Abstract
Fires affect hundreds of millions of hectares annually. Above-ground community composition and diversity after fire have been studied extensively, but effects of fire on soil bacterial communities remain largely unexamined despite the central role of bacteria in ecosystem recovery and functioning. We investigated responses of bacterial community to forest fire in the Greater Khingan Mountains, China, using tagged pyrosequencing. Fire altered soil bacterial community composition substantially and high-intensity fire significantly decreased bacterial diversity 1-year-after-burn site. Bacterial community composition and diversity returned to similar levels as observed in controls (no fire) after 11 years. The understory vegetation community typically takes 20–100 years to reach pre-fire states in boreal forest, so our results suggest that soil bacteria could recover much faster than plant communities. Finally, soil bacterial community composition significantly co-varied with soil pH, moisture content, NH4+ content and carbon/nitrogen ratio (P < 0.05 in all cases) in wildfire-perturbed soils, suggesting that fire could indirectly affect bacterial communities by altering soil edaphic properties.
Fire is one of the most critical threats to forest ecosystems1,2,3 and its overall effects are complex, ranging from the removal of aboveground biomass to altering the physical, chemical and biological components of soil ecosystems2,4. Fire produces a broad spectrum of effects that depend on fuel load and combustion, vegetation type, climate, topography and so on. Changes in the type, intensity, frequency and timing of fire disturbance3,5 due to climate change and human influence may degrade ecosystem function and diversity or perhaps shift the ecosystem to another state.
Despite the essential role that bacteria play in ecosystem recovery6, there is little information about the long-term effects of fire on bacterial communities in soils. Fires likely have large direct and indirect effects on soil bacterial community composition and diversity. Heat from fires can kill soil bacteria, reducing microbial biomass7 and directly impacting bacterial community composition and diversity6. Microbes differ in sensitivity to fire-induced heat8: bacteria tend to be more resistant to heat than fungi, and generally increase in relative abundance after a fire. The rapid capacity to re-colonize soil can be decisive in determining post-fire microbial community structure9. Indirect effects of fire include changes to many soil properties, including consuming organic material and changing soil chemical properties10,11. Most fires result in overall losses of soil C and N, but a pulse of ammonium (inorganic NH4+) often follows fires4,7. Fires can increase N availability to bacteria because of reduced plant uptake and enhanced mineralization10,11. Fires can also create a reactive charcoal layer that affects soil available nitrogen and pH12,13,14. Additionally, severe fires often alter forest canopy, litter layer, and soil permeability, thereby influencing soil moisture content, temperature, and pH15,16. Impacts of fire on bacterial growth and activity can persist for many years after burning17, although little research has gone into understanding the long-term effect of fire on soil bacteria.
The Greater Khingan Mountains harbor the largest forest in China. Forest fire has been an increasingly common phenomenon in the Greater Khingan Mountains region in recent decades. From 1965 to 2009, there were 1,552 fires, and the fire interference area was about 66,000 km2 in the Greater Khingan Mountains18. The objectives of this study were to examine changes in soil bacterial community composition and diversity 1 (short-term) and 11 years (long-term) after the occurrence of forest fire, and to correlate these shifts to edaphic properties. Our work was focused on studying the response of the soil bacteria to wildfire disturbance, emphasizing the main factors driving soil bacterial community composition and diversity after fire, which may improve understanding of post-fire forest ecosystem recovery process.
Results
Effects of fire on soil biogeochemical properties
Fire significantly altered soil biogeochemical properties (Table S1). Burning reduced microbial biomass carbon and nitrogen, with 82% less microbial biomass carbon and 71% less microbial biomass nitrogen in 1-year-post-fire burned soils than unburned samples, and 63% less microbial biomass carbon and 72% less microbial biomass nitrogen in 11-year-post-fire burned soils. Wildfire increased soil pH, available nitrogen and phosphorus, and decreased soil moisture and carbon/nitrogen ratio 1-year-post-fire, but after 11 years, those properties were not significantly different from the levels observed in the unburned control site.
Bacterial community composition
Across all soil samples, we obtained a total of 319,618 quality sequences with 4,123–9,130 sequences per sample (mean 5,327), and were able to classify 84.3% of those sequences. The dominant phyla (or subphyla in the case of Proteobacteria) across the Greater Khingan Mountains soils were Alphaproteobacteria, Actinobacteria, Acidobacteria, Betaproteobacteria and Bacteroidetes, accounting for more than 76% of the bacterial sequences from each of the soils (Fig. S1). In addition, Gammaproteobacteria, Planctomycetes, Chloroflexi, Deltaproteobacteria, Gemmatimonadetes and Firmicutes were present in most soils but at relatively low abundances, and 24 other rare phyla were identified (Table S2).
