Abstract
Mantilla CB, Sieck GC. Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J Appl Physiol 104: 1818–1827, 2008. First published April 10, 2008; doi:10.1152/japplphysiol.01192.2007.—At the time of birth, respiratory muscles must be activated to sustain ventilation. The perinatal development of respiratory motor units (comprising an individual motoneuron and the muscle fibers it innervates) shows remarkable features that enable mammals to transition from in utero conditions to the air environment in which the remainder of their life will occur. In addition, significant postnatal maturation is necessary to provide for the range of motor behaviors necessary during breathing, swallowing, and speech. As the main inspiratory muscle, the diaphragm muscle (and the phrenic motoneurons that innervate it) plays a key role in accomplishing these behaviors. Considerable diversity exists across diaphragm motor units, but the determinant factors for this diversity are unknown. In recent years, the mechanisms underlying the development of respiratory motor units have received great attention, and this knowledge may provide the opportunity to design appropriate interventions for the treatment of respiratory disease not only in the perinatal period but likely also in the adult.
Keywords: respiration, breathing, diaphragm muscle, motor unit
NEURAL CONTROL of respiratory muscles develops perinatally to allow for breathing from the moment of birth onward. The neuronal networks necessary for generation of respiratory rhythm must become sufficiently mature to control gas exchange and maintain acid-base status in the whole body. Motor units comprise a motoneuron and the muscle fibers it innervates. Activation of respiratory motor units must overcome the mechanical limitations imposed by the highly compliant chest wall and high-resistance airways during the postnatal period. Subsequent maturation of motor units increases the range of motor behaviors that can be accomplished by respiratory muscles. For instance, although its major function is in inspiration, the diaphragm muscle (DIAm) participates in coughing, defecation, emesis, micturition, parturition, sneezing, vocalization, and weight lifting. Indeed, resting breathing no longer requires the recruitment of most diaphragm motor units in adult mammals. In this review we will examine the development of respiratory motor units with an emphasis on the development of motor unit diversity.
The DIAm is the major inspiratory muscle in mammals, and its activation is of considerable importance in the postnatal development of ventilatory competence. The DIAm is located between the thoracic and abdominal cavities and is innervated by phrenic motoneurons located in the midcervical spinal cord between C3 and C6. Premotor neurons providing rhythmic excitatory drive to the phrenic motoneuron pool are remotely located in the medulla. DIAm motor units display considerable diversity across motor unit types, yet the properties of muscle fibers and motoneurons within a motor unit are matched. The mechanisms underlying the origin and diversity of motor unit types are the subject of intense investigation. The perinatal development of DIAm motor units will be the focus of this review. Unless otherwise specified the timeline of embryologic events will be based on perinatal development in the rat (Figs. 1 and 2). In most cases, developmental events in the mouse will take place ~2 days before they do in rat and follow a similar transition. The timeline for these developmental benchmarks is more protracted in humans, but convergence of neural and myogenic events also occurs. Therefore, studying the integrated aspects of neural and myogenic development of the DIAm in rodent models is important.
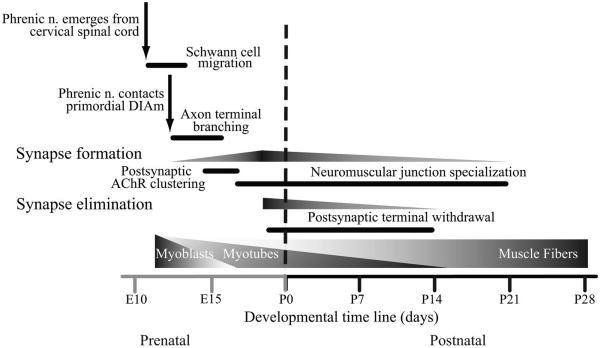
Fig. 1.
Schematic showing the timeline for major events in the development of the rat diaphragm muscle (DIAm). Similar key events occur in other mammals, including humans, during the perinatal development of DIAm motor units. However, temporal characteristics and interrelationships may vary. E and P refer to embryological and postnatal days, respectively.
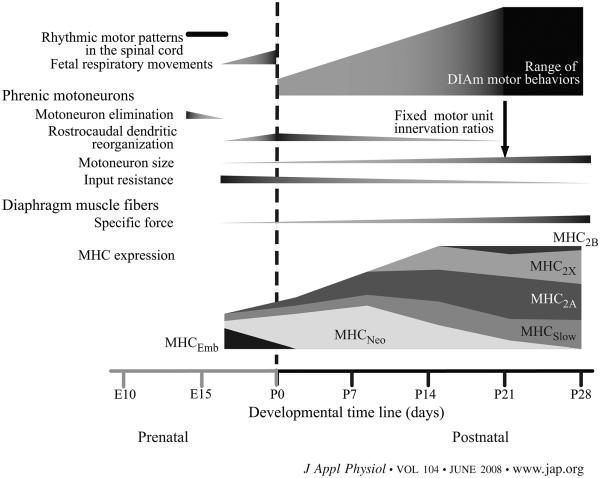
Fig. 2.
Schematic showing the timeline for major events in the development of rat DIAm motor units. MHC, myosin heavy chain.
EMBRYOLOGICAL DEVELOPMENT OF DIAPHRAGM MUSCLE MOTOR UNITS
Phrenic motoneurons
Phrenic motoneurons proliferate in the ventricular zone of the neural tube between embryological day 11 (E11) and E12 (38). They are found in clusters and connected by gap junctions at this stage. Subsequently, motoneurons differentiate into limb and axial motoneurons and assume their topographic organization in distinct columns, based on the interplay between homeodomain (e.g., HB9 and Lhx3) and basic helix-loop-helix (bHLH) transcription factors (83, 95). By E13, a distinct pool of phrenic motoneurons projects to the DIAm primordium (Fig. 1): the pleuroperitoneal fold (1, 2). All regions of the DIAm are innervated by cervical spinal cord segments in a somatotopic pattern (37, 41). The sternal and more ventral aspects of both costal and crural regions of the DIAm are innervated by phrenic motoneurons located in more rostral segments of the cervical spinal cord. At present, it remains unclear whether this somatotopic pattern of DIAm innervation develops before or after initial synapse formation as it could reflect specific neurogenic effects on myoblast migration and/or myogenic influences on motoneuron survival, for instance.
Phrenic motor axons make contact with and start branching within the pleuroperitoneal fold as myoblasts radiate into the dorsocostal, sternocostal, and crural regions of the DIAm (5), although the mechanisms underlying axon targeting and guidance remain unknown. The site of phrenic nerve contact seems to be consistent (medially within the pleuroperitoneal fold) with nerve axons being surrounded by migrating myoblasts. Receptor protein tyrosine phosphatases sigma and delta are complementary in axon targeting to the DIAm. Double mutants, but not single-mutant embryos, display normal cervical spinal cord projections, but the phrenic nerves fail to reach the primordial DIAm, leading to severe muscle abnormalities and motoneuron loss (99). Recent studies suggest a complex interplay between projecting perineurial glia and Schwann cells in axon guidance. Perineurial sheaths may serve as a scaffold for Schwann cell growth and alignment, targeting motor axons toward muscle fibers (58, 96). As phrenic motoneurons make contact with muscle fibers, the number of motoneurons is greatly reduced (Fig. 2). This process of motoneuron elimination takes place between E14 and E16 in rats, and up to 50% of motoneurons are lost by apoptosis at this time (2). It is not clear, however, whether this process depends on the establishment of functional connections between motoneurons and muscle fibers as muscle paralysis induced by curare will rescue motoneurons from programmed death (42).
