Abstract
Online monitoring of serotonin in striatal dialysate from freely moving rats was carried out for more than sixteen hours at one-minute time resolution using microdialysis coupled online to a capillary HPLC system operating at about 500 bar and 50 °C. Several aspects of the system were optimized towards robust, in vivo online measurements. A two-loop, eight-port rotary injection valve demonstrated better consistency of continuous injections than the more commonly used two-loop, ten-port valve. A six-port loop injector for introducing stimulating solutions (stimulus injector) was placed inline between the syringe pump and microdialysis probe. We minimized solute dispersion by using capillary tubing (75 µm i.d., 70 cm long) for the probe inlet and outlet. In vitro assessment of concentration dispersion during transport with 30-s time resolution showed that the dispersion standard deviation for serotonin was well within the desired system temporal resolution. Each 30- or 60-second measurement reflects the integral of the true time response over the measurement time. We have accounted for this mathematically in determining the concentration dispersion during transport. The delay time between a concentration change at the probe and its detection is seven minutes. The timing of injections from the stimulus injector and the cycle time for the HPLC monitoring of the flow stream was controlled. The electrochemical detector contained a 13 µm spacer to minimize detector dead volume. During in vivo experiments, retention time and separation efficiency were stable and reproducible. There was no statistically significant change over 5.5 hours in the electrochemical detector sensitivity factor for serotonin. Dialysate serotonin concentrations change significantly in response to a 120 mM K+ stimulus. Release of serotonin evoked by a ten-minute, 120 mM K+ stimulation, but not for other K+ stimuli, exhibited a reproducible, oscillating profile of dialysate serotonin concentration vs. time. Infusion of fluoxetine, a serotonin uptake inhibitor, increased dialysate serotonin concentrations and stimulated release magnitude. Transient serotonin increases were observed in response to the stress associated with unexpected handling. This system is simple, efficient, reliable, and suitable for study of serotonin neurochemistry associated with emotion and behavior.
Online sampling/analysis is highly desirable for many applications, for example high throughput screening,1 analysis of synthetic reactions in microreactors,2 environmental3 and biological4 substance monitoring, and process analytical chemistry.5 Online sampling especially benefits the analysis of small volume samples since it avoids sample handling procedures. Another area in which it excels is in monitoring dynamic processes such as those occurring in the brain.6 Increasing the temporal resolution of online sampling/analysis will provide more details of dynamic processes.
Serotonin (5-hydroxytryptamine, 5-HT) together with the 5-HT transporter and receptors modulate many brain functions7–10 and intestinal activities.11 Neurological disorders like depression and anxiety are associated with abnormalities of the serotonergic system. Two major tools to study 5-HT neurochemistry in the brain are fast scan cyclic voltammetry12–14 and microdialysis/HPLC. These techniques are complementary. The former has sub-second time resolution while microdialysis-HPLC can perform multi-analyte detection over long timescales with the potential for unambiguous analyte identification.
Online measurements following microdialysis are continuous, e.g., using sensors,15 or discrete, e.g., using a separation.16–19 Here, we focus on the latter. Conventional microdialysis-HPLC is carried out with low temporal resolution. Temporal resolution can be improved by decreasing the scale of the separation method, e.g., capillary HPLC and capillary electrophoresis.20–23 Kennedy and Bowser measured changes in neurotransmitter concentrations (e.g., dopamine, glutamate, aspartate) in response to a stimulus using online microdialysis-capillary electrophoresis with online fluorescent derivatization.24 The temporal resolution was as high as 11 s for amino acids22 and 90 s for dopamine.21 While capillary electrophoresis is powerful, within the neuroscience community HPLC has become the preferred method for research on low concentration neurotransmitters like dopamine and 5-HT25,26 because of its reliability and reproducibility. Using microdialysis-HPLC, Newton and Justice20 studied dopamine dynamics with one-minute temporal resolution by using small, 1 µL samples in conjunction with a 0.5-mm-inner diameter (i.d.) microbore column. Richter et al.27 studied 5-HT and other neurotransmitters with a temporal resolution of 1.5 minutes using 3 µL samples in conjunction with a 2-mm-i.d. column. These researchers recognized the advantage of small diameter columns: they minimize sample dilution during the separation permitting reliable quantitation with small volume injections. However, they performed the chromatography offline. Using even smaller i.d. capillary columns offline Parrot et al.28 with 1 µL samples and Jung et al.29 with 0.5 µL samples achieved low detection limits for 5-HT, but the separations were performed offline and were approximately ten minutes long.
The Andrews lab30 recently reported in vivo, online monitoring of 5-HT with a temporal resolution of three minutes. This is a significant advance. The work was done with commercially available equipment. They applied this fast microdialysis approach to awake animals for times long enough to perceive circadian changes.