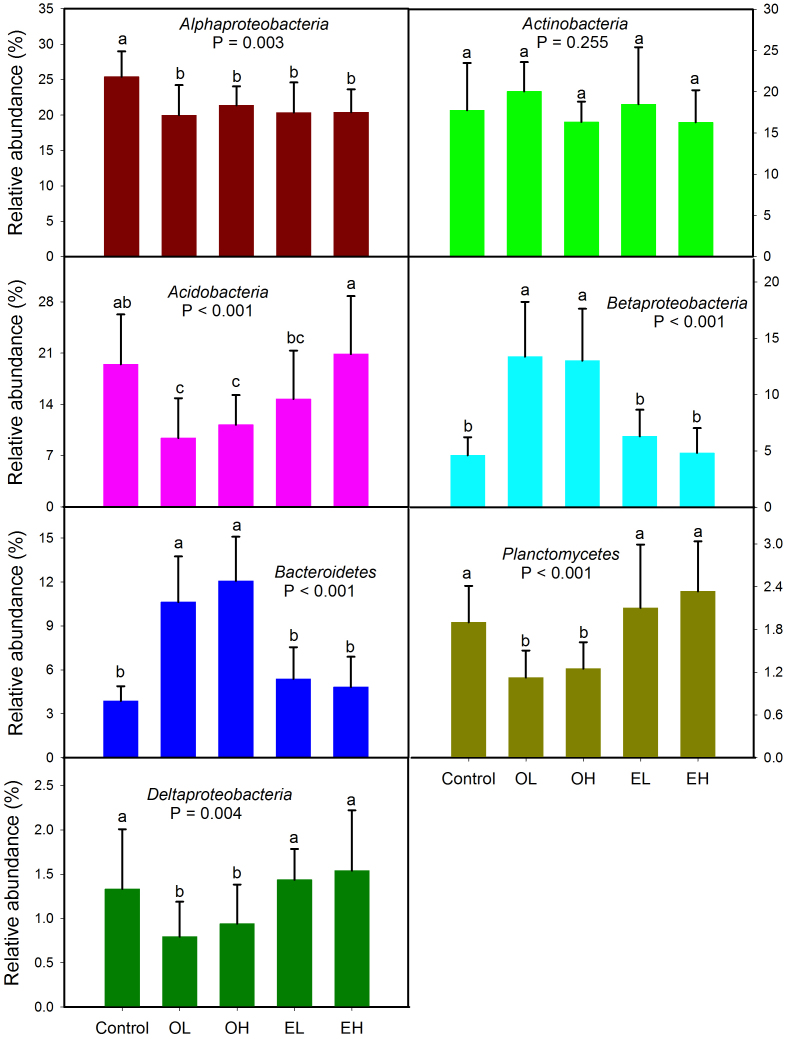
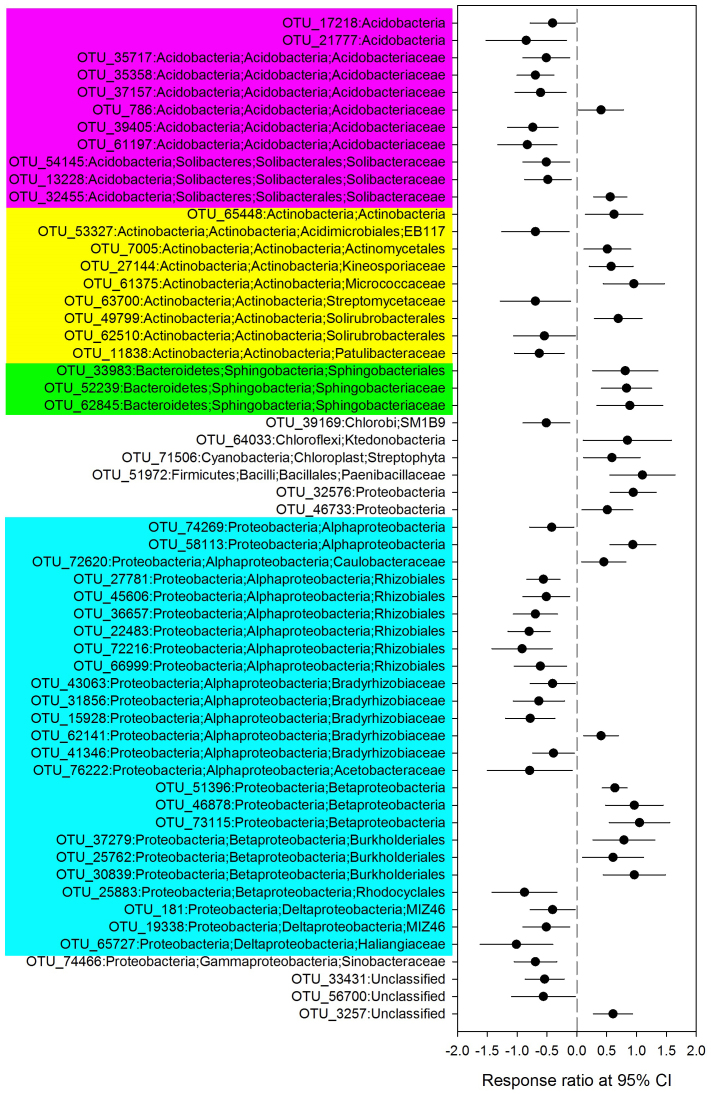
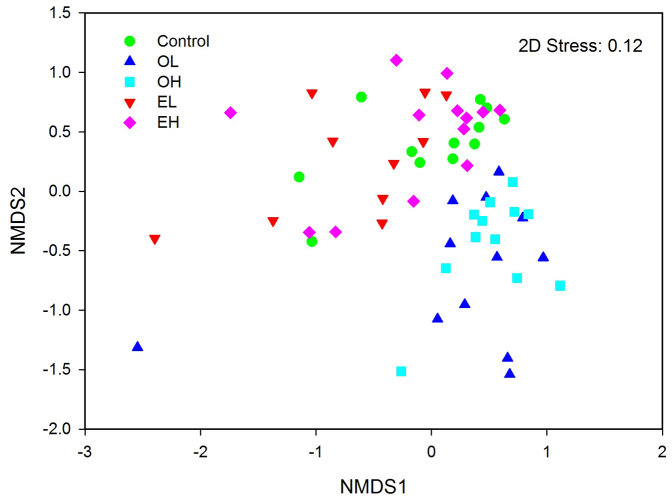
Fire significantly shifted the relative abundance of dominant phyla except Actinobacteria (Fig. 1). Fire greatly increased the relative abundances of Betaproteobacteria and Bacteroidetes and decreased the abundance of Alphaproteobacteria, Acidobacteria, as well as Planctomycetes and Deltaproteobacteria with low abundances 1-year-post-fire. Interestingly, the relative abundances of dominant phyla returned to a similar level to the controls after 11 years, with the exception of Alphaproteobacteria (Fig. 1). However, by comparing OH (1 year post high intensity fire) with control at class or lower levels certain taxa demonstrated significant responses. In the phylum Acidobacteria, all OTUs (operational taxonomic unit) had a significantly lower abundance except OTU_786 and OTU_32455 in response to fire, and for Bacteroidetes, in the family Sphingobacteria, all OTUs had a significantly higher abundance (Fig. 2). In the phylum Proteobacteria, Alphaproteobacteria demonstrated significantly lower abundance, except for OTU_62141, OTU_58113 and OTU_72620; Betaproteobacteria had a significantly greater abundance, except for OTU_25883; while Deltaproteobacteria showed lower abundance in response to fire (Fig. 2). In contrast, Actinobacteria taxa showed a highly variable response (Fig. 2). Similar patterns were observed when comparing OL (1 year post low intensity fire) with the unburned controls (Fig. S2). Bacterial community composition in soils across the Greater Khingan Mountains showed that fire resulted in a dramatic shift in soil bacterial communities 1-year-post-fire (P = 0.001), but after 11 years the communities were indistinguishable from unburned forest soil (P = 0.549, Fig. 3, Table S3). Fire intensity had no significant impact upon recovery (Fig. 3).
Figure 1. The relative abundances of the dominant bacterial phyla in control and post-fire soils.
