Abstract
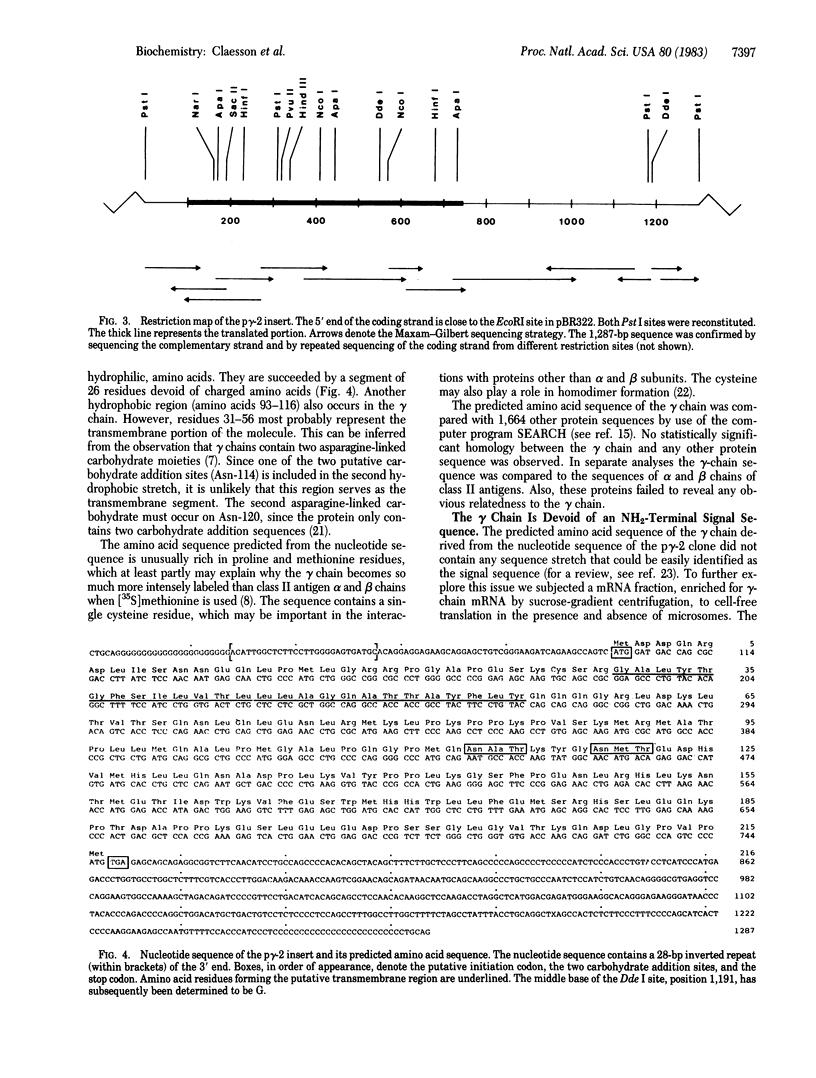
The invariant gamma chain is transitorily associated with class II histocompatibility antigens during intracellular transport. We have isolated and sequenced a cDNA clone corresponding to the human gamma chain. mRNA hybridizing to the cDNA clone translated into a 33,000-dalton chain that associated specifically with class II antigen alpha and beta chains. The gamma chain consists of 216 amino acids. The two N-linked carbohydrates are attached to asparagines 114 and 120. A continuous stretch of hydrophobic and neutral amino acids occurs in positions 31-56 from the NH2 terminus. This region seems to constitute the transmembrane portion of the polypeptide chain. The positions of the carbohydrate moieties and the putative transmembrane segment indicate that the NH2 terminus of the gamma chain resides on the cytoplasmic side of the membrane. Cell-free translations in conjunction with radiochemical amino acid sequence analyses suggest that the gamma chain lacks an NH2-terminal signal sequence.
