Abstract
The process of cell division is highly complex. The DNA of the genome most be accurately replicated and segregated into two precisely equal portions; the cytoskeleton must be actively rearranged; and the cellular motor forces that allow the separation of the replicated chromosomes and the splitting of the mother cell into two daughters must be kept under strict spatial and temporal regulation. Not surprisingly for a process of this complexity, there is a wide range of proteins whose location and activity must be accurately controlled to ensure both efficiency and precision. Although the demands placed on these cell cycle proteins are high, once cells such as neurons differentiate they enter a long non-mitotic phase where evolution has conspired to repurpose many of these proteins, leading them to assume new and often unrelated cellular tasks. In neurons there is a wide range of non-cycling functions for these ‘cell cycle’ proteins and this review covers some of the best-known examples. There is little apparent logic to the second use, but the sheer number of examples suggests that there must be a significant evolutionary advantage to this repurposing strategy.
Introduction
When a construction crew is finished with a building, they are expected to clean up the site and leave no trace of the heavy machinery that was used in the project. This may have esthetic value in an urban environment, but in the cellular environment of the maturing brain, after neurogenesis is finished, the same principles do not apply. Instead the ‘heavy machinery’ that drives the cell cycle during the growth of the CNS is not removed; it is simply repurposed to a variety of new functions in the adult nerve cell. Occasionally this means a modest re-direction of effort – a kinase is after all a kinase. But more often it means that a protein with one function during development adopts an entirely new function in the adult. The metamorphosis can be so dramatic that calls into question the meaning of a label such as “cell cycle protein”.
The machinery of the cell cycle
The normal process of cell division is a complex, interconnected network of biochemical and cell biological events. While the center of the process is the duplication of the cell’s genome, DNA replication must proceed in a cellular context that includes the proper regulation of other cellular events including metabolism, transcription, translation and mitochondrial biogenesis, as well as chromatin condensation, spindle formation and ultimately cytokinesis. Many solid reviews are written each year on this topic and the interested reader is referred to them for more details (1–5); a brief overview will suffice here.
A classic cell cycle is described as having four phases: G1, a growth phase; S, a DNA replication phase; G2, a preparation phase; and M, the phase during which the chromosomes condense and segregate and following cytokinesis two daughter cells are formed. A series of regulatory proteins known as cyclins, activate different members of a family of enzymes known as cyclin dependent kinases (Cdks). These proline-directed serine/threonine protein kinases help to guide the cell through a normal cell cycle. The cyclin/kinase complexes are in turn regulated by a series of Cdk inhibitors (CKIs). To ensure an orderly DNA replication process, an origin recognition complex (ORC) binds at a series of initiation sites known as origins and ensures that each one ‘fires’ only once during a cell cycle. Cdk/cyclin activity is also regulated by protein degradation, initiated by ubiquitination at specific points in the cell cycle. One such ubiquitin ligase is the anaphase promoting complex/cyclosome (APC/C) – a multi-protein complex that can switch between two major activator proteins, Cdc20 and Cdh1, depending on the phase of the cell cycle (6–9). The complex movements of chromosomes and cellular organelles that occurs during M-phase requires many cellular players including cytoskeletal elements such as microtubules and their associated proteins such as tau.
Second careers for the proteins of the cell cycle
A careful examination of any protein will often reveal second functions, some of which are quite dissimilar from the one originally discovered. For example, cytochrome C is an iron-containing protein that is a well known part of the electron transport process; yet students of cell death have come to know it better as the protein that triggers the formation of the apoptosome. In this particular case, there is a clear rationale for linking cell death to the loss of mitochondrial integrity: a cell with badly damaged mitochondria would have limited capacity to produce ATP and thus might be better off eliminated. Despite this ‘logic’, the function of the cytochrome C protein in the two contexts is completely different. Examples of the alternative functions for some of the proteins involved in cell cycle regulation include examples such as this as well as situations where both the contexts and the second functions of the “cell cycle proteins” are unrelated. The only logic to the situation may be that evolution is a conservative process, finding new uses for existing proteins rather than designing new proteins for each new purpose.