Error bars denote standard deviation; different letters represent significant differences from Tukey's HSD comparisons (P < 0.05). OL: one year after low intensity fire; OH: one year after high intensity fire; EL: 11 years after low intensity fire; EH: 11 years after high intensity fire.
Figure 2. OTUs that exhibited significant changes in abundance at 1 year after high intensity fire.
Significance was determined using response ratio methods at a 95% CI (confidence interval).
Figure 3. Bacterial community compositional structure in soils across the Greater Khingan Mountains indicated by non-metric multi-dimensional scaling (NMDS) using Bray-Curtis dissimilarity.
OL: one year after low intensity fire; OH: one year after high intensity fire; EL: 11 years after low intensity fire; EH: 11 years after high intensity fire.
The soil bacterial community was related to soil biogeochemical variables in both pre- and post-fire soils. Mantel tests showed that bacterial community composition was significantly correlated with soil pH in control soils, while the community composition was significantly correlated with soil pH, moisture content, NH4+ content, and C/N ratio in soils 1 and 11 years post fire (Table 1). Among all the measured soil variables, soil pH showed the highest correlation with bacterial community composition in both control and fire-impacted soils (Table 1). Canonical correspondence analysis (CCA) indicated that soil pH had the strongest effect on bacterial community composition, while soil moisture content, NH4+ content, C/N ratio and TN content also had less, but significant effect on the community composition (Fig. S3). In addition, soil pH showed a significant correlation with the relative abundance of Alphaproteobacteria, Actinobacteria, Acidobacteria, Betaproteobacteria, as well as three less abundant phyla (Fig. S4). Soil NH4+ content, C/N ratio, moisture content, TC content and TN content were also significantly correlated with the relative abundance of different dominant phyla (Table S4).