Early in rat development, phrenic motoneurons are recruited by rhythmic motor patterns generated in the spinal cord at ~E13 (40, 77). Embryological maturation of motoneuron pools may depend on these rhythmic motor patterns either directly (via activity-dependent effects) or indirectly (via neurotransmitter-mediated trophic effects). Excitatory neurotransmitter systems change from being predominantly cholinergic to glutamatergic (65, 77). Inhibitory neurotransmitter systems [dependent on glycine and γ-amino-butyric acid (GABA) transmission] also contribute to the global spinal cord patterns of motoneuron activation at ~E16-E17 (77). By E17 in rats, rhythmic fetal respiratory movements are present, driven by medullary premotor drive (40). These fetal respiratory movements increase in frequency and regularity with embryological age, such that the number of rhythmic movements increases 5- to 10-fold by E20 (21, 40, 55). As shown by the use of isolated spinal cord-brain stem preparations in rats, the essential circuitry for the generation and transmission of rhythmic respiratory drive to phrenic motoneurons is present very early in development (28, 65). It is unclear whether synaptic inputs to phrenic motoneurons exert a growth-promoting, trophic influence on phrenic motoneuron growth or dendritic arborization during embryonic development. However, it is important to note that both activity (45, 52) as well as glutamatergic innervation exert important trophic effects on the growth of hindlimb motoneurons and their dendritic arbor postnatally (51).
The dendritic trees of phrenic motoneurons change their predominant orientation within the spinal cord. Initially, dendritic trees are primarily oriented following the migration columns provided by the radial glia, and thus are predominantly ventromedial and dorsolateral (E15). Some extend beyond the ipsilateral gray matter into white matter tracts (92). Following the onset of fetal respiratory movements at ~E17, phrenic dendrites become progressively more rostrocaudally oriented (1), a process that continues postnatally until the adult morphology is obtained (31, 73). The frequency of dendrites projecting to the contralateral ventral horn (64) is also further reduced postnatally, either as a result of dendritic retraction or differential growth of motoneurons relative to the spinal cord. The orientation of dendritic trees is thought to generally follow the predominant synaptic input to motoneurons (101). It is not known whether the prenatal switch in cholinergic to glutamatergic input to phrenic motoneurons or the increase in GABAergic input play a role in their dendritic reorganization. Regardless, the shift in dendritic orientation is thought to 1) help coordinate the activity of phrenic motoneurons, 2) better match the distribution of afferent descending inputs to phrenic motoneurons, and 3) facilitate interactions between the phrenic nucleus and other motor nuclei controlling accessory respiratory muscles (24, 31).
Motoneurons change their complement of ion channels and transporters during embryologic development as their electrophysiological properties become more mature (39, 69). Between E14 and E17, phrenic motoneurons are electrically excitable and can generate action potentials (40). They exhibit depolarized resting membrane potentials and high input impedance (70), which may facilitate activation by generalized rhythmic motor patterns in the spinal cord and the weak inspiratory premotor drive at ~E17 (22). Subsequent increases in Ca2+-activated K+ conductances allow for greatly increased phrenic motoneuron firing rates prenatally (70). Expression of the K+-Cl− cotransporter KCC2 also progressively increases in motoneurons after E17, changing cellular Cl− gradients (93) and making GABA currents hyperpolarizing rather than depolarizing (78). Taken together, these changes may allow for inspiratory recruitment of DIAm motor units at birth and onward. The mechanisms underlying changes in motoneuron electrophysiological properties and their relationship to the concomitant changes in motoneuron morphology deserve further investigation.
Diaphragm muscle
In general terms, all skeletal muscle fibers, including those in the DIAm, undergo 1) commitment when mesodermal progenitor cells transform into myoblasts, and 2) terminal differentiation when myoblasts fuse to form myotubes and myofibers. Whether myogenesis follows an intrinsic genetic program or is subject to extrinsic regulation by positive signaling cues and/or removal of inhibitory signals is controversial (3). However, there is increasing evidence that myogenesis is induced by the expression of bHLH transcription factors known collectively as muscle regulatory factors (MRFs), which include MyoD, myogenin, Myf-5, and MRF4 (105). The MRFs function as molecular switches, initiating myogenesis through binding to cis-acting DNA control elements known as E-boxes (71), which are present in the promoter regions of many skeletal muscle-specific genes (13). DIAm embryogenesis was recently revisited by studies using molecular markers of muscle development (4, 5).
The traditional view of DIAm development called for a complex derivation of the DIAm (106). However, Greer and colleagues (5) showed that the DIAm derives from the pleuroperitoneal fold by examining MyoD and myogenin expression in the primordial DIAm. They reported no evidence for muscle precursors migrating from the septum transversum or esophageal mesenchyme. Accordingly, congenital defects in DIAm development likely arise from malformation of the pleuroperitoneal fold itself rather than from incomplete fusion of DIAm sections. This view is further supported by the similar pathology observed in human tissues and experimental models of congenital DIAm hernias induced by teratogenic, dietary, or genetic manipulations (4, 20). Whether this novel view will lead to much-needed advances in the treatment and prevention of congenital DIAm hernias has to be substantiated in future studies.
In the rat, myoblasts likely migrate from cervical somites (brachial plexus region) and reach the pleuroperitoneal fold at ~E12. Myoblasts then radiate on a mesenchymal substrate into all regions of the DIAm by E17, leaving a small tendinous portion between the costal and crural regions as well as the central tendon amuscular. Myotube formation occurs after myoblasts have migrated from the myotome to their final location in the thoracic or abdominal walls, a process that is marked by expression of Myf-5 and c-met. In the primordial DIAm, Myf-5 and c-met are expressed between E10 and E12. In the absence of c-met (a tyrosine kinase receptor for hepatocyte growth factor/scatter factor), myoblasts fail to migrate (9) and an amuscular diaphragm forms (5). Other factors, present in the extracellular matrix (e.g., fibronectins and laminins), basal lamina (e.g., muscle and neural cell adhesion molecules and M-cadherin), cell membrane (e.g., β1-integrin) and cytoskeleton (e.g., actin and desmin), also modulate terminal differentiation (54). Both proliferation and differentiation of myoblasts are affected by in vitro passive mechanical strain (100). Undoubtedly, terminal differentiation and myotube/myofiber formation result from complex interactions between the differentiating myoblasts and their environment, and much remains to be elucidated.
Importantly, terminal differentiation occurs in concert with intramuscular branching of the phrenic nerve (2), but the exact role of neural influence is not clear. Even though myotubes/myofibers form in vitro in the absence of innervation, they do so at a much slower pace compared with in vivo, indicating that neural influence may facilitate the normal process of terminal differentiation and formation of myotubes/myofibers. This “facilitating” neural influence needs to be explored further. Electrical activity can be elicited in phrenic motoneurons and action potentials generated at the time of intramuscular branching (~E14), raising the prospect of both electrical (activity) and trophic neural effects on muscle development (40). The late embryonic maturation of phrenic motoneurons is accompanied by increases in specific force and twitch contraction times in the DIAm (68). This concerted maturation of motor unit properties may thus permit chest excursion necessary for respiration despite weak inspiratory drive to motoneurons at birth.
Neuromuscular junctions
Coincident with myoblast migration within the primordial DIAm, the phrenic nerves exit the cervical spinal cord and follow migrating myoblasts within the pleuroperitoneal fold (Fig. 1). It is not clear whether the initial targets of phrenic nerve terminals are the pleuroperitoneal membranes rather than myoblasts themselves (2, 38). Regardless, the phrenic nerve radiates within the pleuropericardial folds to a medial location between the pericardial and pleural cavities.