Origin of replication proteins in dendrites
The origin recognition complex (ORC) is a large multisubunit complex that in most eukaryotic cells binds to the sites on the genome where DNA replication is initiated (10). The complex includes 6 ORC proteins as well as others; it identifies sites of replication initiation and prepares the site for DNA synthesis. Far away from the DNA of the nucleus, the neuronal dendrite is a highly complex cytoplasmic extension of the neuronal cytoplasm. Depending on the cell type the furthest extensions of the dendritic tree can be hundreds of microns from the associated cell body. At this distance from the nucleus, there is little reason to expect an origin binding protein would have any function as there is no DNA (at least no nuclear DNA) let alone origins of replication present. Yet ORC2, 3, 4, 5 and 6 are expressed at moderate to high levels in adult brain (11). Using immunocytochemistry, the physical presence of these proteins in the most distal dendritic branches (but not axons) of cultured neurons is striking. Further, the knock down with siRNA of either ORC3 or ORC5 leads to a dramatic dendritic atrophy. The number of dendritic branch points declines by several fold, as does the density of post-synaptic spines. There is as yet no mechanistic understanding as to exactly how these proteins function in spines nor how their DNA replication properties relate to those required for maintaining the integrity of the dendrite. The speculation is that, similar to their association with the spindle mid-zone, cleavage furrow, and centrosomes, the ORC proteins may be involved with the cytoskeletal reorganization needed for proper dendritic maintenance.
The functions of the APC/C complex in adult neurons
To ensure the unidirectional progression of the cell cycle, two principal E3-ligases mediate the stage-specific degradation of the proteins involved in cell cycle control. The two main players in this regard are the SCF complex (Skp1, Cul1, and Rbx1 and an interchangeable F-box protein subunit) and APC/C (anaphase-promoting complex/cyclosome) complex (12). The SCF complex is used throughout the cell cycle whereas APC/C is active from mitosis only through early G1 (13). APC/C is a multi-protein complex made up of 11 different subunits that can switch between two major activator proteins, Cdc20 and Cdh1, depending on the cell cycle phase (6–8, 14); Cdh1-APC functions during late M- and G1 phases, while Cdc20-APC drives anaphase in early mitosis. Beyond these cell cycle functions, both proteins have major post-neurogenic roles in neuronal structure. For example, Cdc20 promotes presynaptic (axonal) differentiation by triggering the degradation of NeuroD2 and thereby promoted presynaptic differentiation (15). In the neuronal dendrite, centrosomal CaMKIIβ drives dendritic retraction and pruning in an action mediated by the Cdc20 form of APC (16). CaMKIIβ-mediated Cdc20 phosphorylation releases it from the centrosome, inhibiting APC activity and triggering a switch from growth to retraction of dendrites. In a complementary way, in the axon, the Cdh1 form of APC/C is an active participant in axonal growth and patterning (9). Inhibition of Cdh1 activity specifically triggers the elongation of axons but not dendrites. Indeed, the Cdh1 form of APC may represent a negative regulator of axonal behavior during development and possibly regeneration. The speculation for both of these observations is that ubiquitin-mediated protein degradation, acting locally or potentially within the nucleus, serves to regulate neuritic growth and branching in a structure specific fashion.
The non-mitotic role of tau
The microtubule associated protein, tau, is well recognized as an important part of the neuronal cytoskeleton. Under most circumstances tau localizes to the axon where its maintenance of proper microtubule stability is essential to axon integrity and efficient axon transport (17). In neurodegenerative conditions such as Alzheimer’s disease, fronto-temporal dementia and others, hyperphosphorylation of tau weakens its microtubule affinity and favors aggregation of the protein into oligomeric and ultimately macromolecular structures recognized as paired-helical filaments (18). Yet tau protein, including its phosphorylated isoforms, is found in the nucleus and associates with the microtubules of the mitotic spindle (19–21). In this case the microtubule stabilizing function of tau is probably similar if not identical in both the axon and the mitotic spindle. Yet it fits will within the classification of a true cell cycle protein with distinct functions in the adult neuron.
