Abstract
Background
Cerebral small vessel disease (SVD) causes lacunar stroke, and more recently has been implicated as a cause of depression. Factors causing reduced quality of life (QoL) in SVD, including the relative contributions of disability and depressive symptoms, remain uncertain.
Hypothesis
Depressive symptoms are a major predictor of reduced QoL in SVD, acting independently of disability.
Methods
The Stroke-Specific QoL scale was completed by 100 patients with SVD (lacunar stroke with MRI lacunar infarct) and 55 controls. We repeated the protocol in 40 patients with the young onset genetic form of SVD, CADASIL, and 35 controls. Disability (modified Rankin Scale), [instrumental] activities of daily living (IADL, ADL), cognition (Mini Mental State Examination) and depressive symptoms (Geriatric Depression Scale, Montgomery-Åsberg Depression Rating Scale) were measured.
Results
QoL was significantly lower in SVD than controls: mean (SD), 196.8 (35.2) versus 226.8(15.3), p<.0001. Depressive symptoms were the major predictor of QoL, accounting for 52.9% of variance. The only other independent predictor of QoL was disability, accounting for an additional 18.4%. A similar pattern was found in CADASIL with reduced QoL (202.0(29.7) versus controls (228.6 (13.1); p<.0001), and depressive symptoms accounting for 42.2% of variance. Disability accounted for an additional 17.6%. Relationships between depression and QoL, and disability and QoL, were independent of one another.
Conclusions
Depressive symptoms, often unrecognized, are a major determinant of reduced QoL in SVD. They account for greater reduction than disability, and the association is independent of disability. This relationship may reflect the proposed causal association between white matter disease and depression. Treatment of depressive symptoms might significantly improve QoL in SVD.
Keywords: Small vessel disease, lacunar stroke, CADASIL, Quality of Life, Depression
Introduction
Cerebral small vessel disease (SVD) accounts for 20% of ischaemic stroke and is the most common cause of vascular cognitive impairment (1). Radiologically, it is characterised by discrete lacunar infarcts, with or without diffuse areas of white matter damage (leukoaraiosis). Associated features include cognitive impairment, particularly involving executive function and information processing speed (2,3) and progressive motor slowing and disability, all factors which might be expected to impact significantly on quality of life (QoL).
Despite its importance, there is little data on QoL in patients presenting with SVD, and the factors which determine any reduction. Where assessments of QoL have been performed in stroke they have been found to be useful indicators of patient recovery and are increasingly being used as outcome measures in research trials. However, QoL studies have tended not to differentiate between stroke subtypes, which show different levels of impairment both in the acute and chronic phases. Due to the characteristic cognitive profile in patients with SVD, and because concomitant lacunar strokes often result in lesser degrees of disability than larger cortical strokes, there might be different determinants of QoL in this condition. In addition, recent evidence suggests SVD is a risk factor for late onset depression (4) which itself may also be an important determinant of QoL. A biological mechanism for this has been suggested with depressive symptoms resulting from disruption of complex cortical-subcortical circuits. Therefore depressive symptoms might be hypothesised to be a major factor influencing QoL in SVD. To investigate this, we performed stroke specific QoL (5) assessments in patients presenting with SVD, and at the same time assessed depression, disability and cognition.
To identify patients with SVD we recruited patients with lacunar infarction and no other obvious cause of stroke. Many such individuals also have white matter damage, identified on scanning as white mater hyperintensities (WMH), or leukoaraiosis. However, SVD is most common in elderly individuals in whom concomitant brain pathology is not-infrequent. Post-mortem studies in patients presenting with presumed vascular dementia, for example, have frequently reported co-existent Alzheimer’s pathology. To allow us to replicate our findings in a group with pure SVD and without concomitant age-related pathology we also studied a group with Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL), a genetic cause of SVD which results in early onset lacunar stroke and cognitive decline. Radiological appearances are similar to those seen in sporadic SVD with lacunar infarction and leukoaraiosis. Due to the younger age of onset, other concomitant brain pathologies are rare, and for this reason CADASIL provides a purer model of SVD pathology. To assess the validity of any findings in the groups presenting with sporadic SVD group, we therefore carried out a replication study in a CADASIL cohort.