As phrenic nerve terminals grow into the primordial DIAm, there is also postsynaptic specialization, with expression and aggregation of cholinergic receptors (Fig. 1). In the rat DIAm, aggregation and clustering of cholinergic receptors is apparent at ~E13. The process of aggregation of cholinergic receptors has received considerable attention, and it is thought that this process involves agrin secretion by the nerve terminal, its incorporation into the basal lamina, subsequent activation of MuSK receptors at the postsynaptic membrane, and finally rapsyn-mediated cholinergic receptor clustering (60, 81). Cholinergic receptor clustering can occur in the absence of neural influence, and presynaptic terminals seemingly incorporate prepatterned cholinergic clusters into a forming neuromuscular junction. Early cholinergic receptor clusters are likely stabilized through the complex interplay of activity and neural-derived influences, e.g., agrin or neuregulin (46).
POSTNATAL DEVELOPMENT OF DIAPHRAGM MUSCLE MOTOR UNITS
Phrenic motoneurons
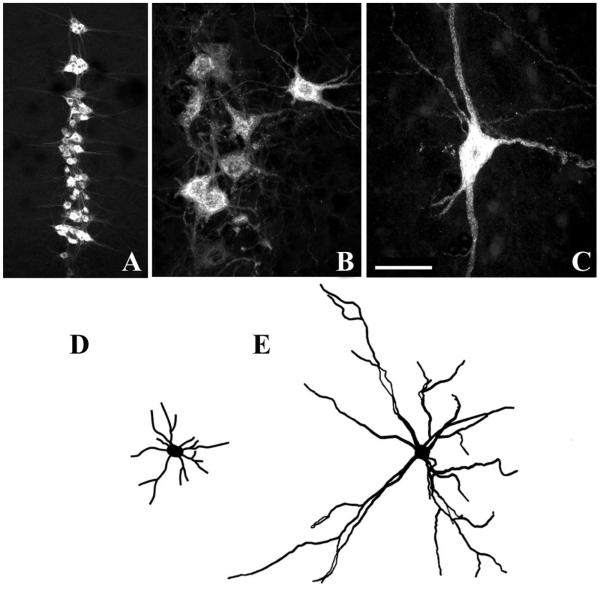
Phrenic motoneurons exhibit significant postnatal growth (16, 17). For example, rat phrenic motoneurons increase in both soma and dendrite dimensions between postnatal day 21 (P21) and adulthood (Fig. 3). Importantly, motoneuron soma volume and surface area increase proportionately to the growth in DIAm fiber cross-sectional area (73), suggesting that even during postnatal development, it is important to match motoneuron and muscle fiber properties.
Fig. 3.
Confocal photomicrographs displaying phrenic motoneurons that were retrogradely labeled by DIAm injection of cholera toxin B-fragment. Motoneurons are shown at postnatal day 10 (P10, A and B) and in the adult rat (C). Neurolucida camera tracings are shown in D and E for P10 and adult motoneurons, respectively. Note differences in cell size (B and C) and dendritic arborization (D and E). Bar indicates 200 μm (A) and 50 μm (B and C).
In the adult, a hallmark of motor control is the match between the phenotypic properties of a motoneuron and the muscle fibers it innervates. The morphology of motoneurons within a pool, including the phrenic pool, varies considerably (16, 17, 73). Differences in soma morphology and dendritic arborization correspond to the diverse intrinsic electrophysiological properties of motoneurons. For instance, motoneurons belonging to slow-twitch (type S) motor units generally have the highest input resistance, lowest rheobase, and slowest axonal conduction velocities among motoneurons. In contrast, motoneurons belonging to fast-twitch fatigable (type FF) motor units are the largest (lowest input resistance) and least excitable (highest rheobase), and display the fastest axonal conduction velocities (15).
During postnatal development, there is significant growth of both motoneurons and muscle fibers (72, 73). According to the “size principle” (44), smaller motoneurons innervating smaller type I and/or IIa fibers comprise type S and fast-twitch fatigue resistant (type FR) motor units that are recruited first during motor behaviors because of the intrinsic size-related electrophysiological properties of the motoneurons. Larger motoneurons innervating larger type IIx and/or IIb fibers comprise fast-twitch fatigue intermediate (type FInt) and type FF motor units that are recruited only during more forceful motor behaviors. During early postnatal development, the extent of DIAm motor unit activation changes to match changing functional demands. For example, in neonates (P0–P7), reduced lung compliance and chest wall stiffness require increased transdiaphragmatic pressure to inflate the lungs, and as a result, near complete activation of DIAm motor units is necessary just to sustain resting ventilation. Later, as lung compliance and chest wall stiffness increase, the transdiaphragmatic pressure needed to inflate the lungs decreases; thus resting ventilatory demand for DIAm contraction decreases. Consequently, more selective activation of DIAm motor units occurs. For example, in adult rat DIAm only ~25–30% of motor units are activated to sustain breathing (84, 86, 88). However, recruitment of additional motor units is required to generate more forceful ventilatory behaviors associated with increased ventilatory demand (e.g., during jumping or running) and some nonventilatory behaviors (e.g., coughing, sneezing).
The more homogeneous and smaller size of phrenic motoneurons during postnatal development is consistent with increased excitability (44, 94) that would lead to more complete and synchronous recruitment of DIAm motor units necessary to sustain resting ventilation in the neonatal rat. The characteristic heterogeneity of motor unit phenotypes present in the adult rat DIAm develops following weaning (~P21) when motor unit innervation ratio (i.e., the number of muscle fibers innervated by a single motoneuron) becomes fixed (Fig. 2). Before weaning, it is difficult to determine whether synapse elimination and/or secondary myogenesis contribute to changes in motor unit size. Synapse elimination may affect motoneuron size via an effect on innervation ratio. For instance, in the adult mouse hindlimb, partial denervation that increases innervation ratio results in an increased size of motoneuron somata (97). In the rat DIAm, synapse elimination continues through P14; thus, if anything, innervation ratio is reduced during this postnatal period. This decrease in innervation ratio would decrease the number of muscle fibers exerting a trophic influence on each motoneuron. However, motoneurons exhibit remarkable growth during this period (16, 17, 73), suggesting that a trophic influence related to innervation ratio is not likely to underlie postnatal changes in phrenic motoneuron size.
Diaphragm muscle
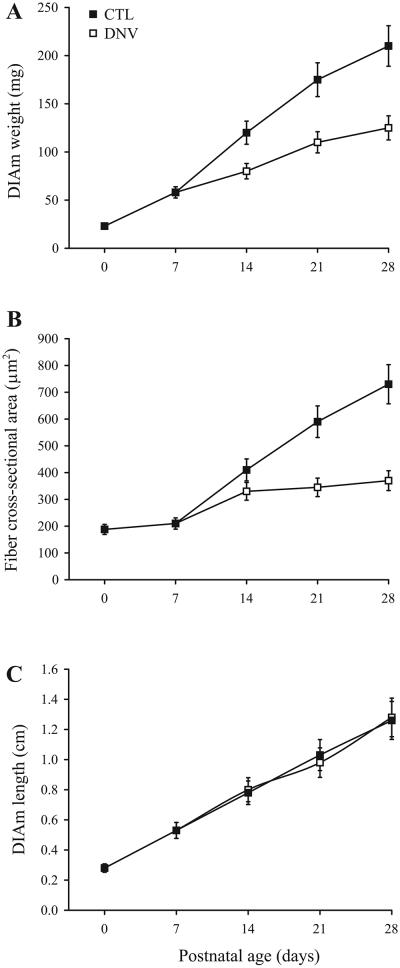
During postnatal development, DIAm mass increases due to an increase in both fiber cross-sectional area and fiber length (Fig. 4). As muscle fibers increase in cross-sectional area, their content of contractile proteins also increases. In fact, specific force (force per cross-sectional area) of DIAm fibers increases approximately twofold during postnatal development (34, 50, 91, 107). Isometric twitch contraction time and half-relaxation time decrease during postnatal development, whereas the force-frequency relationship changes such that the relative tetanic force increases at higher stimulation frequencies (102). Conversely, DIAm fatigability increases during postnatal development (104). Despite the fundamental importance of developmental changes in contractile protein expression, there is currently no information regarding the trophic regulation of contractile protein synthesis and/or degradation during this period of rapid postnatal growth of DIAm fibers.