p27 and p57
The p27 and p57 proteins are members of the Kip family of Cdk inhibitors. Their CKI partners, the INK4 proteins, target CDK4/6-cyclin D during the G1 phase of the cell cycle and inhibit Cdk-cyclin pairing. The Cip/Kip proteins (p21Cip1, p27Kip1, and p57Kip2) inhibit a broader range CDK-cyclin dyads. CDK inhibition by p27 involves tight association with CDK-cyclin complexes leading to kinase inhibition. Both p57 and p27 have been shown to participate in neuronal differentiation and migration through their involvement with changes in the neuronal cytoskeleton (22, 23). In particular, the Cip/Kip family regulates the Rho signaling pathway, with important implications for processes such as coordinating cytoskeletal changes during of nerve cell migration during development, and possibly helping to create the invasive tendency of cancer cells. There is evidence that some cytoskeletal changes that are observed during the cell cycle also require p27; this function may have direct analogy to the migration function post neurogenesis, but it is distinct from the Cdk inhibition. A role as a microtubule binding protein has been proposed for p27, partially explaining its involvement in the nuclear translocation of the developing nervous system as well as in the neuritic branching seen during migration (24). Cells lacking p27 show reduced motility; in the neuronal cytoplasm, when p27 is stabilized by phosphorylation by Cdk5, it binds to cofilin. Cofilin-actin interaction is blocked by this modification allowing migration to proceed (25). In a similar and possibly overlapping fashion, p57 also enhances neurite outgrowth and advances radial migration during development (26)
Cell cycle regulation in the adult
The neurons of the adult CNS are characterized as permanently post-mitotic. The entire concept of a neuronal ‘birthday’ is based on the premise that virtually every neuron in the adult brain goes through a final cell division during development (27, 28). Yet increasing evidence has shown that while adult nerve cells may be mitotically quiescent, there is nothing permanent about their post-mitotic state. They can re-enter a cell cycle process, complete with DNA replication. This attempt at cell division, however, results in the death of the nerve cell. Therefore, it follows that an adult neuron must constantly suppress its cell cycle to remain healthy and survive (29–32). The proteins whose function it is to carry out this life-long suppression are both familiar and unfamiliar.
Retinoblastoma
One familiar protein whose function demonstrates this concept of chronic, life long cell cycle suppression is the tumor suppressor protein, retinoblastoma (RB). Mice with genetic deletions of RB die as embryos, with massive apoptotic death among the recent neuronal emigrants from the ventricular zone (33–35). The timing of these deaths points to a collision between continued unrestrained cell division and the initiation of differentiation and migration. Yet early on it was recognized that RB had developmental functions in addition to its role in cell cycle suppression (36). We now know that this requirement for RB persists into adulthood; acute deletion of RB function results in ectopic neuronal cell cycle protein expression and death (37). Although RB is believed to function by sequestering E2F1, it is interesting that the adult neuronal deaths caused by RB deficiency occur without activation of the E2F-targeted apoptotic genes, Apaf1 and Puma. The concept is expanded further by the association of RB with a second E2F family member, E2F3. In the absence of RB, the process of neuronal migration is severely compromised and this phenotype has been explicitly shown to be distinct from the cell cycle advancing actions of E2F3 (38).
ATM (ataxia-telangiectasia mutated)
The ATM protein is a member of the PI3kinase family whose functions include the induction of cell cycle checkpoint proteins during the repair of DNA damage (39–41). Given this activity, it was a unifying observation that the neuronal death observed in cerebellar neurons and elsewhere is associated (in mouse and human) with ectopic re-activation of the cell cycle (42). As with the retinoblastoma protein, however, there is emerging evidence that the requirement for ATM as a cell cycle suppressor is lifelong. Indeed, a failure of ATM signaling may be one factor leading to the death of neurons in Alzheimer’s disease (43). Yet ATM is also an example of a cell cycle protein whose functions in the adult neuron include other non-cell cycle related events. The ATM protein participates in the trafficking and function of synaptic vesicles and is required by hippocampal neurons to establish the network property known as long term potentiation (44). Looking beyond the CNS it has recently been found that ATM function is needed for proper insulin functioning, both signaling (45) and secretion (46).
Cdk5
Cdk5 is an unexpected entry on any list of cell cycle proteins. Originally, it was identified as an atypical Cdk, with no apparent role in a normal cell cycle process. In recent years, however, it has become clear that Cdk5 functions as a cell cycle suppressor (47). Paradoxically, this function does not rely on its kinase activity. Rather, Cdk5 acts in an RB-like fashion by sequestering E2F1 and preventing its association with its activating partner, DP-1 (48). As with the other cell cycle suppressors cited earlier, the effect is lifelong and there is evidence for a failure of this function during the death of neurons in Alzheimer’s disease (49).
Cdh1
This subunit of the APC/C complex may also have a role in cell cycle suppression in the adult brain (50). Conditional knockouts of the gene encoding Cdh1 (Fzr1) show a late onset development of tumors of a variety of cell types and a hyperproliferation of cells lining the ventricular zone. As they are Sox2/Sox9-positive, these are likely to be neuronal progenitor cells. Associated with these cellular defects, both motor and cognitive performance of the conditional knockouts was degraded significantly. This suggests that the roles of Cdh1, like those of the Cdk5 and ATM, include a cell cycle suppression function in the non-mitotic neurons of the adult.