Methods
Study population 1: Sporadic SVD
Consecutive patients presenting with sporadic SVD were identified using the following inclusion criteria: clinical lacunar stroke syndrome (6) and MRI confirmation of lacunar infarction. Subjects were prospectively recruited from specialised stroke services in 3 district hospitals in South London, UK. In each, recruitment was from both an acute stroke unit and an outpatient minor stroke/TIA service. Exclusion criteria included any cause of stroke other than SVD including any cortical infarct, any large subcortical infarcts (>1.5cm), any cardioembolic source, and extracranial or intracranial large cerebral artery stenosis (>50%). 75% of patients meeting the inclusion criteria agreed to participate. Patients were studied at least 3 months after their last stroke (mean (SD) 3.7 (5.6) years), to avoid any effects on cognition due to acute ischemia. 100 subjects were recruited (mean (SD) age 71.1 (8.5) years; 60 men). All patients had brain MRI. The degree of leukoaraiosis on MRI was graded in the SVD group using the semi-quantitative Fazekas scale (7), modified to separate degrees of confluent leukoaraiosis: grade 0, no leukoaraiosis; grade 1, mild leukoaraiosis; grade 2, moderate confluent leukoaraiosis; and grade 3, severe confluent leukoaraiosis (8).
A control group (N=55, age 70.6 (7.9) years; 30 men), free of stroke and other central nervous system diseases, were recruited from family doctor practices in the same geographical area and from patients’ spouses and friends. Statistical comparisons showed that the control and SVD groups were well matched for age (p=.740) and gender (p=.510).
Study population 2: CADASIL
Subjects with a genetically confirmed diagnosis of CADASIL, based on a typical disease-causing cysteine altering mutation in the notch 3 gene, were recruited prospectively from a national CADASIL referral clinic at St George’s Hospital, London, UK. 78% of patients meeting the inclusion criteria agreed to participate. Forty subjects were recruited with mean (SD) age 51.1 (10.5) years; 15 were male. Of the 40 CADASIL patients, 18 (45%) had previously had a stroke. Seventeen (42.5%) were being treated with antidepressant medication. All had brain MRI.
A young control group consisting of 35 healthy individuals (mean (SD) age 53.0 (8.8) years; 14 men), was also recruited from family doctor practices in South London and from patients’ spouses and friends. Recruits from family doctor practice were contacted by letter and of those contacted approximately 20% responded, and of respondents 85% consented to take part. Statistical comparisons showed that the control and SVD groups were well matched for age (p=.390) and gender (p=.824).
The research was approved by the local research ethics committee and informed written consent was obtained from all participants.
Assessment Measures
All patient and control groups were assessed using the same scale to quantify QoL (Stroke-specific Quality of Life). Patients were also asked to complete activities of daily living (Activities of Daily Living and Instrumental Activities of Daily Living scales) and disability (modified Rankin scale) measures. Depression was assessed in the (older) sporadic SVD group using the age-appropriate Geriatric Depression Scale, and in the (younger) CADASIL cases using the Montgomery-Åsberg Depression Rating Scale (MADRS). All measures were administered by experienced neurologists or psychologists with full medical and medication histories taken; where patients could not recall their medications, prescription lists or hospital records were obtained.
Stroke-specific Quality of Life (SS-QoL) (5)
This scale was developed as a QoL measure for use in stroke trials. It provides different domains of QoL and has established internal reliability and sensitivity to change (5). Due to the chronic nature of SVD, and because the SS-QoL had been developed for a single post acute stroke assessment, the scale items were modified into the present tense to reflect general current experiences (e.g. ‘I often have to stop and rest during the day’). The scores were collapsed into aggregate scores for each domain, where a high score indicates better QoL.
Geriatric Depression Scale (GDS-30) (9)
Depressive symptoms in the SVD group were assessed by the 30-item GDS. This is a self-report measure which includes 30 items with yes/no responses, for example, ‘Do you feel that your life is empty?’ and ‘Are you in good spirits most of the time?’