Table 1. The biogeochemical factors that significantly correlated with bacterial communities were listed below. The correlations (r) and significance (P) were determined by Mantel tests between the community composition and environmental variables. SM: soil moisture; TC: total carbon; TN: total nitrogen; C/N ratio: carbon/nitrogen ratio; DOC: dissolved organic carbon; DON: dissolved organic nitrogen; AP: available phosphorus; MBC: microbial biomass carbon; MBN: microbial biomass nitrogen. OYF: 1-year-post-fire; EYF: 11-years-post-fire.
| Control | OYF | EYF | ||||
|---|---|---|---|---|---|---|
| Variables | r | P | r | P | r | P |
| pH | 0.480 | 0.016 | 0.777 | 0.001 | 0.534 | 0.001 |
| SM (%) | 0.350 | 0.116 | 0.672 | 0.012 | 0.511 | 0.001 |
| NH4+ (mg/kg) | 0.240 | 0.113 | 0.685 | 0.010 | 0.221 | 0.028 |
| C/N Ratio | 0.250 | 0.113 | 0.681 | 0.038 | 0.229 | 0.026 |
| NO3− (mg/kg) | 0.182 | 0.082 | 0.164 | 0.117 | 0.507 | 0.006 |
| TN (%) | 0.181 | 0.772 | 0.153 | 0.127 | 0.022 | 0.378 |
| TC (%) | 0.193 | 0.767 | 0.107 | 0.177 | 0.267 | 0.087 |
| AP (mg/kg) | 0.292 | 0.667 | 0.137 | 0.123 | 0.167 | 0.107 |
| DOC (mg/kg) | 0.166 | 0.672 | 0.030 | 0.407 | 0.166 | 0.084 |
| DON (mg/kg) | 0.147 | 0.683 | 0.078 | 0.202 | 0.019 | 0.447 |
| MBC (mg/kg) | 0.149 | 0.767 | 0.079 | 0.693 | 0.129 | 0.139 |
| MBN (mg/kg) | 0.102 | 0.562 | 0.103 | 0.746 | 0.057 | 0.282 |
| Elevation (m) | 0.196 | 0.235 | 0.112 | 0.463 | 0.128 | 0.368 |
Bacterial diversity
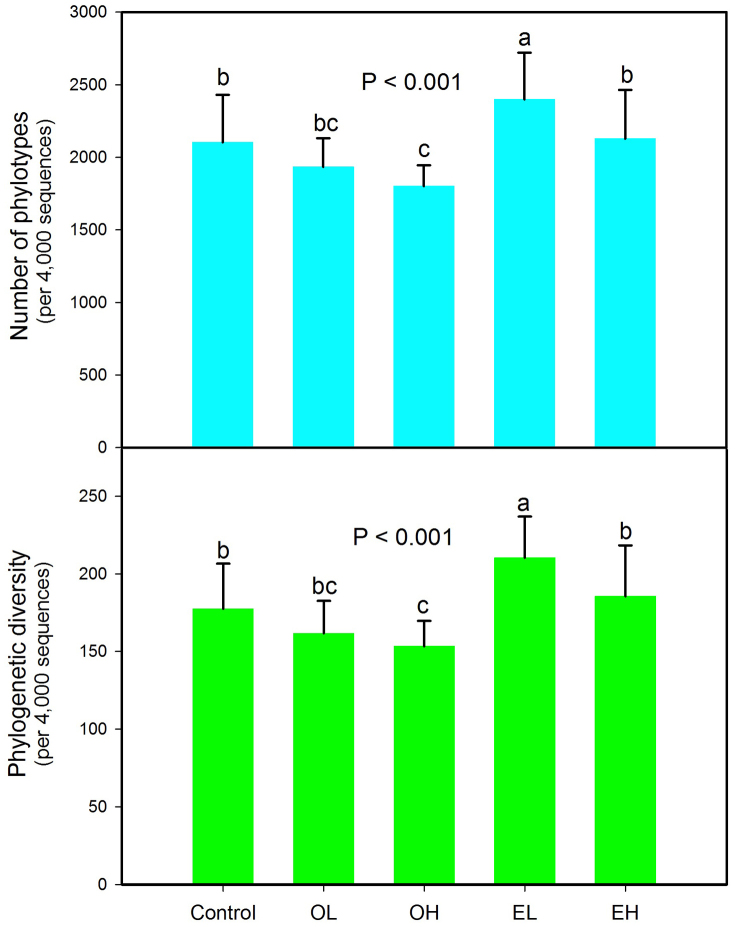
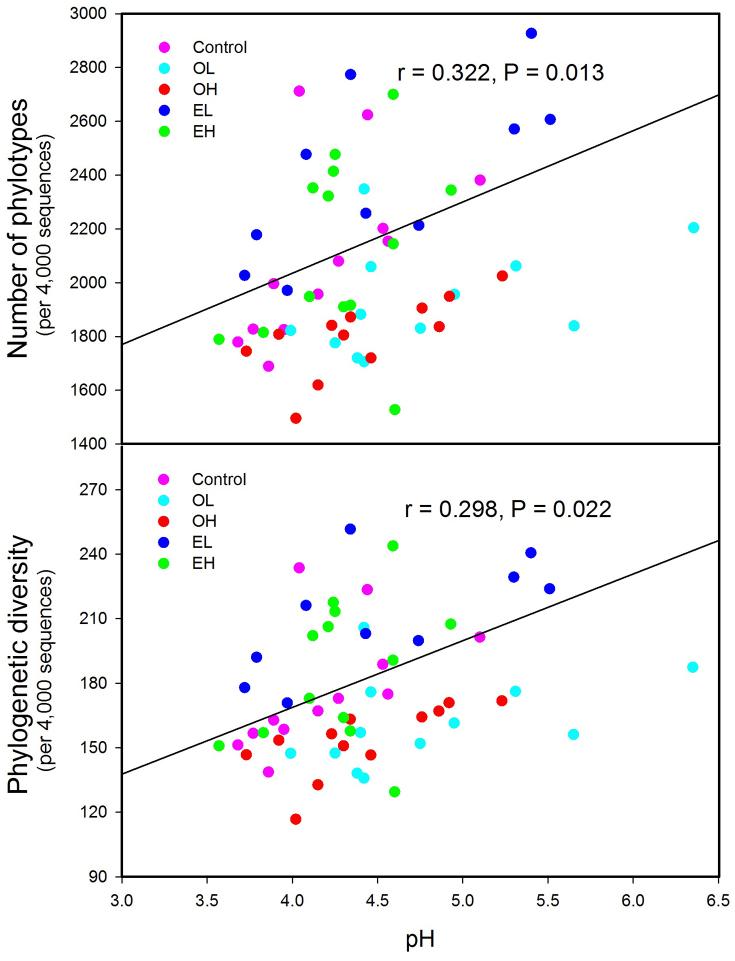