When considering the design of an online separation-based analysis system, it is important to understand the relationships that define and indeed connect the system’s figures of merit. Scheme 1 shows that the concentration of analyte 5-HT in the detector following a separation consists of two independent components: the animal/microdialysis part of the experiment defines the number of moles injected into the separation system and the separation system (here chromatography) dictates the volume of the solution in which the injected moles are found in the detector. We will call the latter volume the peak volume. The number of moles collected in each sample depends on the sample volume and the dialysate concentration, itself a function of the actual concentration in the brain and the microdialysis recovery. The peak volume is defined by the retention time and a number of other parameters related to column efficiency, (which may include extracolumn effects and overloading) as well as the inside diameter of the column. Ultimately, the moles acquired divided by the peak volume must exceed the concentration detection limit of the detector used. The integral relationship between sampling and measurement is even deeper. The microdialysis sample volume and flow rate define the sampling time and thus the temporal resolution. Ideally, the speed of the chromatographic system and the sampling time will “match” so that one chromatogram is achievable in the same time required to collect the sample. The constraints on an optimized system are therefore that under conditions where the sampling time is approximately equal to the separation time, the ratio of moles collected during the sampling time to the peak volume in the separation system must exceed the detector’s detection limit by some factor. While the foregoing is accurate, it should be borne in mind that the system’s time resolution may be limited by the dispersion within the microdialysis flow path (to be discussed below).
Scheme 1.
Yellow outlined boxes correspond to microdialysis; blue boxes correspond to the chromatography. Arrows imply “controls” or “affects”. Black triangles with two “inputs” and one “output” imply “define”. The 5HT concentration in the brain with the microdialysis recovery gives rise to a dialysate. Microdialysis recovery depends on dialysate flow rate. The flow rate of the dialysate and desired sampling time together define the sample volume. The sample volume and dialysate concentration give the moles of 5HT in one sample. Column parameters at a particular temperature, T, limited by available pump pressure, control the number of theoretical plates, N, and the void time, t0. The value of N will be decreased by injecting too much mass or volume. The reduction in efficiency that results from either type of overload depends on the column volume. The void time and the retention factor, k’, give the retention time of the analyte. The chromatographic system yields a peak with a particular volume (peak volume) dependent on a peak width related to bandspreading and column inside diameter. The peak volume and the moles of 5HT from the dialysis give a concentration in the detector that must be greater than the detection limit of the detector. Note that in the optimal case, the sampling time and retention time are similar as well (blue yellow dashed line).
Recently, Liu et al.31 and Zhang et al.26 improved the HPLC analysis speed of 5-HT to the sub-minute level using elevated column pressure and temperature, a capillary column packed with sub-2-µm particles, and a sensitive, low dead-volume electrochemical detector. Using the smallest commercially available packing material at the time, Zhang et al. optimized a chromatographic system as described in Scheme 1 for maximum sensitivity given a required separation power (theoretical plate count), desired separation speed, and sample size. The column diameter is a key parameter and has an optimum value. This is the first time that both the microdialysis sampling time and the analysis time for 5-HT are one minute or less laying the groundwork for in vivo online microdialysis coupled to capillary UHPLC with electrochemical detection (UHPLC-EC) (here we use the rather ill-defined term UHPLC to represent our use of elevated temperature and pressure conditions). In this work, we carried out in vivo online microdialysis coupled to capillary UHPLC to monitor basal 5-HT concentrations and the subsequent changes in response to a stimulus with one-minute temporal resolution in the striatum of freely-moving rats. Optimization of both sampling and analysis were carried out, with emphasis on temporal resolution, long time continuous analysis sensitivity, and robustness. We observed basal concentrations and dynamic changes of 5-HT for up to sixteen hours and forty minutes.
EXPERIMENTAL SECTION
Chemicals and materials
Chemicals and sources were: disodium EDTA, Fisher Scientific (Fair Lawn, NJ); ethanol, Pharmco-AAPER (Shelbyville, KY); sodium acetate and glacial acetic acid, J. T. Baker (Phillipsburg, NJ); acetonitrile, 2-propanol, l-ascorbic acid, serotonin hydrochloride, fluoxetine hydrochloride, and sodium 1-octanesulfonate (SOS), Sigma (St. Louis, MO). All the chemicals were used as received without further purification. Ultra-pure water was obtained from a Millipore Milli-Q Synthesis A10 system (Bedford, MA). Fused silica capillaries were from Polymicro Technologies (Pheonix, AZ).
Chromatography
Capillary columns were packed according to a previously reported procedure.26 Briefly, columns were slurry packed with 1.7 µm BEH C 18 reversed-phase particles (Waters, Milford, MA) using 150 µm i.d., 360 µm outer diameter (o.d.) capillaries as the column blank. The packed capillary columns were connected directly to the injector and flushed with mobile phase overnight before use.
A UHPLC pump (Model nanoLC-Ultra 1D, Eksigent, Dublin, CA), with a maximum pressure limit of 700 bar, was used. The outlet of the pump was connected to an eight-port nanobore injector equipped with two 500 nL sample loops (Valco Instruments, Houston, TX). Isocratic separations of 5-HT were achieved using ion-pair, reversed phase liquid chromatography. The mobile phase was aqueous buffer:acetonitrile in a 96:4 (v/v). The aqueous buffer contained 100 mM sodium acetate, 0.15 mM disodium EDTA and 10.0 mM SOS and glacial acetic acid sufficient to create pH = 4.00. Mobile phase was filtered before use (0.22 µm Nylon filter, Osmonics, Minnetonka, MN). The mobile phase flow rate for the in vivo experiments was 10 µL/min and the operating temperature of the injector and the capillary column was maintained at 50 °C using two homemade heating assemblies26 unless stated otherwise. Analytes were detected at 700 mV vs Ag/AgCl reference electrode by a BASi radial-style electrochemical detection flow cell (West Lafayette, IN) with a 13 µm Teflon gasket. The cell was connected to the column with a 10-cm-long, 25-µm-i.d. capillary. Potential control and data acquisition were done by a BASi Epsilon potentiostat (West Lafayette, IN). Instrument control and data collection was achieved using a PeakSimple Chromatographic Data System (SRI Instruments, Torrance, CA).