Fig. 4.
Timeline of changes in DIAm mass (A), DIAm fiber cross-sectional area (B), and DIAm fiber length (C). Data are means ± SE, summarized from published reports (32, 91, 107).
The diversity of muscle fiber types observed in the adult becomes established during postnatal development of muscle fibers (Fig. 2). Adult skeletal muscle fibers are commonly classified as type I, IIa, and IIb based on the pH lability of myofibrillar ATPase staining (12). This histochemical muscle fiber type classification generally corresponds with the expression of different myosin heavy chain (MHC) isoforms; fibers classified as type I express MHCSlow, IIa fibers express MHC2A, and IIb fibers express MHC2B and/or MHC2X (30, 36). However, this classification of muscle fiber types is not possible during embryonic and neonatal development. The remarkable developmental changes in MHC isoform expression are associated with a high incidence of coexpression of MHC isoforms, which preclude clear distinction of different muscle fiber types (61, 103). In rat, an embryonic MHC isoform (MHCEmb) is predominantly expressed together with MHCSlow and MHC2A during prenatal DIAm development (Fig. 2). At or close to birth, the predominant isoform expression switches to a neonatal MHC isoform (MHCNeo), again together with MHCSlow and MHC2A. Thereafter, MHCNeo expression gradually disappears, becoming undetectable by P28. Expression of MHC2X and MHC2B isoforms emerges only after P14, and the proportion of fibers expressing these isoforms increases until about P28, when the adult pattern of MHC isoform expression is fully established (32, 50, 53, 103). Beyond P28, the relative contribution of each MHC isoform changes due to the disproportionate growth of DIAm fibers, with fibers expressing MHC2X or MHC2B displaying ~2- to 3-fold greater growth than those expressing MHCSlow or MHC2A isoforms (72).
During postnatal development, contractile properties in the rat DIAm correlate with the expression of MHC isoforms. Maximum specific force and maximum unloaded shortening velocity correlate inversely with the MHCNeo isoform expression and positively with MHC2X expression (50). Indeed, when normalized for MHC content per half-sarcomere, fibers expressing MHCSlow and coexpressing MHCNeo produce less force than fibers expressing fast MHC isoforms (34, 90). Fatigue resistance also relates to the relative expression of MHC isoforms. Fatigue resistance of the DIAm decreases with postnatal age (104), as the relative expression of adult MHC isoforms (MHCSlow, MHC2A, MHC2X, and MHC2B) increases relative to developmental isoforms (MHCNeo or MHCEmb). Thus the postnatal changes in MHC isoform expression are important determinants of the contractile and fatigue properties of the DIAm and occur in concert with the establishment of motor unit diversity.
Motor units are commonly categorized into different types based on the mechanical and fatigue characteristics of their muscle fibers (15, 30, 87). Four types of motor units are thus considered: 1) slow twitch, fatigue resistant (type S); 2) fast twitch, fatigue resistant (type FR); 3) fast twitch, fatigue intermediate (type FInt); and 4) fast twitch, fatigable (type FF). This diversity is reflected in the structural and functional differences across muscle fiber types (35, 36, 50, 88). Motor unit composition is critically important in determining overall functional capacity of the DIAm in accomplishing different motor behaviors since the forces generated by a muscle during any motor behavior result from an orderly recruitment of motor units. Consistent with the “size principle,” motor units comprising smaller motoneurons, smaller axons, and thus slower axonal conduction velocities are recruited before units comprising larger motoneurons with faster axonal conduction velocities (23, 44, 48). Thus motor unit recruitment results in activation of muscle fibers in agreement with their mechanical properties and fatigue resistance.
The ability to selectively recruit motor units across different motor behaviors of varying demand develops postnatally. Whereas the neonatal lung is stiffer than in the adult due to greater alveolar recoil partially offset by surfactant expression, the chest and abdominal walls are more compliant. As a result, the DIAm must generate greater relative intrathoracic pressures to produce a given level of inspiratory airflow and tidal volume. Yet, the neonatal DIAm generates much lower maximum tetanic force and has a much slower maximum unloaded shortening velocity compared with the adult (34, 50, 91, 107). Thus the functional reserve capacity of the neonatal DIAm is greatly reduced, and a far greater fraction of maximum power output must be recruited to accomplish ventilation. The more homogeneous phrenic motoneuron and muscle fiber populations would also be consistent with near complete recruitment of all motor units during spontaneous breathing. In addition, polyneuronal innervation of DIAm fibers may be particularly important as a neural strategy to accomplish greater fractional recruitment of DIAm motor units. As synapse elimination proceeds, more selective recruitment of DIAm motor units becomes possible, coinciding with the broader range of functions required of the DIAm (Fig. 2). The increased postnatal expression of MHC2X and MHC2B isoforms in DIAm fibers increases overall functional range, albeit at the expense of lower energy efficiency and greater susceptibility to fatigue (84). Motor units comprising DIAm fibers expressing MHC2X and MHC2B isoforms are likely only recruited during motor behaviors that require high force output and are of short duration, e.g., expulsive behaviors. These DIAm behaviors may be less necessary in the neonate.
Neuromuscular junctions
In the adult rat DIAm, neuromuscular junctions display considerable morphological differences across fiber types. Fiber type differences are evident in the size and complexity (e.g., branching and folding) of both axon terminals and motor endplates of DIAm neuromuscular junctions. Neuromuscular junctions at fibers expressing MHC2X and/or MHC2B are larger and more complex than those at fibers expressing MHCSlow or MHC2A isoforms (74). Synaptic vesicle density at active zones is lower in axon terminals at fibers expressing MHC2X and/or MHX2B isoforms than at those expressing MHCSlow or MHC2A isoforms (66, 79). In addition, mitochondria, rough endoplasmic reticulum, free polysomes, and nuclei are frequently interposed between postsynaptic specializations and myofibrils at fibers expressing MHC2X and/or MHX2B isoforms, not at fibers expressing MHCSlow or MHC2A isoforms. These fiber type differences in neuromuscular junction morphology develop postnatally, with neuromuscular junctions in the neonatal DIAm being smaller and less complex than in the adult. Although much attention has been paid to synapse formation and elimination, the mechanisms underlying neuromuscular junction diversity are poorly understood. It is possible that maturation of all motor unit components results from the matching of muscle fiber and motoneuron properties within a motor unit, either as a result of genetically determined programs or interplay between activity patterns and both myogenic and neurogenic trophic effects. Further studies are needed to address these important questions.
Consistent with the motor unit type-dependent differences in neuromuscular junction morphology, electrophysiological properties of neuromuscular transmission also exhibit considerable diversity across motor units. For instance, the amplitude of excitatory postsynaptic potentials correlates with the input resistance of the motoneuron (15). The safety factor for neuromuscular transmission (defined as the ratio of the excitatory postsynaptic potential amplitude to activation threshold of a muscle fiber) varies across fiber types, being larger for type IIb DIAm fibers than for type I or IIa fibers (25). With repeated activation, the amplitude of excitatory postsynaptic potentials declines to a greater extent at terminals of type I or IIa fibers than at type IIb fibers (79). Taken together, these findings suggest that the probability of synaptic vesicle release is reduced at type I or IIa fibers, which may serve to limit the depletion of synaptic vesicles from these terminals during repeated activation. Importantly, neuromuscular transmission failure in the DIAm depends on the rate of motor axon stimulation (29, 59), with increased failure of action potential propagation and neuromuscular transmission failure with increasing stimulus frequency (29, 49). Accordingly, type FF motor units are more susceptible to neuromuscular transmission failure during repetitive activation than type S or FR motor units in the cat (85).