In terms of both phylotype richness (i.e. number of OTUs) and phylogenetic diversity (Fig. 4), which were surveyed at a depth of 4,000 randomly selected sequences per sample, the diversity of bacterial communities exhibited significant differences (P < 0.001 in both cases). High intensity fire quickly decreased bacterial phylotype richness and phylogenetic diversity, but after 11 years, these parameters returned to match the unburned controls. Interestingly, the highest bacterial diversity was found 11 years after low intensity fire. Soil bacterial phylotype richness was positively correlated with soil pH (P = 0.013, Fig. 5), dissolved organic carbon (P = 0.039, Table S5), and negatively correlated with elevation (P = 0.042, Table S5), while phylogenetic diversity was positively correlated with soil pH (P = 0.022, Fig. 5) and dissolved organic carbon (P = 0.049, Table S5).
Figure 4. Changes in bacterial OTUs phylotype richness and phylogenetic diversity across the different groups.
Diversity indices were calculated using random selections of 4,000 sequences per soil sample. Error bars denote standard deviation; Different letters represent significant differences from Tukey's HSD comparisons (P < 0.05). OL: one year after low intensity fire; OH: one year after high intensity fire; EL: 11 years after low intensity fire; EH: 11 years after high intensity fire.
Figure 5. The relationship between soil pH and bacterial OTUs phylotype richness and phylogenetic diversity by linear regression analyses.
The communities were randomly sampled at the 4,000 sequences level. Individual points represent different samples across all the treatments. OL: one year after low intensity fire; OH: one year after high intensity fire; EL: 11 years after low intensity fire; EH: 11 years after high intensity fire. P < 0.05, significant convention.