Montgomery-Åsberg Depression Rating Scale (MADRS) (10)
Depressive symptoms in the CADASIL group were assessed using the MADRS, a clinician-administered scale with 10 questions relating to depressive symptoms. Responses range from 0-6 points dependent on the severity of the symptom.
Activities of Daily Living (ADL) (11)
ADLs were measured using Barthel’s index. The scale assesses the level of basic function and independent living.
Instrumental Activities of Daily Living (IADL) (see (12))
The IADL scale measures higher level everyday function related to independent living, such as the ability to manage finances or use public transportation.
Modified Rankin Scale (mRS) (13)
Functional status was measured using the mRS, a six-point rating scale developed to measure disability following stroke.
Mini-Mental State Exam (MMSE) (14)
General cognition was measured using the mini-mental state examination. A widely used test of dementia, the scale comprises a 30-point evaluation of the patient’s cognitive function, incorporating memory, concentration, copying, object recognition and time/place orientation tasks.
Data completeness
In the SVD group SSQoL data was available for all cases and all controls. Additional tests were completed by 98 of the 100 SVD cases, but GDS30 scores were not available in a further 9 of these, and ADL was not available for one further patient. In the CADASIL study SSQoL data was available for all cases and all controls. Additional measures were also completed by all CADASIL patients.
Statistical analysis
1. QoL outcomes in SVD compared with a normal population
Independent t-tests were used to compare differences between each patient group and their respective controls on total QoL scores and for the QoL subscales. The total QoL score was calculated pro-rata to account for intermittent missing responses (no. subtests * mean subtests). Effect sizes (Glass’s Δ) were calculated as (control group mean - patient mean)/control SD). Scale reliability score for each group and the total sample were calculated using Cronbach’s alpha.
2. Predictors of QoL outcomes in SVD
Stepwise regressions were performed to examine the relative contributions of disability, depression, and activities of daily living to quality of life in SVD and CADASIL. Absolute relationships between the SSQoL and measures of disability, depression and cognition, as well as leukoaraiosis grade, were assessed using Pearson’s correlation coefficients. The same analysis was also repeated with the mood subscale removed from the SSQoL, based on the notion that the SSQoL is sensitive to mood state (15) and this might result in inflated associations between depression and QoL due to the same thing being measured in each case.
3. Predictor independence
To examine whether the relationship between depression and QoL was mediated by disability, the interaction between depression and disability was calculated using moderation (16) and mediation analyses.
4. Model replication