The neonatal rat DIAm is more susceptible to neuromuscular transmission failure than the adult (7, 27, 29, 89), which may relate to the more extensive branching of phrenic axons (due to polyneuronal innervation) with an associated greater likelihood of failure of action potential propagation at axonal branch points (29, 56, 57, 89). Spontaneous miniature endplate potential frequency and amplitude and evoked endplate potential amplitude are reduced in the neonatal DIAm compared with the adult, suggesting that either reduced neurotransmitter release and/or disproportionately smaller endplate regions (with correspondingly fewer cholinergic receptors) may be present (7, 27, 29). Detailed, quantitative studies at the neuromuscular junction are needed to illuminate this issue.
MECHANISMS REGULATING POSTNATAL MOTOR UNIT DEVELOPMENT
Although diversity in motor unit phenotype could be determined genetically and established early during embryonic development, based on the temporal associations between innervation and the major developmental events in muscle specialization (Figs. 1 and 2), it seems reasonable that the nervous system can either directly (e.g., activity, nerve traffic) or indirectly (e.g., via secretion of trophic factors) influence DIAm development. However, the precise mechanisms by which innervation, activity, and/or trophic influences regulate DIAm development remain largely unknown.
Role of innervation
The consistent matching of motoneuron and muscle fiber properties within motor units suggests an important influence of motoneurons on muscle fibers. During DIAm development, single myofibers are contacted by multiple motoneurons (i.e., polyneuronal innervation) until ~P14 (8). Synapse elimination occurs through competition among motor axons for target cell innervation, depending on the relative activity of the competing terminals (14, 81). Accordingly, axon terminals of more active motoneurons will prevail over those of less active motoneurons. However, motor units with the largest innervation ratio (i.e., number of muscle fibers innervated by a single motoneuron) are those that are least active in the adult; i.e., motoneurons belonging to type FInt and type FF motor units (10, 30). Thus either the activity levels of these motoneurons change dramatically during development (changing from most to least active) or the number of muscle fibers innervated by these motoneurons must be greater initially (accommodating the subsequent greater loss of synaptic contacts). It is also possible that the pattern of activity may be more important than the total level of activity in defining synapse elimination (6). In addition, differences in muscle fiber lineage and/or MHC isoform expression may contribute to synapse elimination as motor unit innervation becomes established.
Unilateral denervation of the rat DIAm alters normal transitions of MHC isoform expression and affects postnatal changes in maximum specific force and maximum unloaded shortening velocity (91). With phrenic denervation at P7, the postnatal growth of DIAm fibers is blunted (Fig. 4). Expression of MHCNeo persists, and emergence of MHC2X and MHC2B is delayed (32, 33). At both P21 and P28, maximum specific force is reduced and maximum unloaded shortening velocity is slowed compared with control DIAm (91). Clearly, the communication between motoneuron and muscle fibers can take the form of neural electrical activity (i.e., propagated action potentials) and/or a neurogenic trophic influence. However, a variety of environmental factors may also be altered by denervation including external load, muscle blood flow, local oxygen tension, and accumulation of metabolites. Any or all of these factors may influence the properties of motor units. Studies in which the emergence of specific neural properties is disrupted (e.g., by preventing expression of Ca2+-activated K+conductances necessary for increased firing rates perinatally) are needed to refine our understanding of motoneuron effects on the development of motor unit types.
Role of activity
At the present time, any activity-dependent effects on motoneuron growth or remodeling are incompletely understood. A multiplicity of factors likely determine the size of dendritic arbors in motoneurons (52). However, it is worth noting that those motoneurons belonging to the least active motor units (type FInt and FF motor units) are much larger than motoneurons belonging to type S and FR motor units. In addition, the determinants of matching motoneuron and muscle fiber properties need further study. Whether activity levels or specific patterns of activity determine the concerted growth of all components of motor units (independent for instance of innervation ratio) is presently unknown.
Activity-dependent adaptations of muscle fibers may be mediated by changes in myoplasmic Ca2+ homeostasis. For example, calcineurin is a Ca2+ binding protein that dephosphorylates cytosolic members of the NF-AT family (nuclear factors of activated T-cells) causing their nuclear translocation. In the myonucleus, NF-ATs stimulate transcription of slow-fiber genes by acting on their promoter/enhancer regions (18, 82). Another target of calcineurin signaling is the peroxisomeproliferator receptor-γ-coactivator 1 (PGC-1α), which acting in concert with Mef2 stimulates transcriptional activation of slow fiber genes (63). It is currently not known whether similar Ca2+-mediated effects of activity are at play in the postnatal differentiation of muscle fibers.
In the adult rat, inactivity of the different components of the motor unit results in distinct adaptations of DIAm neuromuscular junctions (67, 75, 76). Inactivity of both axon terminals and muscle fibers (as a result of tetrodotoxin blockade of action potential propagation) significantly alters neuromuscular junction morphology and reduces synaptic vesicle pool size and neuromuscular transmission. In contrast, additional inactivation of the phrenic motoneuron soma (as a result of unilateral spinal cord hemisection at C2) has minimal impact on neuromuscular junction morphology or synaptic vesicle pool size and improves neuromuscular transmission. The molecular mechanisms underlying the matching of motor unit properties are not known, but the use of similar experimental models may offer insight into the postnatal maturation of motor unit properties. It is possible that activity may play a role in the postnatal differentiation of neuromuscular junctions and contribute to fiber type differences in neuromuscular junction structure and function, but this has not been examined to date.
Role of trophic factors
Trophic factors derived from motoneurons or muscle fibers may be important in determining postnatal motor unit structure and function. Although a number of growth factors are known to be involved in motoneuron survival and formation of the neuromuscular junction, far less is known about the role of trophic factors in the growth of motoneurons or muscle fibers. In addition, trophic factors may display differential developmental effects depending on motor unit type, possibly as a result of selective expression of factors, their receptors, or individual signaling pathway components. Several trophic factors are interesting candidate molecules that may contribute to the concerted maturation and growth of all components of a motor unit.
Neuregulin is a member of the larger epidermal growth factor family of trophic factors that activate receptor tyrosine kinases of the ErbB family (26, 98, 108). Neuregulin is expressed by motoneurons during embryonic and early postnatal development (80) and induces acetylcholine receptor expression at embryonic and perhaps adult neuromuscular junctions by its action on Schwann cells (47). Both ErbB2 and ErbB3 are expressed in mature skeletal muscle (62), including rat DIAm (43). Neuregulin-induced phosphorylation of ErbB receptors can lead to subsequent activation of the phosphoinositide-3-kinase (PI3K)/Akt intracellular pathway, which appears to be a critical regulator of muscle protein synthesis and hypertrophy (11). All four members of the ErbB receptor family can recruit PI3K, and ErbB2/ErbB3 heterodimer is a very strong activator of PI3K (19). Indeed, neuregulin is a potent inducer of protein synthesis in the postnatal DIAm (43). Whether neuregulin constitutes a nerve-derived trophic influence for muscle fiber growth during perinatal development remains to be determined. Developmental and motor unit type differences in neuregulin and ErbB receptor expression need to be explored.