Discussion
Records of wildfire occurrences provided an opportunity to examine the effects of fire on soil bacterial community composition, diversity and succession. In this study, we found Proteobacteria, Actinobacteria, Acidobacteria and Bacteroidetes were the main phyla in boreal forest soil (Fig. S1), similar to observations from other soils collected from Arctic and subalpine soil environments19,20, showing that dominant bacterial phyla in soils are similar. We found that burning had a dramatic impact on the soil bacterial community composition and diversity 1 year following a fire (Fig. 1, 3, 4). Bacterial communities from 1 and 11 years post-burn were significantly different not only in the OTUs present, but also in the proportional abundances of phyla (Fig. 1, 4, S1; Table S2). An earlier study21 found that fire dramatically altered soil bacterial community composition and diversity 4 and 16 weeks after fire and our results showed that the effect of fire on bacterial community could last for more than 1 year, suggesting that fire had a strong impact on bacterial community. Our results go beyond these findings by showing that the composition of bacterial community 11 years after fire returned to a similar state compared to the unburned control site (Fig. 3, Table S3). In addition, bacterial diversity decreased 1-year-post-fire but the diversity recovered 11-year-post-fire with the highest diversity at low intensity fire (Fig. 4), which might be due to the successful colonization and survival of many rare phyla into soil during the process of bacterial succession post fire. Other studies have shown that the post-fire understory vegetation community reaches its pre-fire level in boreal forest 20–100 years after a fire, depending on pre-fire stand age and site conditions22,23. Our results therefore suggest that bacterial communities may recover much faster than understory vegetation. In the present study, we have only two time points after fire occurrence (1 and 11 years post fire), and in future study more time points post fire might be needed to clarify when the bacterial communities recover to the unburned level and how bacterial communities succeed after forest fire. Human influence and global warming are rapidly increasing the frequency of forest fire, therefore understanding different recovery rate between soil microbial community and vegetation community in response to wildfire may be important for understanding the recovery of forest ecosystems as a whole.
The results in this study showed that bacterial community composition and diversity were mainly correlated with soil pH. The relative abundance of dominant phyla was also correlated with soil pH. For example, the relative abundance of Acidobacteria has been shown to increase with decreased pH (Table S4), which is consistent with most of previous studies20,24. However, the relative abundance of Alphaproteobacteria was shown to decrease toward higher pH in our study (Table S4), which is contrary to other studies in different systems19,25. These results indicated that although bacterial community composition was clearly influenced by pH, there were some differences in the responses of specific phylum to changes in soil pH. The overriding importance of soil pH has been demonstrated as a key factor in driving soil bacterial distribution across a variety of spatial scales, including continents19,26, national24, land-use types at a given location27, small and sub-meter scales28, and even along elevational gradient20. In this study, we observed that the bacterial community composition and diversity were primarily correlated with soil pH in both control and post fire soils (Fig S3; Table 1), suggesting that pH might have predictive power for bacterial distribution in not only undisturbed but also recently fire-perturbed ecosystems.
As noted above, disturbance may trigger both direct and indirect effects on soil microbial community structure. We found that wildfire altered soil pH, moisture content, NH4+ content and C/N ratio which were significantly co-varied with bacterial community composition in soils both 1 and 11 years after fire (Table 1). These results might suggest that fire could indirectly affect bacterial communities by altering soil properties. In addition, the significance of fire as a shaper of vegetation composition and structure is well known. Major links between plant species and soil microorganisms include the quantity of resources produced, competition for nutrients, quality of resources and mutualism6. How the successional growth of vegetation after a wildfire will influence and possibly be influenced by soil microbial community structure is a topic warranting future investigation in this and other study systems.
Methods
Site selection and soil sampling
Our study area was located in the Greater Khingan Mountains in northeast China (51°17′N 122°42′E to 51°56′N 123°18′E), and encompassed approximately 167,213 ha. The area has a cold, continental climate, with average annual temperature declining from 1°C at its southern extremes to −6°C at its northern extremes, and precipitation declining from 442 mm in the south to 240 mm in the north. More than 60% of the annual precipitation falls in the summer season from June to August18. The vegetation of this area is representative of cool temperate coniferous forests, forming the southern extension of the eastern Siberian boreal forests. Historically, fires were caused primarily by lightning18. Dendrochronological studies have indicated that the historical fire regime was characterized by frequent surface fires, mixed with infrequent stand-replacing fires, with the interval between fires ranging from 30 to 120 years18,29. However, forest harvesting and fire suppression have altered fire regimes in this region30.