To test the reliability of the regression model, the predicted QoL scores were calculated for all participants first using the regression equation derived from the sporadic cases and then using the regression equation derived from the genetic cases. This was correlated to give a measure of model stability.
Results
Demographics of the patient and control groups are shown in Table 1.
Table 1.
Patient demographics and results from the different assessments. Values are number (%) or mean (SD) as appropriate.
SSQoL: Stroke-specific Quality of Life. GDS-30: Geriatric Depression Scale. MADRS: Montgomery-Åsberg Depression Rating Scale. ADL: Activities of Daily Living. IADL: Instrumental Activities of Daily Living. MMSE: Mini-Mental State Exam. *Significantly different from control group, p<.0001
| Sporadic SVD study | CADASIL study | |||
|---|---|---|---|---|
| SVD cases (n=100) |
Controls (n=55) |
CADASIL (n=40) |
Controls (n=35) |
|
|
| ||||
| Mean (SD) age Age range (years) |
71.1 (8.5) 46-89 |
70.6 (7.9) 44-86 |
51.1 (10.5) 34-70 |
53.0 (8.8) 36-69 |
|
| ||||
| Male gender | 60 (60%) | 30 (55 %) | 15 (38%) | 14 (40%) |
|
| ||||
| Lacunar stroke | 100 (100%) | 18 (45%) | ||
|
| ||||
| Time since last stroke (mean years (SD)) |
3.7 (5.6) | - | 4.5 (38) | - |
|
| ||||
| Leukoaraiosis grade | ||||
| 0 | 6% | - | 0% | - |
| 1 | 19% | - | 5% | - |
| 2 | 45% | - | 35% | - |
| 3 | 30% | - | 60% | - |
|
| ||||
| Treated Hypertension | 95 (95%) | 19 (34.5%) | 12 (30%) | 7 (20%) |
|
| ||||
| Diabetes mellitus | 23 (23%) | 4 (7.3%) | 1 (2.5%) | 1 (2.9%) |
|
| ||||
| Smoking | ||||
| Never | 41 (41%) | 20 (36.4%) | 23 (57.5%) | 20 (57.1%) |
| Ex | 39 (39%) | 29 (52.7%) | 14 (35%) | 12 (34.3%) |
| Current | 20 (20%) | 6 (10.9%) | 3 (7.5%) | 3 (8.6%) |
|
| ||||
| Treated depression | 4 (4%) | - | 17 (42.5%) | - |
|
| ||||
| SSQoL | 196.8 (35.2) * | 226.8 (15.3) | 202.0 (29.7)* | 228.6 (13.1) |
|
| ||||
| GDS-30 | 8.2 (6.5) | - | - | - |
|
| ||||
| MADRS | - | - | 9.4 (9.2) | - |
|
| ||||
| ADL | 18.9 (2.1) | - | 19.3 (1.7) | - |
|
| ||||
| IADL | 7.2 (1.6) | - | 7.4 (1.6) | - |
|
| ||||
| Modified Rankin score | 1.2 (1.3) | - | 0.6 (1.1) | - |
|
| ||||
| MMSE | 27.4 (2.9) | - | 28.4 (2.9) | - |
Study 1: Sporadic SVD cohort
The mean (SD) total SSQoL score was significantly lower for sporadic SVD patients than for controls, 196.8 (35.2) versus 226.8 (15.3), p<.0001. This reduction was seen across all sub-domains of the SSQoL scale (Table 2). The modified SSQoL showed high internal consistency for controls (α = .91), SVD patients (α = .96), and for all participants combined (α = .97). 34% of the SVD patients fitted the GDS criteria for depression (9) and around half of those who fell in the normal range reported 5 or more depressive symptoms.
Table 2.
Comparison of Stroke-specific Quality of Life (SSQoL) scores in the cases and controls for the sporadic SVD study and the CADASIL study, mean (SD).
| Sporadic SVD | CADASIL | |||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls (older) |
Independent samples t-tests |
Δ | Cases | Controls (younger ) |
Independent samples t-tests |
Δ | |
| SSQol Total | 196.8 (35.2) |
226.8 (15.3) |
p<.0001 | −2.0 | 202.0 (29.3) |
228.6 (13.1) |
p<.0001 | −2.0 |
| Energy | 3.2 (13) |
