- Mundy GR, Elefteriou F. Boning up on ephrin signaling. Cell 2006;126:441–443. [DOI] [PubMed] [Google Scholar]
- Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M et al. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem 2009;284:14637–14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takyar FM, Tonna S, Ho PW, Crimeen-Irwin B, Baker EK, Martin TJ et al. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J Bone Miner Res 2013;28:912–925. [DOI] [PubMed] [Google Scholar]
- Furuya Y, Inagaki A, Khan M, Mori K, Penninger JM, Nakamura M et al. Stimulation of bone formation in cortical bone of mice treated with a receptor activator of nuclear factor-kappaB ligand (RANKL)-binding peptide that possesses osteoclastogenesis inhibitory activity. J Biol Chem 2013;288:5562–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K, Saito H, Itzstein C, Ishiguro M, Shibata T, Blanque R et al. A TNF receptor loop peptide mimic blocks RANK ligand-induced signaling, bone resorption, and bone loss. J Clin Invest 2006;116:1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chan E, Wang SX, Li B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology 2003;144:2068–2074. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature 2006;441:1075–1079. [DOI] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004;103:3258–3264. [DOI] [PubMed] [Google Scholar]
- Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 2007;109:3706–3712. [DOI] [PubMed] [Google Scholar]
- Panaroni C, Wu J. Interactions between B lymphocytes and the osteoblast lineage in bone marrow. Calcif Tissue Int 2013;93:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Takagi K, Kitaoka M, Iyama KI, Usuku G. Influence of monocyte-macrophage lineage cells on alkaline phosphatase activity of developing osteoblasts derived from rat bone marrow stromal cells. Nihon Seikeigeka Gakkai Zasshi 1993;67:480–489. [PubMed] [Google Scholar]
- Rifas L, Cheng SL, Shen V, Peck WA. Monokines produced by macrophages stimulate the growth of osteoblasts. Connect Tissue Res 1989;23:163–178. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Pettit AR, Raggatt LJ, Jacobsen RN, Forristal CE, Barbier V et al. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia 2012;26:1594–1601. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010;116:4815–4828. [DOI] [PubMed] [Google Scholar]
- Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012;30:762–772. [DOI] [PubMed] [Google Scholar]
- Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM et al. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One 2012;7:e39871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun 1999;265:144–150. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Stein E, Colmenero P, Purath U, Muller-Ladner U, de Matos CT et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci USA 2010;107:13028–13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wythe SE, Nicolaidou V, Horwood NJ. Cells of the immune system orchestrate changes in bone cell function. Calcif Tissue Int (e-pub ahead of print 3 August 2013; 10.1007/s00223-013-9764-0). [DOI] [PubMed] [Google Scholar]
- von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol 2010;11:344–349. [DOI] [PubMed] [Google Scholar]
- Horwood NJ, Elliott J, Martin TJ, Gillespie MT. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol 2001;166:4915–4921. [DOI] [PubMed] [Google Scholar]
- Bougeret C, Mansur IG, Dastot H, Schmid M, Mahouy G, Bensussan A et al. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J Immunol 1992;148:318–323. [PubMed] [Google Scholar]
- Alnaeeli M, Penninger JM, Teng YT. Immune interactions with CD4+ T cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J Immunol 2006;177:3314–3326. [DOI] [PubMed] [Google Scholar]
- Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 2004;104:4029–4037. [DOI] [PubMed] [Google Scholar]
- Speziani C, Rivollier A, Gallois A, Coury F, Mazzorana M, Azocar O et al. Murine dendritic cell transdifferentiation into osteoclasts is differentially regulated by innate and adaptive cytokines. Eur J Immunol 2007;37:747–757. [DOI] [PubMed] [Google Scholar]
- Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest 2000;106:1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggatt LJ, Alexander KA, Kaur S, Wu AC, MacDonald KP, Pettit AR. Absence of B cells does not compromise intramembranous bone formation during healing in a tibial injury model. Am J Pathol 2013;182:1501–1508. [DOI] [PubMed] [Google Scholar]
- Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC et al. Receptor activator of nuclear factor kappaB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem 2012;287:29851–29860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 2000;21:115–137. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Ng KW, Sims NA. Basic principles of bone cell biology. In: Karsenty G (ed)Translational Endocrinology of Bone Elsevier: Amsterdam, 2013; pp 5–26. [Google Scholar]
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ et al. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell 2007;12:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 2007;9:1273–1285. [DOI] [PubMed] [Google Scholar]
- Zhou H, Mak W, Zheng Y, Dunstan CR, Seibel MJ. Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through Wnt signaling. J Biol Chem 2008;283:1936–1945. [DOI] [PubMed] [Google Scholar]
- Miao D, He B, Jiang Y, Kobayashi T, Soroceanu MA, Zhao J et al. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1–34. J Clin Invest 2005;115:2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]