Soil samples were collected on July 24th to August 19th of 2011 in the Huzhong National Natural Reserve of the Greater Khingan Mountains. The study area is primarily covered by mature larch (Larix gmelinii) forest with little human disturbances since the establishment of the Reserve29. The parent material is granite bedrock and the soil is a dark brown forest soil31. We used a stratified sampling design to select sample plots based on fire history and fire severity. Fire history (surface fire) has three levels: 1-year-after-fire, 11-year-after-fire and unburned control. Fire severity was defined into two levels: low and high severities. Fire severity levels were defined based on differenced normalized burn ratio (dNBR) of remote sensing Landsat TM images, which have been proved applicable to our study area29. The dNBR32,33 was calculated using the equation NBRpre-fire − NBRpost-fire, while NBR was calculated using the equation (TM4 − TM7)/(TM4 + TM7). TM4 and TM7 refer to Thematic Mapper bands 4 (the near-infrared wave) and 7 (the medium-infrared wave), respectively, which were calculated according to pre- and post-fire images. In the stratified random sampling design, the dNBR value was classified into two levels according to its histogram: high severity (≥743) and low severity (<743)29. In addition, we selected 12 unburned locations (plots), which were classified as mature forest without fire disturbance scattered among fire occurrence region as control. In summary, those samples included no fire (control), 1 year after low intensity fire (OL), 1 year after high intensity fire (OH), 11 years after low intensity fire (EL) and 11 years after high intensity fire (EH). A total of 59 selected samples, 12 from unburned soils and 47 (10 for EL, 13 for EH, 12 for OL and 12 for OH) from burned soils, were analyzed in this study. In each plot (40 m × 40 m), soil was collected from five points (four vertices and the center) at a depth of 0–5 cm and then mixed as one sample. After sampling, the soils were kept in a cooler and shipped refrigerated to the lab. The samples were thoroughly mixed and sieved to remove grassroots and stone, and divided into two parts: one part was stored at 4°C for biogeochemical analysis; the other was stored at −40°C for DNA analysis.
Soil nutrients and microbial biomass analyses
Soil pH was measured using a pH Meter after shaking a soil water suspension (1:5 wt/vol) for 30 minutes. Soil moisture was measured gravimetrically. Total carbon (TC) and total nitrogen (TN) were determined by dichromate oxidation and titration with ferrous ammonium sulfate34. Soil dissolved organic C (DOC) and dissolved total N (DTN) and mineral nitrogen were extracted by adding 50 ml of 0.5 M K2SO4 to 10 g fresh soil, shaking for 1 h and then vacuum filtering through glass fiber filters (Fisher G4, 1.2 μm pore space). Ammonium (NH4+) and nitrate (NO3−) contents in the extracts were determined colourimetrically by automated segmented flow analysis (Bran + Luebbe AAIII, Germany) using the salicylate/dichloroisocyanuric acid and cadmium column/sulphanilamide reduction methods, respectively. DOC and DTN were determined using a TOC-TN analyzer (Shimadzu, Kyoto, Japan). Dissolved organic N (DON) was calculated as follows: DON = DTN − (NH4+ − N) − (NO3− − N). Microbial biomass C (MBC) and biomass N (MBN) were analyzed by the chloroform fumigation and extraction method35, and the final values were calculated using 0.35 (kC) and 0.4 (kN) correction factors36.
Soil DNA extraction
Soil DNA was extracted from the 0.5 g soil after sieving using a FastDNA® SPIN Kit for soil (MP Biomedicals, Santa Ana, CA) according to the manufacturer's instructions. The extracted soil DNA was dissolved in 60 μl TE buffer, quantified by NanoDrop and stored at −20°C.
Bacterial 16S rRNA genes amplification and 454 Sequencing
An aliquot (50 ng) of purified DNA from each sample were used as template for amplification. The V4–V5 hypervariable regions of the bacterial 16S rRNA genes (Escherichia coli positions 515–907) were amplified using the primer set: F515: GTGCCAGCMGCCGCGG with the Roche 454 ‘A' pyrosequencing adapter, and a unique 7 bp barcode sequence, while primer R907: CCGTCAATTCMTTTRAGTTT contained the Roche 454 ‘B' sequencing adapter at the 5′-end of each primer, respectively. The targeted gene region has been shown to be the most appropriate for the accurate phylogenetic reconstruction of bacteria37. Each sample was amplified in triplicate with 50 μl reaction under following: 35 cycles of denaturation at 94°C for 45 s, annealing at 55°C for 45 s, and extension at 72°C for 45 s; with a final extension at 72°C for 10 min. PCR products were pooled together and purified by Agarose Gel DNA purification kit (TaKaRa). An equal amount of PCR product for each sample qualitative determination by bioanalyzer (Agilent 2100) and quantitative analysis by NanoDrop was combined in a single tube, and run on a Roche FLX 454 pyrosequencing machine (Roche Diagnostics Corporation, Branford, CT, USA), producing reads from the forward direction F515.
Processing of pyrosequencing data
Data were processed by the Quantitative Insights Into Microbial Ecology (QIIME) pipeline38. Specifically, bacterial sequences with the same barcode were assigned to the same sample after denoising by denoiser v. 0.9139. The barcode and primer sequences were removed, and only the first 350 bp after the proximal PCR primer was included for further analysis. Bacterial phylotypes were identified using uclust40 and assigned to operational taxonomic units (OTUs, 97% similarity). Representative sequences from each phylotype were aligned using PyNAST41,42. The taxonomic identity of each phylotype was determined using the ribosomal database project (RDP) Classifier43. To correct for survey effort, we used a randomly selected subset of 4,000 sequences per sample to compare relative difference between samples.