4.1 (0.9) |
p<.0001 | −0.5 | 3.1 (13) |
4.0 (1.1) |
p=.003 | −0.8 |
| Language | 4.5 (0.6) |
4.8 (0.2) |
p<.0001 | −1.5 | 4.5 (0.5) |
4.8 (0.3) |
p=.002 | −1.0 |
| Mobility | 4.0 (1.0) |
4.7 (0.4) |
p<.0001 | −1.8 | 4.5 (0.6) |
4.9 (0.2) |
p=.001 | −2.0 |
| Mood | 4.1 (1.1) |
4.7 (0.5) |
p<.0001 | −1.2 | 4.1 (0.9) |
4.7 (0.5) |
p<.0001 | −1.2 |
| Personality | 3.8 (1.2) |
4.5 0.6) |
p<.0001 | −1.2 | 3.6 (1.3) |
4.2 (0.8) |
p=.017 | −0.8 |
| Self Care | 4.6 (0.7) |
5.0 (0.1) |
p<.0001 | −4.0 | 4.8 (0.6) |
4.9 (0.1) |
p=.045 | −1.0 |
| Social roles | 3.2 (1.2) |
4.2 (0.9) |
p<.0001 | −1.1 | 3.5 (1.3) |
4.2 (0.9) |
p=.005 | −0.8 |
|
Upper
function |
4.4 (0.9) |
4.9 (0.3) |
p<.0001 | −1.7 | 4.6 (0.8) |
4.9 (0.1) |
p=.008 | −3.0 |
| Vision | 4.7 (0.6) |
5.0 (0.2) |
p=.003 | −1.5 | 4.9 (0.3) |
5.0 (0.1) |
p=.099 | −1.0 |
| Family life | 3.9 (1.1) |
4.7 (0.5) |
p<.0001 | −1.6 | 3.8 (1.2) |
4.8 (0.4) |
p<.0001 | −2.5 |
| Thinking | 3.2 (1.2) |
4.0 (0.9) |
p<.0001 | −0.9 | 2.8 (1.4) |
4.2 (1.0) |
p<.0001 | −1.4 |
| Work | 3.8 (1.2) |
4.7 (0.4) |
p<.0001 | −2.8 | 4.2 (0.9) |
4.9 (0.3) |
p<.0001 | −2.3 |
Total scores and scores for each individual domain are given. Δ= Glass’s effect size
Predictors of quality of life in SVD
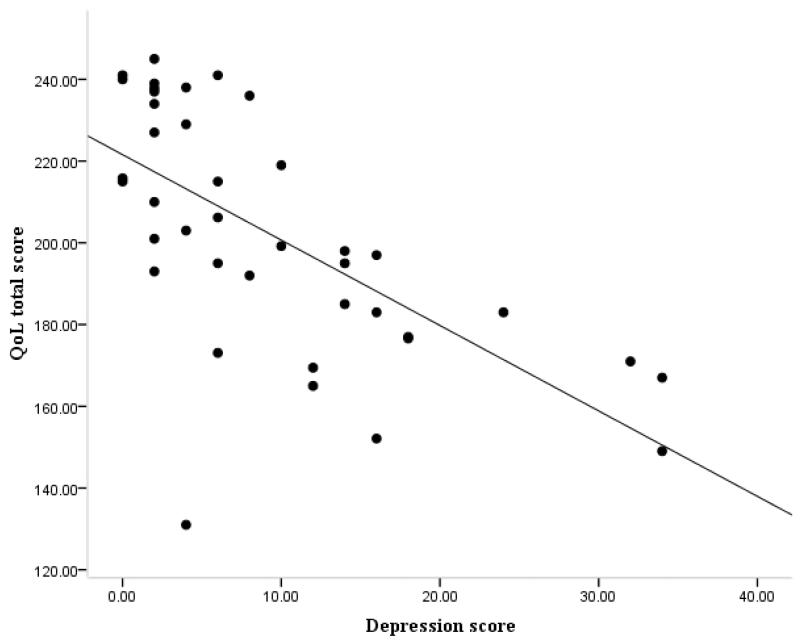
There were significant correlations between all of the different assessment measures and SSQoL (GDS r= −0.748, p<0.0001; mRS r= −0.567, p<0.0001; ADL r= 0.449, p<0.00001; IADL r= 0.453, p<0.0001; MMSE r= .235, p<0.021). To determine which of these factors best predicted QoL in SVD, a stepwise multiple regression was carried out entering IADL, ADL, mRS, MMSE and GDS30 into the model. The final model accounted for 71.3% of the total variance in SSQoL, with GDS30 accounting for the highest proportion (52.9%), and mRS accounting for the remaining 18.4% (Table 3). When mood related items (5/49) were excluded in calculating the overall QoL score, the model remained significant, accounting for 70.8% of the variance with depression accounting for the highest proportion (47%).
Table 3.
Stepwise regression models for the prediction of QoL in SVD and CADASIL. GDS-30: Geriatric Depression Scale. MADRS: Montgomery-Åsberg Depression Rating Scale. mRS: Madified Rankin Score
| R2 | R2 change |
Standardised β coefficient |
Model significance |
|
|---|---|---|---|---|
| SVD | ||||
| GDS30 | .529 | .529 | −.639 | p<.0001 |
| GDS30 + mRS | .713 | .184 | −.438 | |
|
| ||||
| CADASIL | ||||
| MADRS | .422 | .422 | −.584 | p<.0001 |
| MADRS + mRS | .597 | .176 | −.424 | |
Independence of predictor variables
Moderated regressions for the effect of disability on the depression/QoL relationship showed that the interaction term (mRScGDS30c) did not correlate with QoL (r= −.041, p=.352) and the regression model remained unchanged (R2= .713, p<.0001), when mRScGDS30c was included in the model. Additional partial correlations confirmed that the relationship between GDS30 and SSQoL was not mediated by mRS scores (r= −.763, p<.0001). Similarly, the relationship between mRS score and QoL was not mediated by GDS30 (r= −.636, p<.0001).