Microdialysis Probe Fabrication
Microdialysis probes32 were prepared with Spectra-Por RC hollow fiber dialysis membrane (4 mm long; 200-µm i.d., 216-µm o.d.; molecular weight cut-off (MWCO) 13 kD; Spectrum Laboratories Inc., Rancho Dominguez, CA) and fused-silica inlet/outlet tubing (70 cm long; 75 µm i.d., 150 µm o.d.), which were sealed in a 3.5-cm-long stainless steel hypodermic tubing body (B000FMWJWK, Small Parts, Logansport, IN) with 2 Ton epoxy (ITW Devcon, Danvers, MA). The hollow fiber membrane is shipped filled with a water-insoluble ester, isopropyl myristate, to prevent membrane deformation. Probes were activated by flushing with 1.0 mL ethanol to remove the long chain ester before use. The alcohol was removed by rinsing with artificial cerebrospinal fluid (aCSF) (containing NaCl 144 mM, KCl 2.7 mM, CaCl2 1.2 mM, MgCl2 1 mM, NaH2PO4 2 mM at pH 7.4).
In Vitro Online Microdialysis Coupled to a Capillary UHPLC-EC System
A microdialysis probe was placed in a 100 mL beaker filled with aCSF solution (Figure S1). A concentration step was generated through the addition of a concentrated 5-HT solution to the stirred contents of the beaker. The 5-HT concentration was monitored with a 30 seconds analysis time interval. The chromatographic conditions were slightly different from the in vivo experiments in order to achieve the 30 s time resolution. Mobile phase flow rate was 12 µL/min with column and injector temperatures at 70 °C.
In Vitro Assessment of Solute Dispersion during Sample Transfer
The microdialysis probe was replaced by a loop injector fitted with a 6 µL injection loop. The concentration profile resulting from injecting the loop contents into the outlet capillary was monitored with one-minute time resolution. It is important to control the time of the injection with respect to the continuous, one-minute cycle time of the monitoring. In this experiment, we altered the time between the injection and the UHPLC injection cycle to 5 s, 25 s and 45 s for three separate concentration steps.
Surgical Procedure
All procedures involving animals were carried out with the approval of the Institutional Animal Care and Use Committee of the University of Pittsburgh. Male Sprague-Dawley rats (250–375 g, Hilltop, Scottsdale, PA) were anesthetized with isoflurane (0.5% by volume, Baxter Healthcare, Deerfield, IL). A homoeothermic blanket (EKEG Electronics; Vancouver, BC, Canada) kept the body temperature at 37 °C. A stereotaxic frame (David Kopf Instruments; Tujunga, CA) was used for all surgeries. (over the striatum. A guide cannula MD-2251, Bioanalytical Systems Inc.; West Lafayette, IN) was implanted into the striatum (1.2 mm anterior of bregma, 2.5 mm lateral of bregma), and lowered to 1 mm beneath the surface of the brain. Jeweler’s screws with dental cement were used to hold the cannula in place. Rats recovered for a minimum of three days. Then a second procedure was performed to implant the microdialysis probe. Rats were anesthetized with isoflurane and microdialysis probes were slowly lowered vertically over thirty minutes into the striatum (7.0 mm below dura) and secured to the guide cannula with epoxy cement. Once the probe was implanted, anesthesia was removed and the rats were placed in a Raturn chamber (Bioanalytical Systems, West Lafayette, IN) for the duration of the experiment.
In Vivo Online Microdialysis Coupled with Capillary UHPLC-EC System
Figure 1 shows a diagram for the in vivo online microdialysis-UHPLC-EC system. A PicoPlus syringe pump (Harvard Apparatus, Holliston, MA) with a 1.0 mL gas-tight syringe delivered the microdialysis perfusion fluid, aCSF. A two-position “stimulus” loop injector was placed between the syringe and dialysis probe for the introduction of another solution. To minimize Taylor-Aris dispersion and other sources of dispersion, 75 µm i.d. capillary tubing (10 cm) connected the stimulus valve to the microdialysis inlet capillary, the inlet capillary (70 cm) to the dialysis tubing, the outlet capillary (70 cm) to a connector capillary (10 cm) connected to the HPLC injection valve. All connections were made with MicroTight Unions (IDEX Health & Science, Oak Harbor, WA).
Figure 1.
In vivo online microdialysis-UHPLC-EC experimental set up.
Probe implantation was done early in the morning and online experiments were initiated in the afternoon of the same day. Probes were perfused with aCSF at 0.6 µL/min for at least 2 hours after implantation prior to connection to the online chromatographic system. Standards (100 nM 5-HT) were analyzed before and after the online experiments for calibration. The sampling volume was 600 nL which was selected to slightly overfill the 500 nL sample loop volume to increase injection volume precision33. Stable basal 5-HT concentrations were observed for at least twenty minutes prior to carrying out an experiment such as K+ stimulation. Potassium stimulations of three or ten minutes were conducted with either 70 mM K+ or 120 mM K+. The selective serotonin reuptake inhibitor fluoxetine was introduced by perfusion with 10 µM fluoxetine dissolved in aCSF.