Other trophic factors (e.g., insulin-like growth factor-1, IGF-1) also increase protein synthesis and induce muscle hypertrophy. Indeed, IGF-1 and neuregulin share similar intracellular signaling pathways, including PI3K/Akt activation, which result in increased protein synthesis and muscle growth. In this sense, it is interesting to speculate that postnatal growth of DIAm fibers may be differentially regulated by neuregulin and IGF-1 such that nerve-derived neuregulin may contribute to cross-sectional growth whereas IGF-1 contributes to the longitudinal growth (Fig. 4).
CONCLUSIONS AND FUTURE DIRECTIONS
Despite the vital importance of respiratory muscles, previous studies have provided mostly descriptive information about its development and growth. Accumulating information regarding neural and muscle development derived from in vitro model systems may prove applicable to the mechanisms regulating motor unit development in vivo. Important adaptations occur perinatally in all of the components of a motor unit: the motoneuron, the neuromuscular junction and the target muscle fibers, allowing for an increased range of possible ventilatory and nonventilatory behaviors. In this sense, an integrative approach to the study of postnatal development of respiratory muscles, such as the DIAm, is direly needed, one which would also take into account the maturation of other systems, e.g., the central nervous system, the lung, and the thoracic and abdominal walls.
Acknowledgments
GRANTS
This work was supported by National Institutes of Health Grants AR-51173 and HL-37680 and by the Mayo Foundation.
REFERENCES
- 1.Allan DW, Greer JJ. Development of phrenic motoneuron morphology in the fetal rat. J Comp Neurol. 1997;382:469–479. doi: 10.1002/(sici)1096-9861(19970616)382:4<469::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Allan DW, Greer JJ. Embryogenesis of the phrenic nerve and diaphragm in the fetal rat. J Comp Neurol. 1997;382:459–468. doi: 10.1002/(sici)1096-9861(19970616)382:4<459::aid-cne3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Arnold HH, Braun T. Genetics of muscle determination and development. Curr Top Dev Biol. 2000;48:129–165. doi: 10.1016/s0070-2153(08)60756-5. [DOI] [PubMed] [Google Scholar]
- 4.Babiuk RP, Greer JJ. Diaphragm defects occur in a CDH hernia model independently of myogenesis and lung formation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1310–L1314. doi: 10.1152/ajplung.00257.2002. [DOI] [PubMed] [Google Scholar]
- 5.Babiuk RP, Zhang W, Clugston R, Allan DW, Greer JJ. Embryological origins and development of the rat diaphragm. J Comp Neurol. 2003;455:477–487. doi: 10.1002/cne.10503. [DOI] [PubMed] [Google Scholar]
- 6.Barber MJ, Lichtman JW. Activity-driven synapse elimination leads paradoxically to domination by inactive neurons. J Neurosci. 1999;19:9975–9985. doi: 10.1523/JNEUROSCI.19-22-09975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bazzy AR, Donnelly DF. Failure to generate action potentials in newborn diaphragms following nerve stimulation. Brain Res. 1993;600:349–352. doi: 10.1016/0006-8993(93)91396-a. [DOI] [PubMed] [Google Scholar]
- 8.Bennett MR, Lavidis NA. Segmental motor projections to rat muscles during the loss of polyneuronal innervation. Dev Brain Res. 1984;13:1–7. doi: 10.1016/0165-3806(84)90070-1. [DOI] [PubMed] [Google Scholar]
- 9.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 10.Bodine SC, Roy RR, Eldred E, Edgerton VR. Maximal force as a function of anatomical features of motor units in the cat tibialis anterior. J Neurophysiol. 1987;57:1730–1745. doi: 10.1152/jn.1987.57.6.1730. [DOI] [PubMed] [Google Scholar]
- 11.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 12.Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- 13.Buckingham M. Which myogenic factors make muscle? Curr Biol. 1994;4:61–63. doi: 10.1016/s0960-9822(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 14.Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 15.Burke RE. Handbook of Physiology. The Nervous System. Motor Control. II. Am. Physiol. Soc.; Bethesda, MD: 1981. Motor units: anatomy, physiology and functional organization; pp. 345–422. sect. 1. pt. 1, chapt. 10. [Google Scholar]
- 16.Cameron WE, Brozanski BS, Guthrie RD. Postnatal development of phrenic motoneurons in the cat. Dev Brain Res. 1990;51:142–145. doi: 10.1016/0165-3806(90)90269-5. [DOI] [PubMed] [Google Scholar]
- 17.Cameron WE, He F, Kalipatnapu P, Jodkowski JS, Guthrie RD. Morphometric analysis of phrenic motoneurons in the cat during postnatal development. J Comp Neurol. 1991;314:763–776. doi: 10.1002/cne.903140409. [DOI] [PubMed] [Google Scholar]
- 18.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Weiguang Z, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 20.Clugston RD, Klattig J, Englert C, Clagett-Dame M, Martinovic J, Benachi A, Greer JJ. Teratogen-induced, dietary and genetic models of congenital diaphragmatic hernia share a common mechanism of pathogenesis. Am J Pathol. 2006;169:1541–1549. doi: 10.2353/ajpath.2006.060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Pasquale E, Monteau R, Hilaire G. In vitro study of central respiratory-like activity of the fetal rat. Exp Brain Res. 1992;89:459–464. doi: 10.1007/BF00228263. [DOI] [PubMed] [Google Scholar]
- 22.Di Pasquale E, Tell F, Monteau R, Hilaire G. Perinatal developmental changes in respiratory activity of medullary and spinal neurons: an in vitro study on fetal and newborn rats. Brain Res Dev Brain Res. 1996;91:121–130. doi: 10.1016/0165-3806(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 23.Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity for supraspinally driven diaphragmatic motor units. J Neurophysiol. 1987;57:245–259. doi: 10.1152/jn.1987.57.1.245. [DOI] [PubMed] [Google Scholar]
- 24.Ellenberger HH, Vera PL, Haselton JR, Haselton CL, Schneiderman N. Brainstem projections to the phrenic nucleus: an anterograde and retrograde HRP study in the rabbit. Brain Res Bull. 1990;24:163–174. doi: 10.1016/0361-9230(90)90201-a. [DOI] [PubMed] [Google Scholar]
- 25.Ermilov LG, Mantilla CB, Rowley KL, Sieck GC. Safety factor for neuromuscular transmission at type-identified diaphragm fibers. Muscle Nerve. 2007;35:800–803. doi: 10.1002/mus.20751. [DOI] [PubMed] [Google Scholar]
- 26.Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 27.Feldman JD, Bazzy AR, Cummins TR, Haddad GG. Developmental changes in neuromuscular transmission in the rat diaphragm. J Appl Physiol. 1991;71:280–286. doi: 10.1152/jappl.1991.71.1.280. [DOI] [PubMed] [Google Scholar]
- 28.Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci. 1985;5:1993–2000. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fournier M, Alula M, Sieck GC. Neuromuscular transmission failure during postnatal development. Neurosci Lett. 1991;125:34–36. doi: 10.1016/0304-3940(91)90124-c. [DOI] [PubMed] [Google Scholar]
- 30.Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol. 1988;59:1055–1066. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- 31.Furicchia FV, Goshgarian HG. Dendritic organization of phrenic motoneurons in the adult rat. Exp Neurol. 1987;96:621–634. doi: 10.1016/0014-4886(87)90224-x. [DOI] [PubMed] [Google Scholar]