Age as a covariate
Age and QoL did not correlate significantly (r=−.108, p=.289). Further partial correlations confirmed that including age as a covariate had no effect on the relationship between QoL and mRS score (r= −.592, p<.0001) or QoL and GDS30 (r=.739, p<.0001).
Leukoaraiosis grade and QoL
Leukoraiosis grade correlated significantly with SSQoL (r= −.283, p= .006), mRS (r= .241, p= .018), ADL (r= −.216, p= .037), and GDS30 (r= .208, p= .05), but did not correlate with IADL (r = −.177, p= .087) or MMSE (r= −.012, p=.911).
Study 2: CADASIL cohort
The mean (SD) total SSQoL score was significantly lower for CADASIL patients than for controls (202.0 (29.7) versus 228.6 (13.1), p<.0001). Analyses across subscales also revealed significant (p<.05) deficits in energy, language, mobility, mood, personality, self care, social roles, upper extremity function, family life, thinking, and work, but not for vision (see Table 2). The modified SSQoL showed high internal validity when used with controls (α = .87), CADASIL patients (α = .96), and for all participants combined (α = .95). 40% of the CADASIL patients met the MADRS criteria for mild or moderate depression (17).
Predictors of quality of life in CADASIL
MADRS, IADL, mRS and MMSE correlated significantly with overall quality of life (MADRS r= −.649, p<.0001; mRS r= −.515, p=.001; IADL r=− .392, p=.012; MMSE r=.434; p=.005). The correlation with ADL approached significance (r= .311, p=.051). A stepwise multiple regression was carried out to determine the best predictors of quality of life in CADASIL. The predictor variables entered into the model were IADL, ADL, mRS, MMSE and MADRS. The final model accounted for 59.7% of the variance in SSQOL, with depressive symptoms accounting for the majority of this (42.2%), and the remaining variance accounted for by mRS score (see Table 3 for the model summary). When mood related items were excluded from the total SSQoL, the model remained significant, accounting for 58.5% of the variance with depression accounting for the highest proportion (36.6%).
Moderation and mediation analyses
The interaction term between the MADRS and Rankin scores (mRScMADRSc) did not correlate with QoL (r= −.003, p=.984) and the regression model remained unchanged (R2= .597, p<.0001), when mRScMADRSc was included in the model. Additional partial correlations confirmed that the relationship between MADRS scores and SSQoL was not mediated by mRS scores (r= −.673, p<.0001). Similarly, the relationship between mRS scores and QoL was not mediated by MADRS scores (r= −.551, p<.0001).
Age as a covariate
The results showed that the relationships between QoL and mRS score (r= −.425, p<.004) and QoL and MADRS (r= −.629, p<.0001) remained significant when age was held as a covariate.
Leukoaraiosis grade and QoL
Leukoraiosis grade correlated significantly with MMSE (r= −.320, p=.044) but did not correlate significantly with SSQoL (r= −.277, p= .084), mRS (r= .298, p= .086), IADL (r= −.275, p= .062) or MADRS (r= −.253, p= .115), or ADL (r = −.277, p= .084).
Model replication
The regression model for the SVD group showed a striking similarity to the model for the younger CADASIL group. Cross validation revealed that the predictions from the two independently calculated models were highly correlated (r= .972, p<.0001 and r= .977, p<.0001), indicating model stability (see table 4).
Table 4.
Cross validation of QoL models in SVD and CADASIL
| SVD patient group | CADASIL patient group |
|
|---|---|---|
| SVD model 239.405 + (−3.327 * depression) + (−11.686 * Rankin) |
R2=.713 | R2=.570 |
| CADASIL model 226.431 + (−1.879 * depression) + (−11.885 * Rankin) |
R2=.674 | R2=.597 |
| Model correlations per patient group | r=.972 | r=.977 |
Post hoc analysis
As MMSE did not remain in the stepwise regression model, correlations with the depression indices were calculated for both groups in order to examine whether the depression scores were a consequence of reduced cognition. The results showed that depression and MMSE score did not significantly correlate for either group (SVD r = −.079; p=.468; CADASIL r= −.289; p= .071).