Peak area was used for concentration calibration. To integrate the areas under the 5-HT peaks, a standard Savitzky-Golay, second derivative, quadratic smooth was employed, using an 81 point window.34,35 Prior to determining the integration limits, an approximate window defined to contain the eluted peak was identified. The start integration point and the stop integration point were manually determined. A linear baseline between the start and stop points was created and the integration routine was programmed in the MATLAB environment (Mathworks, Natick, MA).
RESULTS AND DISCUSSION
Valve Selection and Stimulus Introduction
For continuous sampling/analysis, two-position, electronically actuated valves with dual sample loops are employed. Each of the valve positions dictates which loop is in the “load” position while the other is in the “inject” position. Our initial online experiments had a ten-port injection valve. However, the ten-port valve was found to be less desirable than an eight-port injection valve. The ten-port valve has an extra length of tubing, a so-called jumper loop (Figure S3). During the injection process, the jumper loop’s contents, mobile phase, follow the contents of the sample loop in the “A” position. However, the jumper loop’s contents precede, and thus mix with the leading edge of, injections from the “B” position. We speculate that the additional extra-column bandspreading seen when injecting from the “B” position resulted from poor on-column focusing resulting from the higher eluting strength of the mixed sample/mobile phase as it enters the column while the leading edge of the samples injected from the “A” position were not mixed with mobile phase prior to entering the column. While peak areas are not significantly different, peak heights for 5-HT standards injected from the “A” and “B” positions were different (paired Student’s t, p < 0.05). The eight-port injection valve has an asymmetrical flow path but no jumper loop (Figure 1). Using the eight-port valve, peak heights for 5-HT standards injected from the A and B loops were not found to be statistically different (paired Student’s t, p > 0.05).
Initially, we set up the perfusion system with a pair of syringes in a syringe pump connected to a four-port switching valve to switch between syringes. However, we noted that three- and five-minute K+ stimulations in awake animals both gave increased 5-HT over a nine-minute period. We speculate that two factors contribute to this. One is the large, 12.4 µL swept volume of the four-position switching valve. The other is the change in back pressure felt by the syringe that is switched into the flow stream. We replaced the two-syringe-pump arrangement a single syringe followed by a low dead-volume (90 nL), six-port loop injection valve (Figure 1) with a loop made from tubing with a larger i.d. (150 µm) than the inlet and outlet tubing (75 µm i.d.). The loop is also shorter than the transport tubing. Recall that for constant flow rate, the change in pressure is proportional to the fourth power of the change in radius. Thus a factor of two change in radius corresponds to a factor of sixteen in pressure. In combination with the shorter length, we estimate that the backpressure with the loop inline is only 2% larger than with the loop out of the flow path. Therefore, the microdialysis flow rate remains stable and the introduction of a chemical stimulus is reproducible.
In-house Microdialysis Probe Characterization and System Temporal Resolution
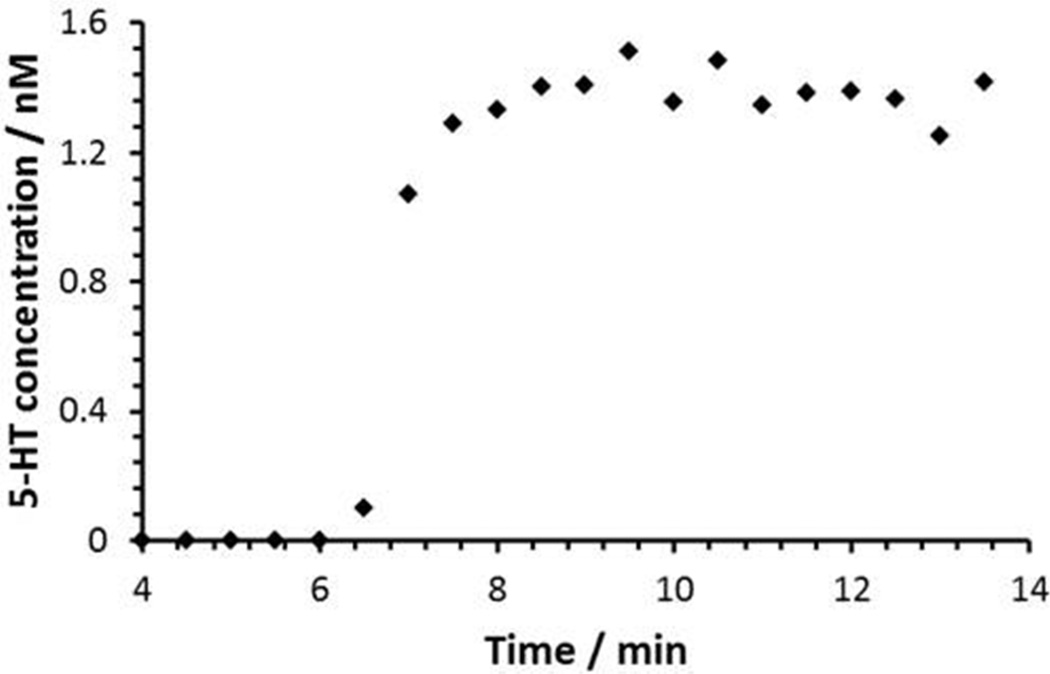
Initially, we used commercial microdialysis probes, but observed column pressure increases, and significant solute dispersion due to the relatively large outlet tubing (120 µm i.d.) in in vivo experiments. Dispersion during transport of the samples from the dialysis probe to the analysis system needs to be carefully controlled because it can be the limiting factor15,18,36 in the overall temporal resolution of online measurements as sampling and analysis speeds are improved. In general, use of smaller microdialysis probe internal volume, shorter, narrower i.d. outlet tubing with fewer connections and zero dead-volume connectors will help to minimize the concentration dispersion during transport of samples from the brain to the instrument. On the other hand, the microdialysis probe is prone to clogging for outlet tubing with internal diameters less than 25 µm. Also, the pressure stemming from narrow outlet tubing can have an impact on both the relative and absolute recoveries of the probe.37 Therefore, the outlet tubing i.d. and internal