Concluding comments
The examples of repurposed proteins cited here come from all phases of the cell cycle. Their functions in the adult neuron range widely as well (Figure 1). If there were a common theme that characterized the non-mitotic uses of cell cycle proteins, it might be neuronal cytoskeletal integrity. In this small sample, it would appear that axonal and presynaptic functions tend to be the province of proteins whose cell cycle activity tended to be in the G2 and M cell cycle phases, while those involved with migration and dendritic structure are mostly G1 and S phase proteins. From an evolutionary perspective, it should be noted that the sheer number of proteins that fit in this category suggests that finding new uses for the heavy machinery of the cell cycle is a process with significant adaptive advantage for the neuron. And in truth, the concept of repurposing of construction machinery when its initial function is complete is not a new one (51).
Figure 1.
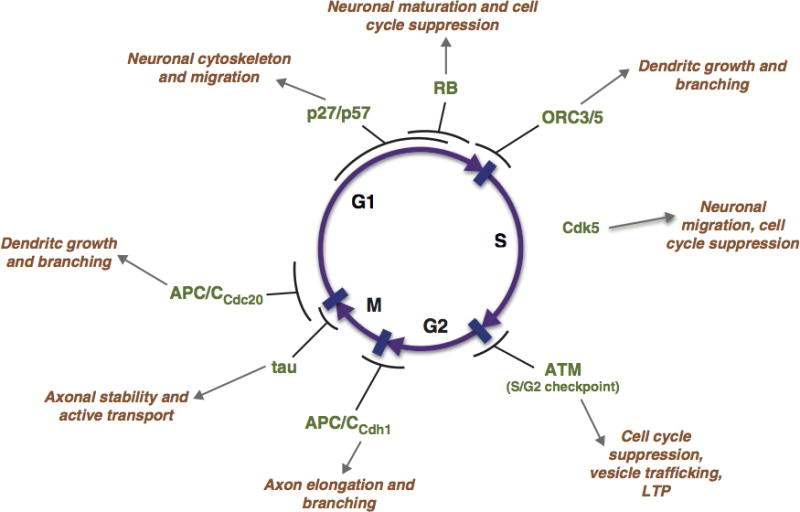
The phases of the cell cycle are indicated in the center of the circle. Around the perimeter, the proteins described in this review are indicated in green font with their approximate point of action in the cell cycle indicated with a black arc. Outside of these, the adult neuronal functions of the same proteins are indicated in a golden font. Cdk5, which has no known role in a normal cell cycle process, is represented next to (but not connected with) S-phase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8(2):149–60. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 2.Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol. 2008;9(11):910–6. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- 3.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18(22):2699–711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 4.Nevins JR. The Rb/E2F pathway and cancer. Hum Mol Genet. 2001;10(7):699–703. doi: 10.1093/hmg/10.7.699. [DOI] [PubMed] [Google Scholar]
- 5.Wong JV, Dong P, Nevins JR, Mathey-Prevot B, You L. Network calisthenics: control of E2F dynamics in cell cycle entry. Cell Cycle. 2011;10(18):3086–94. doi: 10.4161/cc.10.18.17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2(2):163–71. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- 7.Visintin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278(5337):460–3. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Summers MK, Pham V, Lill JR, Liu J, Lee G, et al. Deubiquitinase USP37 is activated by CDK2 to antagonize APC(CDH1) and promote S phase entry. Mol Cell. 2011;42(4):511–23. doi: 10.1016/j.molcel.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 9**.Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303(5660):1026–30. doi: 10.1126/science.1093712. This paper was the first to suggest that the APC/C complex proteins played a formative role in the post-mitotic differentiation of neurons. [DOI] [PubMed] [Google Scholar]
- 10.Stillman B. Origin recognition and the chromosome cycle. FEBS letters. 2005;579(4):877–84. doi: 10.1016/j.febslet.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 11**.Huang Z, Zang K, Reichardt LF. The origin recognition core complex regulates dendrite and spine development in postmitotic neurons. J Cell Biol. 2005;170(4):527–35. doi: 10.1083/jcb.200505075. The observations of origin recognition complex proteins (ORCs) in dendrites and their requirement for dendritic integrity represents perhaps the most dramatic example of cell cycle protein re-purposing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ang XL, Wade Harper J. SCF-mediated protein degradation and cell cycle control. Oncogene. 2005;24(17):2860–70. doi: 10.1038/sj.onc.1208614. [DOI] [PubMed] [Google Scholar]