Full text
PDF


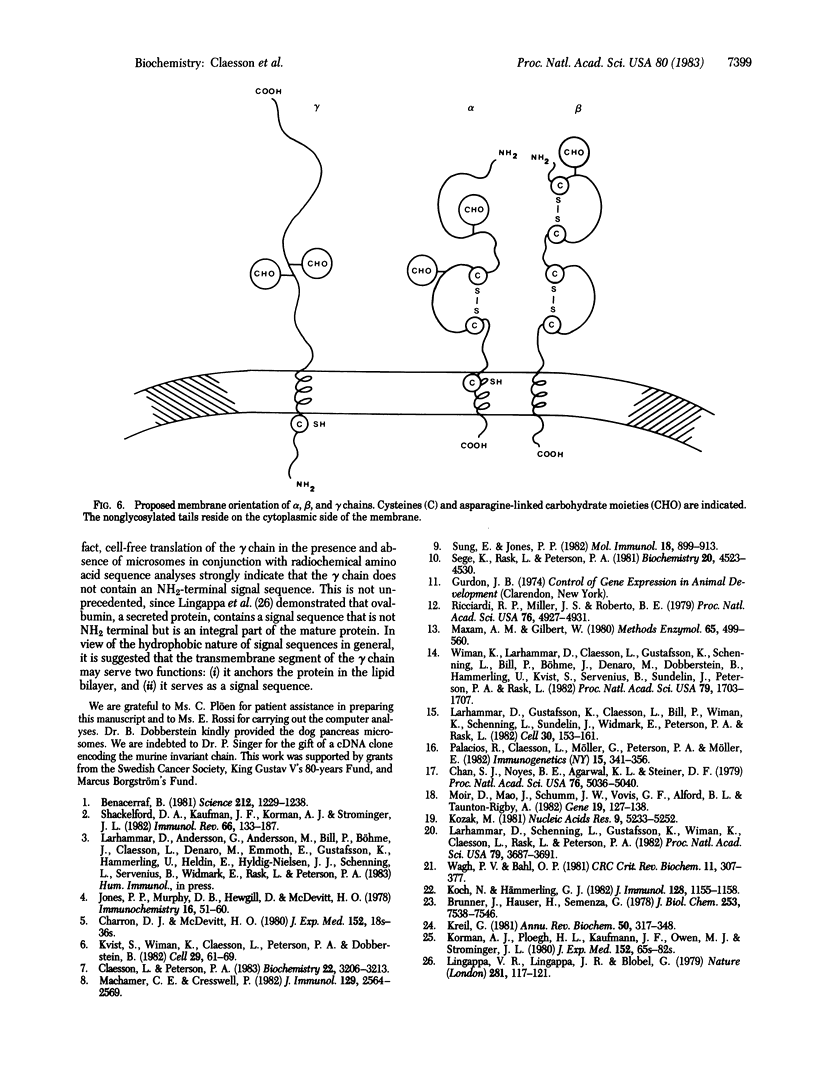
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Brunner J., Hauser H., Semenza G. Single bilayer lipid-protein vesicles formed from phosphatidylcholine and small intestinal sucrase.isomaltase. J Biol Chem. 1978 Oct 25;253(20):7538–7546. [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Characterization of HLA-D-region antigens by two-dimensional gel electrophoresis. Molecular-genotyping. J Exp Med. 1980 Aug 1;152(2 Pt 2):18s–36s. [PubMed] [Google Scholar]
- Claesson L., Peterson P. A. Association of human gamma chain with class II transplantation antigens during intracellular transport. Biochemistry. 1983 Jun 21;22(13):3206–3213. doi: 10.1021/bi00282a026. [DOI] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Koch N., Hämmerling G. J. Structure of Ia antigens: identification of dimeric complexes formed by the invariant chain. J Immunol. 1982 Mar;128(3):1155–1158. [PubMed] [Google Scholar]
- Korman A. J., Ploegh H. L., Kaufman J. F., Owen M. J., Strominger J. L. Cell-free synthesis and processing of the heavy and light chains of HLA-DR antigens. J Exp Med. 1980 Aug 1;152(2 Pt 2):65s–82s. [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Gustafsson K., Claesson L., Bill P., Wiman K., Schenning L., Sundelin J., Widmark E., Peterson P. A., Rask L. Alpha chain of HLA-DR transplantation antigens is a member of the same protein superfamily as the immunoglobulins. Cell. 1982 Aug;30(1):153–161. doi: 10.1016/0092-8674(82)90021-6. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Schenning L., Gustafsson K., Wiman K., Claesson L., Rask L., Peterson P. A. Complete amino acid sequence of an HLA-DR antigen-like beta chain as predicted from the nucleotide sequence: similarities with immunoglobulins and HLA-A, -B, and -C antigens. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3687–3691. doi: 10.1073/pnas.79.12.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Cresswell P. Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens. J Immunol. 1982 Dec;129(6):2564–2569. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moir D., Mao J., Schumm J. W., Vovis G. F., Alford B. L., Taunton-Rigby A. Molecular cloning and characterization of double-stranded cDNA coding for bovine chymosin. Gene. 1982 Jul-Aug;19(1):127–138. doi: 10.1016/0378-1119(82)90197-4. [DOI] [PubMed] [Google Scholar]
- Palacios R., Claesson L., Möller G., Peterson P. A., Möller E. The alpha chain, not the beta chain of HLA-DR antigens participates in activation of T cells in autologous mixed lymphocyte reaction. Immunogenetics. 1982;15(4):341–356. doi: 10.1007/BF00364258. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sege K., Rask L., Peterson P. A. Role of beta2-microglobulin in the intracellular processing of HLA antigens. Biochemistry. 1981 Aug 4;20(16):4523–4530. doi: 10.1021/bi00519a003. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Kaufman J. F., Korman A. J., Strominger J. L. HLA-DR antigens: structure, separation of subpopulations, gene cloning and function. Immunol Rev. 1982;66:133–187. doi: 10.1111/j.1600-065x.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Sung E., Jones P. P. The invariant chain of murine Ia antigens: its glycosylation, abundance and subcellular localization. Mol Immunol. 1981 Oct;18(10):899–913. doi: 10.1016/0161-5890(81)90013-4. [DOI] [PubMed] [Google Scholar]
- Wagh P. V., Bahl O. P. Sugar residues on proteins. CRC Crit Rev Biochem. 1981;10(4):307–377. doi: 10.3109/10409238109113602. [DOI] [PubMed] [Google Scholar]
- Wiman K., Larhammar D., Claesson L., Gustafsson K., Schenning L., Bill P., Böhme J., Denaro M., Dobberstein B., Hammerling U. Isolation and identification of a cDNA clone corresponding to an HLA-DR antigen beta chain. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1703–1707. doi: 10.1073/pnas.79.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]