volume of the microdialysis probe itself should be carefully chosen. Figure 2 demonstrates a rapid response to concentration changes using the in-house microdialysis probe with a 75 µm i.d., 70 cm long outlet tubing. Data were acquired at 30 seconds intervals using a 200 nL sample loop at a microdialysis flow rate of 0.6 µL/min. Seven minutes after the change in 5-HT concentration in the beaker the change was detected in the UHPLC system. Solute dispersion resulted chiefly from two factors, namely inside the hollow fiber probe itself and within the outlet tubing. Assuming that the concentration changes as an instantaneous step function from 0 to C0 in the beaker, the normalized concentration C/C0 vs. time function when the step arrives at the UHPLC injector will be an error function38, eq. 1, as expected for Taylor-Aris dispersion.39 C is the concentration at time t, tc is the time at the inflection point of the error function, and σ is the concentration dispersion standard deviation in units of time. Because each injection has the number of moles of 5-HT in a particular 200 nL segment of the flow stream, each UHPLC peak’s magnitude is proportional to the area under a particular segment of the concentration-time function. The area (A) from the integration of the normalized concentration C/C0 profile for a certain time window (t1 to t2) in equation 2 represents the measured concentration for a single peak in the chromatogram. Using the solver function in Excel, the concentration dispersion standard deviation σ was found to be 11.6 s by fitting eq. 2 to the measured concentration distribution. This means that it took about 46.4s (−2σ to 2σ ) for a concentration change to reach steady state (~ 2% to ~ 98% of maximum), well within the one minute temporal resolution of the system.
| 1 |
| 2 |
Figure 2.
Solute dispersion of a concentration step by using an in-house microdialysis probe. Concentration changes were measured at 30 s intervals.
We also studied the solute dispersion in the outlet tubing and connections alone by creating a concentration step using a six-port loop injector injecting directly into the 75 µm i.d., 70 cm long outlet capillary. Here we also explored attaining greater time resolution by observing multiple transients with different phase shift compared to a one-minute separation cycle time (Figure S2). Three consecutive concentration steps were created with 5 s, 25 s, and 45 s between 5-HT injections and the UHPLC injection cycle (Figure S4). The concentration values in the transient region were dependent on the phase difference between the injection time and the UHPLC injection cycle. Combination of the three concentration step profiles gave a concentration step with 20 s resolution (Figure S5). This demonstrates how to increase the time resolution when the experiment involves the introduction of a change in concentration at a known time. These data (Figure S5) tell us about dispersion in the outlet tube alone. Using the solver function in Excel, the concentration dispersion standard deviation was found to be 7.6 s by fitting eq. 2 to the measured concentration distribution. This was in reasonable agreement with a calculated value of the standard deviation from Taylor dispersion, 6.2 s, based on a diffusion coefficient40 for 5-HT of 5.4 × 10−10 m2s−1 in aqueous samples. If we make the assumption that the observed total solute dispersion has independent contributions from the hollow fiber probe and the capillary tubing, then the variances from each contribution will add to yield the total observed variance of 134.6 s2 (i.e., (11.6 s)2) We can thus deduce that the hollow fiber probe’s concentration dispersion standard deviation is 8.7 s. Note that these dispersion experiments used concentrations of 5-HT in the physiological range (~1.5 nM), important to get a realistic measurement. The response time can be concentration dependent when the solute adsorbs to sampling system components. Use of a high concentration will lead to lower dispersion, and an overly optimistic picture.
Switching to the in-house probe also solved the column clogging problem41 present with the commercial probes (30 kDa MWCO, 4 mm long poly(acrylonitrile). The problem of the sample clogging the column is more severe when using capillary columns because the sample is not significantly diluted upon injection. The in vitro recovery of 5-HT with these microdialysis probes was about 35% at a perfusion flow rate of 0.6 µL/min. In total, fourteen in-house probes were used for in vivo online experiments. Twelve out of the fourteen showed no column clogging.