- 13.Solomon MJ, Lee T, Kirschner MW. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell. 1992;3(1):13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7(9):644–56. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 15*.Yang Y, Kim AH, Yamada T, Wu B, Bilimoria PM, Ikeuchi Y, et al. A Cdc20-APC ubiquitin signaling pathway regulates presynaptic differentiation. Science. 2009;326(5952):575–8. doi: 10.1126/science.1177087. The complementarity between Cdh1 and Cdc20 in the APC/C complex is further extended here into their post-mitotic function in brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puram SV, Kim AH, Ikeuchi Y, Wilson-Grady JT, Merdes A, Gygi SP, et al. A CaMKIIbeta signaling pathway at the centrosome regulates dendrite patterning in the brain. Nat Neurosci. 2011;14(8):973–83. doi: 10.1038/nn.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24(8):1079–85. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8(9):663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 19*.Pope W, Lambert M, Leypole B, Seupaul R, Sletten L, Krafft G, et al. Microtubule-associated protein tau is hyperphosphorylated during mitosis in the human neuroblastoma cell line SH-SY5Y. Experimental Neurology. 1994;126:185–94. doi: 10.1006/exnr.1994.1057. This paper is one example of the dynamic way in which the microtubule associate protein, tau, is modified by phosphorylation during a cell cycle. [DOI] [PubMed] [Google Scholar]
- 20.Illenberger S, Zheng-Fischhofer Q, Preuss U, Stamer K, Baumann K, Trinczek B, et al. The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer’s disease. Mol Biol Cell. 1998;9(6):1495–512. doi: 10.1091/mbc.9.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Vincent I, Zheng JH, Dickson DW, Kress Y, Davies P. Mitotic phosphoepitopes precede paired helical filaments in Alzheimer’s disease. Neurobiol Aging. 1998;19(4):287–96. doi: 10.1016/s0197-4580(98)00071-2. During mitosis tau is transiently phosphorylated in a number of specific serines. This paper demonstrates that these epitopes unexpectedly appear in the supposedly non-mitotic neurons of the Alzheimer’s disease brain. [DOI] [PubMed] [Google Scholar]
- 22.Tury A, Mairet-Coello G, DiCicco-Bloom E. The multiple roles of the cyclin-dependent kinase inhibitory protein p57(KIP2) in cerebral cortical neurogenesis. Dev Neurobiol. 2012;72(6):821–42. doi: 10.1002/dneu.20999. [DOI] [PubMed] [Google Scholar]
- 23.Besson A, Assoian RK, Roberts JM. Regulation of the cytoskeleton: an oncogenic function for CDK inhibitors? Nature reviews Cancer. 2004;4(12):948–55. doi: 10.1038/nrc1501. [DOI] [PubMed] [Google Scholar]
- 24.Godin JD, Thomas N, Laguesse S, Malinouskaya L, Close P, Malaise O, et al. p27(Kip1) is a microtubule-associated protein that promotes microtubule polymerization during neuron migration. Dev Cell. 2012;23(4):729–44. doi: 10.1016/j.devcel.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 25*.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8(1):17–26. doi: 10.1038/ncb1338. A demonstration of a role for the CKI, p27, in the maintenance of cytoskeletal integrity during the non-mitotic process of neuronal migration. [DOI] [PubMed] [Google Scholar]
- 26.Tury A, Mairet-Coello G, DiCicco-Bloom E. The cyclin-dependent kinase inhibitor p57Kip2 regulates cell cycle exit, differentiation, and migration of embryonic cerebral cortical precursors. Cereb Cortex. 2011;21(8):1840–56. doi: 10.1093/cercor/bhq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caviness VS, Jr, Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol. 1973;148(2):141–51. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- 28.Rakic P, Sidman RL. Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci U S A. 1973;70(1):240–4. doi: 10.1073/pnas.70.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arendt T. Dysregulation of neuronal differentiation and cell cycle control in Alzheimer’s disease. J Neural Transm Suppl. 2002;(62):77–85. doi: 10.1007/978-3-7091-6139-5_8. [DOI] [PubMed] [Google Scholar]
- 30.Kruman Why do neurons enter the cell cycle? Cell Cycle. 2004;3(6):769–73. [PubMed] [Google Scholar]
- 31.Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci. 2007;8(5):368–78. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- 32.Nagy Z, Esiri MM, Smith AD. The cell division cycle and the pathophysiology of Alzheimer’s disease. Neuroscience. 1998;87(4):731–9. doi: 10.1016/s0306-4522(98)00293-0. [DOI] [PubMed] [Google Scholar]