Discussion
This study shows that in patients with both sporadic SVD, and younger onset SVD due to CADASIL, depressive symptoms are a major predictor of the reduced QoL. They are a more important predictor than disability and act independently of disability.
In both patient groups, depressive symptoms accounted for approximately half of the variance in QoL. The strikingly similar findings in the sporadic SVD and CADASIL groups, suggest that this association is a consistent feature of SVD. The QoL measure we used does include some indicators sensitive to mood; however excluding these from the analysis did not significantly alter the results. The only other variable which remained significantly associated with QoL was disability, assessed by the modified Rankin scale, but this accounted for a lesser degree of variance in QoL. Depression and disability were found to predict QoL independently of one another.
Many recent studies have associated white matter ischemia with late-onset depression (4,18) and depression has been found to be a prominent feature of CADASIL, where it frequently presents before clinical symptoms such as stroke (19). This has led to the hypothesis that white matter ischemia and subsequent white matter tract damage results in disruption of cortical-subcortical connections underlying complex networks involved in the regulation of mood (20,21). MRI was performed in all patients allowing us to grade the degree of leukoaraiois and determine whether it also related to quality of life. In the sporadic SVD group, leukoaraiosis grade was positively related to QoL. We also confirmed a graded relationship between increasing degree of leukoaraiosis and depression and disability. This is consistent with previous work showing that increased leukoaraiosis is an important predictor of older-onset depression and disability (22). In the CADASIL group we found a significant correlation between leukoaraiosis grade and MMSE but no significant correlations between other functional measures and leukoariosis grade, although the absolute r-values were similar to those seen in the sporadic SVD group. The lack of association may partly reflect the smaller sample size. Of note, the finding of similar associations in the CADASIL group relating to depression and QoL enhances the importance of vascular factors in the pathogenesis of the dysexecutive-depressive syndrome of SVD, underlining the usefulness of comparing these two groups of patients, with CADASIL not complicated by neurodegenerative changes associated with old age.
A limitation of this study is that the scope of our observations are confined to the domains examined. For example, apathy and motivation are features not investigated in the current study, and other groups have reported an association between apathy and QoL in CADASIL (23).
Additionally, it could be argued that mood state, including levels of depression, contributes to quality of life and for this reason QoL scales may include mood-related items. To explore this we removed depression items from the quality of life analysis and obtained similar results. Nevertheless, there remains the issue that lower mood might result in people reporting experiencing a low quality of life, whilst this may not necessarily by the case. Similarly, measurement of activities of daily living was based on self report and this might be influenced by mood state or lack of awareness of disability by the patient. To address this issue, informant-based measurement of these aspects could be used, although it should be recognised that psychosocial factors have also been found to impact on informant ratings in this regard (24).
Our results have a number of clinical implications. Firstly, not surprisingly they highlight that SVD is associated with a markedly reduced QoL. More interestingly, they demonstrate the importance of depressive symptoms in the reduction. This may reflect the suggested causal biological associations between white matter disease and depression. Importantly, we also discovered that depression was often not clinically recognised or treated in the sporadic SVD group: only 4% of the patients were receiving antidepressant medication at the time of the study, although 34% met the criteria for depression on the GDS. It is possible that more intensive treatment may result in significant improvements, not only in mood, but also in QoL in this patient group. Further work is therefore required to assess the causal relationship through investigation of the impact of treating depression, which is often undiagnosed in this patient group.
Figure 1.
Correlation between depression (GDS30) and quality of life (SSQoL) scores in the SVD group
Figure 2.
Correlation between depression (MADRS) and quality of life (SSQoL) scores in the CADASIL group
Acknowledgements
Recruitment to the study was supported by the English National Institute of Health Research Clinical Stroke Research Network. We also thank Prof. Lalit Kalra and Dr Anthony Rudd for help with recruitment, and Zuzana Winter, Susie Tinkler for assistance with testing and Andrew Lawrence for assistance with testing and advice on moderation analysis.