Online Measurement of Basal Serotonin Concentration in Striatum
Microdialysate contains electroactive components at concentrations greater than 5-HT. Many of them are anionic, eluting prior to 5-HT, e.g., 5-hydroxyindole-3-acetic acid (5-HIAA), 3,4-dihydroxyphenylacetic aicd (DOPAC), and homovanillic acid (HVA) which are usually in the 200 nM to 5 µM range. However, one particular anionic compound, ascorbate, was the most bothersome during the initial chromatographic optimization experiments. For clarity, this discussion will refer to “ascorbate” and an “ascorbate peak” recognizing that in fact all of the anionic, electroactive components of the sample contribute to the low-retention-time signal. As seen in Figure 3 (dotted line), ascorbate is poorly retained. The concentration of ascorbate in the microdialysate is roughly 50 µM. The peak appears wide especially when observed with a detection sensitivity sufficient to see 5-HT at about 105 lower concentration. The ascorbate peak tails with the result that the “baseline” for 5-HT is actually part of the tail of the ascorbate peak. This tail leads to signal drift in the region of 5-HT elution. This problem was not seen for offline analysis with commercial probes in our previous study26,31 (Figure 3 solid line) where ascorbate was apparently at a much lower concentration due to the reduction in observed peak tailing. We also found that the ascorbate concentration was in between these two extremes for online analysis with commercial probes (Figure 3 dashed line). We think this ascorbate tailing is particularly associated with using capillary columns in which mass capacity is low compared to conventional columns. Ascorbate is oxidized by O2 during sample transfer in the outlet tubing, and in offline collection and storage of the microdialysis sample. The oxidation rate depends on oxygen partial pressure, temperature,42 pH43 and oxygen permeability of the tubing wall materials.44 Offline microdialysis samples are usually acidified45 in the collection vial and stored frozen before analysis which decreases but cannot completely stop ascorbate oxidation/degradation. The Teflon tubing used by most commercial microdialysis probes is O2 permeable, and the large surface area to volume ratio of the outlet tubing creates an ideal environment for ascorbate oxidation. In our current online experiment, the transfer from microdialysis to capillary UHPLC takes only about seven minutes and the outlet tubing is fused silica which is not O2 permeable. As seen in Figure 4, the baseline is sloping and the 5-HT peak is not quantifiable with a column temperature of 60 °C. To solve this problem, 5-HT retention was increased by lowering the column temperature to 50 °C so it will elute at a later time (0.9 minutes) where the contribution of ascorbate is not as significant.
Figure 3.
Chromatograms of microdialysis samples. The large peaks starting from about 0.05 minutes include ascorbic acid, the most abundant oxidizable molecule in the chromatogram. Dotted line: online UHPLC analysis of microdialysis samples using an in-house microdialysis probe. Dashed line: online UHPLC analysis of microdialysis samples using a commercial microdialysis probe. Solid line: offline UHPLC analysis of microdialysis samples using a commercial microdialysis probe.
Figure 4.
Chromatograms of microdialysis samples at 50 °C and 60 °C.
System Stability
Column performance deterioration and detection sensitivity loss with time is a concern for in vivo experiments. We did four injections of a 100 nM standard before and after 5.5 hours of continuous in vivo experiments. Table 1 shows that the 5-HT retention time, peak width, and column plate number were reproducible. There was no significant change in the peak area when comparing standard injections before and after the in vivo experiments (Student’s t test, p > 0.05, Table S1).
Table 1.
Column performance evaluation before and after in vivo measurements.
| Retention time / s |
fwhm/ s | Plate number |
|
|---|---|---|---|
| Before | 52.0 ± 0.3 | 2.2 | 3089 ± 37 |
| After | 51.8 ± 0.1 | 2.2 | 3071 ± 15 |
In Vivo Monitoring and Serotonin Dynamics in Response to a Stimulus
Concentrations were determined using external calibration without correction for the probe recovery. A linear calibration curve was created using peak area vs 5-HT standard concentration from 0 to 100 nM (r2 = 0.998). The detection limit for 5-HT was 0.16 nM (signal three times greater than rms noise). Figure 5 is part of a series of chromatograms showing 5-HT concentration changes caused by a three-minute 120 mM K+ stimulation. The basal 5-HT concentration measured for this rat was 0.64 ± 0.04 nM (standard error based on eight samples). Introduction of 120 mM K+ induces 5-HT release into the extracellular space resulting in the microdialysate concentration increasing to 29.2 nM.
Figure 5.
Typical chromatogram of continuous 5-HT measurements. Arrows indicate the 5-HT peak. The rat was under three-minute high K+ stimulation which resulted in 5-HT release.
Figure 6 shows a typical run of nearly six hours. Each data point represents a single chromatogram. The figure shows several 5-HT transients caused by K+ stimulation. Typically, the increase in the 5-HT concentration was about four times higher for three-minute 120 mM K+ stimuli than for a three-minute 70 mM K+ stimulus. We will return to the ten-minute 120 mM K+ below. To confirm that we are measuring 5-HT, we infused an SSRI, fluoxetine and observed increased 5-HT concentration46 (Figure S6). Infusion of fluoxetine also increased transient 5-HT concentration from high potassium stimulation (Figure 7), and decreased extraction efficiency of infused 5-HT (Figure S7).
Figure 6.
Typical online in vivo 5-HT measurement of one rat. Three-minute 70 mM K+, three-minute and ten-minute 120 mM K+ stimulations were carried out. Perfusion of aCSF containing 10 µM fluoxetine started at 06:05 PM. 6 µL 100 nM 5-HT standard was introduced to the perfusate by the six-port injector valve at 07:43 PM. A typical chromatogram for basal 5-HT is shown in the up right corner.
Figure 7.
The effect of fluoxetine on 5-HT release resulted from K+ stimulations.