- 33.Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, et al. Requirement for a functional Rb-1 gene in murine development [see comments] Nature. 1992;359(6393):328–30. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- 34.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse [see comments] Nature. 1992;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 35.Lee EY, Chang CY, Hu N, Wang YC, Lai CC, Herrup K, et al. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992;359(6393):288–94. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 36**.Lee EY, Hu N, Yuan SS, Cox LA, Bradley A, Lee WH, et al. Dual roles of the retinoblastoma protein in cell cycle regulation and neuron differentiation. Genes Dev. 1994;8(17):2008–21. doi: 10.1101/gad.8.17.2008. An early demonstration of how a protein that is closely associate with cell cycle regulation, retinoblastoma, can also play a post-mitotic role in neuronal differentiation. [DOI] [PubMed] [Google Scholar]
- 37.Andrusiak MG, Vandenbosch R, Park DS, Slack RS. The retinoblastoma protein is essential for survival of postmitotic neurons. J Neurosci. 2012;32(42):14809–14. doi: 10.1523/JNEUROSCI.1912-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClellan KA, Ruzhynsky VA, Douda DN, Vanderluit JL, Ferguson KL, Chen D, et al. Unique requirement for Rb/E2F3 in neuronal migration: evidence for cell cycle-independent functions. Mol Cell Biol. 2007;27(13):4825–43. doi: 10.1128/MCB.02100-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nature reviews Cancer. 2003;3(3):155–68. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 40.McKinnon PJ. ATM and ataxia telangiectasia. EMBO Rep. 2004;5(8):772–6. doi: 10.1038/sj.embor.7400210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6(8):931–42. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Herrup K. Loss of neuronal cell cycle control in ataxia-telangiectasia: a unified disease mechanism. J Neurosci. 2005;25(10):2522–9. doi: 10.1523/JNEUROSCI.4946-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrup K, Li J, Chen J. The role of ATM and DNA damage in neurons: Upstream and downstream connections. DNA Repair (Amst) 2013 doi: 10.1016/j.dnarep.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Li J, Han YR, Plummer MR, Herrup K. Cytoplasmic ATM in neurons modulates synaptic function. Curr Biol. 2009;19(24):2091–6. doi: 10.1016/j.cub.2009.10.039. ATM function is primarily seen as cell cycle suppression during the DNA damage response. This work shows that it serves the unrelated function of regulating LTP and vesicle dynamics in non-mitotic nerve cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2(12):893–8. doi: 10.1038/35046542. The appearance of ATM in cytoplasm had been seen with EM immunohistochemistry. This paper was one of the first to demonstrate a non-nuclear function for ATM and spell out its mechanism. [DOI] [PubMed] [Google Scholar]
- 46.Miles PD, Treuner K, Latronica M, Olefsky JM, Barlow C. Impaired insulin secretion in a mouse model of ataxia telangiectasia. Am J Physiol Endocrinol Metab. 2007;293(1):E70–4. doi: 10.1152/ajpendo.00259.2006. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Herrup K. Nucleocytoplasmic Cdk5 is involved in neuronal cell cycle and death in post-mitotic neurons. Cell Cycle. 2011;10(8):1208–14. doi: 10.4161/cc.10.8.15328. [DOI] [PubMed] [Google Scholar]
- 48*.Zhang J, Li H, Yabut O, Fitzpatrick H, D’Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J Neurosci. 2010;30(15):5219–28. doi: 10.1523/JNEUROSCI.5628-09.2010. The Cdk5 protein, related to other Cdks but viewed as having no role in a normal cell cycle, in fact is critical for maintaining the non-proliferative state in adult neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, Cicero SA, Wang L, Romito-Digiacomo RR, Yang Y, Herrup K. Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proc Natl Acad Sci U S A. 2008;105(25):8772–7. doi: 10.1073/pnas.0711355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Higuera I, Manchado E, Dubus P, Canamero M, Mendez J, Moreno S, et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol. 2008;10(7):802–11. doi: 10.1038/ncb1742. [DOI] [PubMed] [Google Scholar]
- 51.Burton VL. Mike Mulligan and His Steam Shovel. New York: Hougton Mifflin; 1938. [Google Scholar]