- 32.Geiger PC, Bailey JP, Mantilla CB, Zhan WZ, Sieck GC. Mechanisms underlying myosin heavy chain expression during development of the rat diaphragm muscle. J Appl Physiol. 2006;101:1546–1555. doi: 10.1152/japplphysiol.00221.2006. [DOI] [PubMed] [Google Scholar]
- 33.Geiger PC, Bailey JP, Zhan WZ, Mantilla CB, Sieck GC. Denervation-induced changes in myosin heavy chain expression in the rat diaphragm muscle. J Appl Physiol. 2003;95:611–619. doi: 10.1152/japplphysiol.00862.2002. [DOI] [PubMed] [Google Scholar]
- 34.Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Mechanisms underlying increased force generation by rat diaphragm muscle fibers during development. J Appl Physiol. 2001;90:380–388. doi: 10.1152/jappl.2001.90.1.380. [DOI] [PubMed] [Google Scholar]
- 35.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- 36.Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol. 1999;87:1894–1900. doi: 10.1152/jappl.1999.87.5.1894. [DOI] [PubMed] [Google Scholar]
- 37.Gordon DC, Richmond FJ. Topography in the phrenic motoneuron nucleus demonstrated by retrograde multiple-labelling techniques. J Comp Neurol. 1990;292:424–434. doi: 10.1002/cne.902920308. [DOI] [PubMed] [Google Scholar]
- 38.Greer JJ, Allan DW, Martin-Caraballo M, Lemke RP. An overview of phrenic nerve and diaphragm muscle development in the perinatal rat. J Appl Physiol. 1999;86:779–786. doi: 10.1152/jappl.1999.86.3.779. [DOI] [PubMed] [Google Scholar]
- 39.Greer JJ, Funk GD. Perinatal development of respiratory motoneurons. Respir Physiol Neurobiol. 2005;149:43–61. doi: 10.1016/j.resp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Greer JJ, Smith JC, Feldman JL. Respiratory and locomotor patterns generated in the fetal rat brain stem-spinal cord in vitro. J Neurophysiol. 1992;67:996–999. doi: 10.1152/jn.1992.67.4.996. [DOI] [PubMed] [Google Scholar]
- 41.Hammond CG, Gordon DC, Fisher JT, Richmond FJ. Motor unit territories supplied by primary branches of the phrenic nerve. J Appl Physiol. 1989;66:61–71. doi: 10.1152/jappl.1989.66.1.61. [DOI] [PubMed] [Google Scholar]
- 42.Harris AJ, McCaig CD. Motoneuron death and motor unit size during embryonic development of the rat. J Neurosci. 1984;4:13–24. doi: 10.1523/JNEUROSCI.04-01-00013.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellyer NJ, Mantilla CB, Park EW, Zhan WZ, Sieck GC. Neuregulin-dependent protein synthesis in C2C12 myotubes and rat diaphragm muscle. Am J Physiol Cell Physiol. 2006;291:C1056–C1061. doi: 10.1152/ajpcell.00625.2005. [DOI] [PubMed] [Google Scholar]
- 44.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- 45.Inglis FM, Zuckerman KE, Kalb RG. Experience-dependent development of spinal motor neurons. Neuron. 2000;26:299–305. doi: 10.1016/s0896-6273(00)81164-2. [DOI] [PubMed] [Google Scholar]
- 46.Jaworski A, Burden SJ. Neuromuscular synapse formation in mice lacking motor neuron- and skeletal muscle-derived Neuregulin-1. J Neurosci. 2006;26:655–661. doi: 10.1523/JNEUROSCI.4506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jo SA, Zhu X, Marchionni MA, Burden SJ. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature. 1995;373:158–161. doi: 10.1038/373158a0. [DOI] [PubMed] [Google Scholar]
- 48.Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol. 1987;58:105–124. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- 49.Johnson BD, Sieck GC. Differential susceptibility of diaphragm muscle fibers to neuromuscular transmission failure. J Appl Physiol. 1993;75:341–348. doi: 10.1152/jappl.1993.75.1.341. [DOI] [PubMed] [Google Scholar]
- 50.Johnson BD, Wilson LE, Zhan WZ, Watchko JF, Daood MJ, Sieck GC. Contractile properties of the developing diaphragm correlate with myosin heavy chain phenotype. J Appl Physiol. 1994;77:481–487. doi: 10.1152/jappl.1994.77.1.481. [DOI] [PubMed] [Google Scholar]
- 51.Kalb RG. Regulation of motor neuron dendrite growth by NMDA receptor activation. Development. 1994;120:3063–3071. doi: 10.1242/dev.120.11.3063. [DOI] [PubMed] [Google Scholar]
- 52.Kalb RG, Hockfield S. Electrical activity in the neuromuscular unit can influence the molecular development of motor neurons. Dev Biol. 1994;162:539–548. doi: 10.1006/dbio.1994.1107. [DOI] [PubMed] [Google Scholar]
- 53.Kelly AM, Rosser BW, Hoffman R, Panettieri RA, Schiaffino S, Rubinstein NA, Nemeth PM. Metabolic and contractile protein expression in developing rat diaphragm muscle. J Neurosci. 1991;11:1231–1242. doi: 10.1523/JNEUROSCI.11-05-01231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knudsen KA. Cell adhesion molecules in myogenesis. Curr Opin Cell Biol. 1990;2:902–906. doi: 10.1016/0955-0674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi K, Lemke RP, Greer JJ. Ultrasound measurements of fetal breathing movements in the rat. J Appl Physiol. 2001;91:316–320. doi: 10.1152/jappl.2001.91.1.316. [DOI] [PubMed] [Google Scholar]
- 56.Krnjevic K, Miledi R. Failure of neuromuscular propagation in rats. J Physiol. 1958;140:440–461. [PMC free article] [PubMed] [Google Scholar]
- 57.Krnjevic K, Miledi R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kucenas S, Takada N, Park HC, Woodruff E, Broadie K, Appel B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci. 2008;11:143–151. doi: 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuei JH, Shadmehr R, Sieck GC. Relative contribution of neurotransmission failure to diaphragm fatigue. J Appl Physiol. 1990;68:174–180. doi: 10.1152/jappl.1990.68.1.174. [DOI] [PubMed] [Google Scholar]
- 60.Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 61.LaFramboise WA, Daood MJ, Guthrie RD, Schiaffino S, Moretti P, Brozanski B, Ontell MP, Butler-Browne GS, Whalen RG, Ontell M. Emergence of the mature myosin phenotype in the rat diaphragm muscle. Dev Biol. 1991;144:1–15. doi: 10.1016/0012-1606(91)90473-g. [DOI] [PubMed] [Google Scholar]
- 62.Lebrasseur NK, Cote GM, Miller TA, Fielding RA, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003;284:C1149–C1155. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]
- 63.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 64.Lindsay AD, Greer JJ, Feldman JL. Phrenic motoneuron morphology in the neonatal rat. J Comp Neurol. 1991;308:169–179. doi: 10.1002/cne.903080204. [DOI] [PubMed] [Google Scholar]
- 65.Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- 66.Mantilla CB, Rowley KL, Fahim MA, Zhan WZ, Sieck GC. Synaptic vesicle cycling at type-identified diaphragm neuromuscular junctions. Muscle Nerve. 2004;30:774–783. doi: 10.1002/mus.20173. [DOI] [PubMed] [Google Scholar]
- 67.Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience. 2007;146:178–189. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 68.Martin-Caraballo M, Campagnaro PA, Gao Y, Greer JJ. Contractile and fatigue properties of the rat diaphragm musculature during the perinatal period. J Appl Physiol. 2000;88:573–580. doi: 10.1152/jappl.2000.88.2.573. [DOI] [PubMed] [Google Scholar]
- 69.Martin-Caraballo M, Greer JJ. Development of potassium conductances in perinatal rat phrenic motoneurons. J Neurophysiol. 2000;83:3497–3508. doi: 10.1152/jn.2000.83.6.3497. [DOI] [PubMed] [Google Scholar]