Figure 8 shows a portion of a set of data (full 16.7 h trace is Fig S8). We noticed that the 120 mM K+, ten minute stimulation led to transient changes in the 5-HT concentration. These, to our knowledge, have not been observed before. We controlled the time between stimulation and the UHPLC injection cycle to 5 seconds for all K+ stimulations. Because of this, samples were acquired with a consistent time relationship to the onset of the stimulus. When we overlaid the four responses from the high potassium, ten minute stimulations seen in Figure 8, we found that the 5-HT oscillations were quite reproducible for repeated stimulations (Figure 9). The reproducibility coupled with the absence of similar fluctuations for three-minute infusions of K+, and for ten-minute 5-HT infusions suggests that these observations represent real changes in extracellular 5-HT rather than an experimental artifact.
Figure 8.
Online in vivo 5-HT measurements with attempted i.p. injection and repeated ten-minute 120 mM K+ stimulations.
Figure 9.
Comparison of the 5-HT release in response to four ten-minute 120 mM K+ stimulations. One of the 5-HT release profile (triangle) missed a data point because the UHPLC system stopped to refill the reservoir so there was no analysis for that sample. The data before and after that missing data are emphasized by enlarged triangles. Curves are cubic splines
We made an interesting, if accidental, observation. None of the rats used in the experiments were accustomed to handling, thus it was not unusual to notice some unwillingness on the part of a particular animal during the handling required to make an intraperitoneal (i.p.) injection. We noticed considerable animal-to-animal variability in the signs of stress during handling. The 5-HT vs. time trace showed a transient elevation of 5-HT concentration correlated with the handling of one animal. Fortuitously, another animal undergoing the same handling appeared relaxed and exhibited no signs of stress, and there was no 5-HT elevation observed. A third animal exhibited less stress than the first animal, and this animal’s 5-HT also spiked transiently as shown in Figure 8. We do not attempt to draw biological conclusions from these observations. The point is that the rapid response time of the system reveals such correlations. These observations are similar to those of Yang et al. who recently pointed out the value of six minute, on-line sampling by observing transients from i.p. injections of saline which could not be observed for longer sampling times30.
CONCLUSIONS
The potential for higher time resolution over long times in awake animals has been in existence for some time. We have realized this potential based on prior work on optimizing chromatographic conditions.26,31 Of course, speeding up the chromatography is necessary but not sufficient to establish a useful method. All components of the system must be compatible with the higher time resolution and the smaller column volume. As discussed by Yang et al.,30 the higher time resolution may make possible the investigation of some aspects of 5HT dynamics and also changes in 5HT during behavior.
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate the financial support from the National Institute of Mental Health (National Institutes of Health) through grant MH083134 (SGW) and MH075989, NS081744 (ACM). Helpful discussions with Prof. Anne Andrews, UCLA are gratefully acknowledged. We also thank Dr. Ed Bouvier of Waters Corp. for the gift of packing materials and Steve Groskreutz for many editorial suggestions.
LITERATURE CITED
- 1.Zhou J-L, An J-J, Li P, Li H-J, Jiang Y, Cheng J-F. J. Chromatogr. A. 2009;1216:2394–2403. doi: 10.1016/j.chroma.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Fang H, Xiao Q, Wu F, Floreancig PE, Weber SG. J. Org. Chem. 2010;75:5619–5626. doi: 10.1021/jo100981e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sameenoi Y, Koehler K, Shapiro J, Boonsong K, Sun Y, Collett J, Volckens J, Henry CS. J. Am. Chem. Soc. 2012;134:10562–10568. doi: 10.1021/ja3031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dishinger JF, Kennedy RT. Anal. Chem. 2007;79:947–954. doi: 10.1021/ac061425s. [DOI] [PubMed] [Google Scholar]
- 5.Workman J, Koch M, Lavine B, Chrisman R. Anal. Chem. 2009;81:4623–4643. doi: 10.1021/ac900778y. [DOI] [PubMed] [Google Scholar]
- 6.Schultz KN, Kennedy RT. Annu. Rev. Anal. Chem. 2008;1:627–661. doi: 10.1146/annurev.anchem.1.031207.113047. [DOI] [PubMed] [Google Scholar]
- 7.Murphy DL, Lesch K. Nat. Rev. Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 8.Naughton M, Mulrooney JB, Leonard BE. Hum. Psychopharmacol. 2000;15:397–415. doi: 10.1002/1099-1077(200008)15:6<397::AID-HUP212>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 9.Hahn MK, Blakely RD. Pharmacogenomics J. 2002;2:217–235. doi: 10.1038/sj.tpj.6500106. [DOI] [PubMed] [Google Scholar]
- 10.Gainetdinov RR, Caron MG. Annu. Rev. Pharmacol. Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. 1 plate. [DOI] [PubMed] [Google Scholar]