Funding: This research was supported by grants from the Stroke Association (TSA 2006/12) and the Wellcome Trust (081589/Z/06/Z).
Footnotes
Conflicts of interest: None
Reference List
- 1.Erkinjuntti T, Rockwood K. Vascular dementia. Semin Clin Neuropsychiatry. 2003;8:37–45. doi: 10.1053/scnp.2003.50004. [DOI] [PubMed] [Google Scholar]
- 2.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–36. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan M, Morris RG, Markus HS. Brief cognitive assessment for patients with cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2005;76:1140–5. doi: 10.1136/jnnp.2004.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien JT, Firbank MJ, Krishnan MS, van Straaten EC, van der Flier WM, Petrovic K, et al. White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. Am J Geriatr Psychiatry. 2006;14:834–41. doi: 10.1097/01.JGP.0000214558.63358.94. [DOI] [PubMed] [Google Scholar]
- 5.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–9. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 6.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–6. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 7.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol. 1987;149:351–6. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 8.Hassan A, Hunt BJ, O’Sullivan M, Parmar K, Bamford JM, Briley D, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003;126:424–32. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 9.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 11.Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 12.Pantoni L, Basile AM, Pracucci G, Asplund K, Bogousslavsky J, Chabriat H, et al. Impact of age-related cerebral white matter changes on the transition to disability -- the LADIS study: rationale, design and methodology. Neuroepidemiology. 2005;24:51–62. doi: 10.1159/000081050. [DOI] [PubMed] [Google Scholar]
- 13.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Scott Med J. 1957;2:200–15. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Williams LS, Weinberger M, Harris LE, Biller J. Measuring quality of life in a way that is meaningful to stroke patients. Neurology. 1999;53:1839–43. doi: 10.1212/wnl.53.8.1839. [DOI] [PubMed] [Google Scholar]
- 16.Dux MC, Woodard JL, Calamari JE, Messina M, Arora S, Chik H, et al. The moderating role of negative affect on objective verbal memory performance and subjective memory complaints in healthy older adults. J Int Neuropsychol Soc. 2008;14:327–36. doi: 10.1017/S1355617708080363. [DOI] [PubMed] [Google Scholar]
- 17.Muller MJ, Szegedi A, Wetzel H, Benkert O. Moderate and severe depression. Gradations for the Montgomery-Asberg Depression Rating Scale. J Affect Disord. 2000;60:137–40. doi: 10.1016/s0165-0327(99)00162-7. [DOI] [PubMed] [Google Scholar]
- 18.Teodorczuk A, Firbank MJ, Pantoni L, Poggesi A, Erkinjuntti T, Wallin A, et al. Relationship between baseline white-matter changes and development of late-life depressive symptoms: 3-year results from the LADIS study. Psychol Med. 2009:1–8. doi: 10.1017/S0033291709990857. [DOI] [PubMed] [Google Scholar]
- 19.Valenti R, Poggesi A, Pescini F, Inzitari D, Pantoni L. Psychiatric disturbances in CADASIL: a brief review. Acta Neurol Scand. 2008;118:291–5. doi: 10.1111/j.1600-0404.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 20.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–95. [PubMed] [Google Scholar]
- 22.Inzitari D, Pracucci G, Poggesi A, Carlucci G, Barkhof F, Chabriat H, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339:b2477. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes S, Viswanathan A, Godin O, Dufouil C, Benisty S, Hernandez K, et al. Apathy: a major symptom in CADASIL. Neurology. 2009;72:905–10. doi: 10.1212/01.wnl.0000344166.03470.f8. [DOI] [PubMed] [Google Scholar]
- 24.Clare L, Nelis SM, Martyr A, Roberts J, Whitaker CJ, Markova IS, et al. The influence of psychological, social and contextual factors on the expression and measurement of awareness in early-stage dementia: testing a biopsychosocial model. Int J Geriatr Psychiatry. doi: 10.1002/gps.2705. In press. [DOI] [PubMed] [Google Scholar]