- 70.Martin-Caraballo M, Greer JJ. Electrophysiological properties of rat phrenic motoneurons during perinatal development. J Neurophysiol. 1999;81:1365–1378. doi: 10.1152/jn.1999.81.3.1365. [DOI] [PubMed] [Google Scholar]
- 71.Olson EN. Signal transduction pathways that regulate skeletal muscle gene expression. Mol Endocrinol. 1993;7:1369–1378. doi: 10.1210/mend.7.11.8114752. [DOI] [PubMed] [Google Scholar]
- 72.Prakash YS, Fournier M, Sieck GC. Effects of prenatal undernutrition on developing rat diaphragm. J Appl Physiol. 1993;75:1044–1052. doi: 10.1152/jappl.1993.75.3.1044. [DOI] [PubMed] [Google Scholar]
- 73.Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol. 2000;89:563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- 74.Prakash YS, Miller SM, Huang M, Sieck GC. Morphology of diaphragm neuromuscular junctions on different fibre types. J Neurocytol. 1996;25:88–100. doi: 10.1007/BF02284788. [DOI] [PubMed] [Google Scholar]
- 75.Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 76.Prakash YS, Zhan WZ, Miyata H, Sieck GC. Adaptations of diaphragm neuromuscular junction following inactivity. Acta Anat (Basel) 1995;154:147–161. doi: 10.1159/000147762. [DOI] [PubMed] [Google Scholar]
- 77.Ren J, Greer JJ. Ontogeny of rhythmic motor patterns generated in the embryonic rat spinal cord. J Neurophysiol. 2003;89:1187–1195. doi: 10.1152/jn.00539.2002. [DOI] [PubMed] [Google Scholar]
- 78.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 79.Rowley KL, Mantilla CB, Ermilov LG, Sieck GC. Synaptic vesicle distribution and release at rat diaphragm neuromuscular junctions. J Neurophysiol. 2007;98:478–487. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- 80.Sandrock AW, Jr, Dryer SE, Rosen KM, Gozani SN, Kramer R, Theill LE, Fischbach GD. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science. 1997;276:599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- 81.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 82.Serrano AL, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lomo T, Schiaffino S. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc Natl Acad Sci USA. 2001;98:13108–13113. doi: 10.1073/pnas.231148598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- 84.Sieck GC. Neural control of the inspiratory pump. News Physiol Sci. 1991;6:260–264. [Google Scholar]
- 85.Sieck GC, Fournier M. Changes in diaphragm motor unit EMG during fatigue. J Appl Physiol. 1990;68:1917–1926. doi: 10.1152/jappl.1990.68.5.1917. [DOI] [PubMed] [Google Scholar]
- 86.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- 87.Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neurosci Lett. 1989;97:29–34. doi: 10.1016/0304-3940(89)90134-1. [DOI] [PubMed] [Google Scholar]
- 88.Sieck GC, Fournier M, Prakash YS, Blanco CE. Myosin phenotype and SDH enzyme variability among motor unit fibers. J Appl Physiol. 1996;80:2179–2189. doi: 10.1152/jappl.1996.80.6.2179. [DOI] [PubMed] [Google Scholar]
- 89.Sieck GC, Prakash YS. Fatigue at the neuromuscular junction. Branch point vs. presynaptic vs. postsynaptic mechanisms. Adv Exp Med Biol. 1995;384:83–100. [PubMed] [Google Scholar]
- 90.Sieck GC, Prakash YS, Han YS, Fang YH, Geiger PC, Zhan WZ. Changes in actomyosin ATP consumption rate in rat diaphragm muscle fibers during postnatal development. J Appl Physiol. 2003;94:1896–1902. doi: 10.1152/japplphysiol.00617.2002. [DOI] [PubMed] [Google Scholar]
- 91.Sieck GC, Zhan WZ. Denervation alters myosin heavy chain expression and contractility of developing rat diaphragm muscle. J Appl Physiol. 2000;89:1106–1113. doi: 10.1152/jappl.2000.89.3.1106. [DOI] [PubMed] [Google Scholar]
- 92.Song A, Ashwell KW, Tracey DJ. Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Anat Embryol (Berl) 2000;202:159–177. doi: 10.1007/s004290000096. [DOI] [PubMed] [Google Scholar]
- 93.Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- 94.Su CK, Mellen NM, Feldman JL. Intrinsic and extrinsic factors affecting phrenic motoneuronal excitability in neonatal rats. Brain Res. 1997;774:62–68. doi: 10.1016/s0006-8993(97)81688-5. [DOI] [PubMed] [Google Scholar]
- 95.Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- 96.Thompson DM, Buettner HM. Neurite outgrowth is directed by schwann cell alignment in the absence of other guidance cues. Ann Biomed Eng. 2006;34:161–168. doi: 10.1007/s10439-005-9013-4. [DOI] [PubMed] [Google Scholar]
- 97.Tissenbaum HA, Parry DJ. The effect of partial denervation of tibialis anterior muscle on the number and sizes of motoneurons in the motor nucleus of normal and dystrophic mice. Can J Physiol Pharmacol. 1991;69:1769–1773. doi: 10.1139/y91-261. [DOI] [PubMed] [Google Scholar]
- 98.Trinidad JC, Fischbach GD, Cohen JB. The Agrin/MuSK signaling pathway is spatially segregated from the neuregulin/ErbB receptor signaling pathway at the neuromuscular junction. J Neurosci. 2000;20:8762–8770. doi: 10.1523/JNEUROSCI.20-23-08762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci. 2006;26:5872–5880. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vandenburgh HH, Hatfaludy S, Karlisch P, Shansky J. Skeletal muscle growth is stimulated by intermittent stretch-relaxation in tissue culture. Am J Physiol Cell Physiol. 1989;256:C674–C682. doi: 10.1152/ajpcell.1989.256.3.C674. [DOI] [PubMed] [Google Scholar]
- 101.Vaughn JE, Barber RP, Sims TJ. Dendritic development and preferential growth into synaptogenic fields: a quantitative study of Golgi-impregnated spinal motor neurons. Synapse. 1988;2:69–78. doi: 10.1002/syn.890020110. [DOI] [PubMed] [Google Scholar]
- 102.Watchko JF, Brozanski BS, O’Day TL, Guthrie RD, Sieck GC. Contractile properties of the rat external abdominal oblique and diaphragm muscles during development. J Appl Physiol. 1992;72:1432–1436. doi: 10.1152/jappl.1992.72.4.1432. [DOI] [PubMed] [Google Scholar]
- 103.Watchko JF, Daood MJ, Sieck GC. Myosin heavy chain transitions during development. Functional implications for the respiratory musculature. Comp Biochem Physiol. 1998;119:459–470. doi: 10.1016/s0305-0491(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 104.Watchko JF, Sieck GC. Respiratory muscle fatigue resistance relates to myosin phenotype and SDH activity during development. J Appl Physiol. 1993;75:1341–1347. doi: 10.1152/jappl.1993.75.3.1341. [DOI] [PubMed] [Google Scholar]
- 105.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, Zhuang Y, Lassar A. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 106.Wells LJ. Development of the human diaphragm and pleural sacs. Contrib Embryol. 1954;35:107–134. [Google Scholar]
- 107.Zhan WZ, Watchko JF, Prakash YS, Sieck GC. Isotonic contractile and fatigue properties of developing rat diaphragm muscle. J Appl Physiol. 1998;84:1260–1268. doi: 10.1152/jappl.1998.84.4.1260. [DOI] [PubMed] [Google Scholar]
- 108.Zhu X, Lai C, Thomas S, Burden SJ. Neuregulin receptors, erbB3 and erbB4, are localized at neuromuscular synapses. EMBO J. 1995;14:5842–5848. doi: 10.1002/j.1460-2075.1995.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]