- 11.Bian X, Patel B, Dai X, Galligan JJ, Swain G. Gastroenterology. 2007;132:2438–2447. doi: 10.1053/j.gastro.2007.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood KM, Hashemi P. ACS Chem. Neurosci. 2013;4:715–720. doi: 10.1021/cn4000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman RM. Anal. Chem. 2009;81:9462–9471. doi: 10.1021/ac9018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunin MA, Prioleau C, Mailman RB, Wightman RM. J. Neurochem. 1998;70:1077–1087. doi: 10.1046/j.1471-4159.1998.70031077.x. [DOI] [PubMed] [Google Scholar]
- 15.Rogers ML, Feuerstein D, Leong CL, Takagaki M, Niu X, Graf R, Boutelle MG. ACS Chem. Neurosci. 2013;4:799–807. doi: 10.1021/cn400047x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandi P, Lunte SM. Anal. Chim. Acta. 2009;651:1–14. doi: 10.1016/j.aca.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry M, Li Q, Kennedy RT. Anal. Chim. Acta. 2009;653:1–22. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandi P, Scott DE, Desai D, Lunte SM. Electrophoresis. 2013;34:895–902. doi: 10.1002/elps.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott DE, Grigsby RJ, Lunte SM. ChemPhysChem. 2013;14:2288–2294. doi: 10.1002/cphc.201300449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton AP, Justice JB., Jr Anal. Chem. 1994;66:1468–72. doi: 10.1021/ac00081a018. [DOI] [PubMed] [Google Scholar]
- 21.Shou M, Ferrario CR, Schultz KN, Robinson TE, Kennedy RT. Anal. Chem. 2006;78:6717–6725. doi: 10.1021/ac0608218. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien KB, Esguerra M, Miller RF, Bowser MT. Anal. Chem. 2004;76:5069–5074. doi: 10.1021/ac049822v. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Hershey ND, Mabrouk OS, Kennedy RT. Anal. Bioanal. Chem. 2011;400:2013–2023. doi: 10.1007/s00216-011-4956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowser MT, Kennedy RT. Electrophoresis. 2001;22:3668–3676. doi: 10.1002/1522-2683(200109)22:17<3668::AID-ELPS3668>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Jaquins-Gerstl A, Shu Z, Zhang J, Liu Y, Weber SG, Michael AC. Anal. Chem. 2011;83:7662–7667. doi: 10.1021/ac200782h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Liu Y, Jaquins-Gerstl A, Shu Z, Michael AC, Weber SG. J. Chromatogr. A. 2012;1251:54–62. doi: 10.1016/j.chroma.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. J Physiol. 1999;514(Pt 2):567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parrot S, Lambas-Senas L, Sentenac S, Denoroy L, Renaud B. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2007;850:303–309. doi: 10.1016/j.jchromb.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 29.Jung MC, Shi G, Borland L, Michael AC, Weber SG. Anal. Chem. 2006;78:1755–1760. doi: 10.1021/ac051183g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Thompson AB, McIntosh BJ, Altieri SC, Andrews AM. ACS Chem. Neurosci. 2013;4:790–798. doi: 10.1021/cn400072f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Zhang J, Xu X, Zhao MK, Andrews AM, Weber SG. Anal. Chem. 2010;82:9611–9616. doi: 10.1021/ac102200q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamford JA. Monitoring Neuronal Activity. Oxford: Oxford University Press; 1992. pp. 149–155. [Google Scholar]
- 33.Dolan JW. LC-GC. 1996;14:562–566. [Google Scholar]
- 34.Savitzky A, Golay MJE. Anal. Chem. 1964;36:1627–1639. [Google Scholar]
- 35.Thekkudan DF, Rutan SC. In: Comprehensive Chemometrics. Brown S, Tauler R, Walczak B, editors. Oxford: Elseiver; 2009. pp. 9–24. [Google Scholar]
- 36.Lada MW, Vickroy TW, Kennedy RT. Anal. Chem. 1997;69:4560–4565. doi: 10.1021/ac970518u. [DOI] [PubMed] [Google Scholar]
- 37.Bungay PM, Wang T, Yang H, Elmquist WF. J. Membr. Sci. 2010;348:131–149. doi: 10.1016/j.memsci.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nunge RJ, Gill WN. Ind. Eng. Chem. 1969;61:33–49. [Google Scholar]
- 39.Beisler AT, Schaefer KE, Weber SG. J. Chromatogr., A. 2003;986:247–251. doi: 10.1016/s0021-9673(02)02018-6. [DOI] [PubMed] [Google Scholar]
- 40.Gerhardt G, Adams RN. Anal. Chem. 1982;54:2618–2620. [Google Scholar]
- 41.Chaurasia CS, Chen C-E, Ashby CR., Jr J. Pharm. Biomed. Anal. 1999;19:413–422. doi: 10.1016/s0731-7085(98)00182-4. [DOI] [PubMed] [Google Scholar]
- 42.Penicaud C, Bohuon P, Peyron S, Gontard N, Guillard V, et al. Ind. Eng. Chem. Res. 2012;51:1131–1142. [Google Scholar]
- 43.Tikekar RV, Anantheswaran RC, LaBorde LF. J. Food Sci. 2011;76:H62–H71. doi: 10.1111/j.1750-3841.2010.02015.x. [DOI] [PubMed] [Google Scholar]
- 44.Dupertuis YM, Ramseyer S, Fathi M, Pichard C. JPEN J Parenter Enteral Nutr. 2005;29:125–130. doi: 10.1177/0148607105029002125. [DOI] [PubMed] [Google Scholar]
- 45.Roig MG, Rivera ZS, Kennedy JF. Int J Food Sci Nutr. 1995;46:107–115. doi: 10.3109/09637489509012538. [DOI] [PubMed] [Google Scholar]
- 46.Koch S, Perry KW, Bymaster FP. Neuropharmacology. 2004;46:232–242. doi: 10.1016/j.neuropharm.2003.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.