Statistical analysis
Phylogenetic diversities (PD) were estimated by Faith's index44, which provides an integrated index of the phylogenetic breadth across taxonomic levels. The relationships between the taxonomic diversity for the group with geochemical features were tested with linear regression analyses using SPSS 17.0 for Windows. The response ratio (RR), calculated using the SAS program (SAS version 9.1. SAS Institute, Cary, North Carolina, USA), was used to analyze the effects of fire on phylogenetic composition and structure of bacterial communities45. NMDS (Non-metric multidimensional scaling) using Bray-Curtis dissimilarity and ANOSIM (Analysis of Similarity) based on the OTU table were completed in the vegan package (Version 2.0-2) of R v.2.8.1 project (R Development Core Team. Vienna, Austria) to compare community composition in burned and unburned samples. Mantel tests46 were performed in the vegan package (Version 2.0-2) of R v.2.8.1 project (R Development Core Team. Vienna, Austria) were used to identify environmental factors that significantly correlated with community composition (abundance of OTUs), and the factors that significantly correlated with the bacterial community composition were tested by variance inflation factor (VIF), which is used to judge the colinearity. The VIF value of factors less than 20 were selected to perform canonical correspondence analysis (CCA) in the vegan package (Version 2.0-2) of R v.2.8.1 project (R Development Core Team. Vienna, Austria).
Author Contributions
H.C., X.X. and J.Y. initiated and designed the research; X.X., J.Y. and J.K. collected soil samples; X.X., Y.S. and J.K. performed research; X.X., H.C., H.Z. and Y.S. analyzed the data and wrote the paper. J.Y., J.Z. and X.L. also revised and edited the manuscript.
Supplementary Material
supporting information
Acknowledgments
This work was supported by National Natural Science Foundation of China to H. Chu (41071167) and J. Yang (41071121) and the Hundred Talents Program of Chinese Academy of Sciences to H. Chu. We thank Zhihua Liu and Weili Liu for assistance in sampling. We also thank Jinbo Xiong for useful discussion.
References
- Bond W. J. & Keeley J. E. Fire as a global ‘herbivore': the ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 20, 387–394 (2005). [DOI] [PubMed] [Google Scholar]
- Certini G. Effects of fire on properties of forest soils: a review. Oecologia 143, 1–10 (2005). [DOI] [PubMed] [Google Scholar]
- Barcenas-Moreno G., Garcia-Orenes F., Mataix-Solera J., Mataix-Beneyto J. & Baath E. Soil microbial recolonisation after a fire in a Mediterranean forest. Biol. Fert. Soils 47, 261–272 (2011). [Google Scholar]
- Neary D. G., Klopatek C. C., DeBano L. F. & Ffolliott P. F. Fire effects on belowground sustainability: a review and synthesis. Forest Ecol. Manag. 122, 51–71 (1999). [Google Scholar]
- Smithwick E. A. H., Naithani K. J., Balser T. C., Romme W. H. & Turner M. G. Post-Fire Spatial Patterns of Soil Nitrogen Mineralization and Microbial Abundance. Plos One 7, e50597 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart S. C., DeLuca T. H., Newman G. S., MacKenzie M. D. & Boyle S. I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. Forest Ecol. Manag. 220, 166–184 (2005). [Google Scholar]
- Wang Q. K., Zhong M. C. & Wang S. L. A meta-analysis on the response of microbial biomass, dissolved organic matter, respiration, and N mineralization in mineral soil to fire in forest ecosystems. Forest Ecol. Manag. 271, 91–97 (2012). [Google Scholar]
- Dunn P. H., Barro S. C. & Poth M. Soil-Moisture Affects Survival of Microorganisms in Heated Chaparral Soil. Soil Biol. Biochem. 17, 143–148 (1985). [Google Scholar]
- Pietikäinen J. & Fritze H. Clear-Cutting and Prescribed Burning in Coniferous Forest - Comparison of Effects on Soil Fungal and Total Microbial Biomass, Respiration Activity and Nitrification. Soil Biol. Biochem. 27, 101–109 (1995). [Google Scholar]
- Choromanska U. & DeLuca T. H. Prescribed fire alters the impact of wildfire on soil biochemical properties in a ponderosa pine forest. Soil Sci. Soc. Am. J. 65, 232–238 (2001). [Google Scholar]
- Fernández I., Cabaneiro A. & Carballas T. Organic matter changes immediately after a wildfire in an Atlantic forest soil and comparison with laboratory soil heating. Soil Biol. Biochem. 29, 1–11 (1997). [Google Scholar]
- DeLuca T. H. & Sala A. Frequent fire alters nitrogen transformations in ponderosa pine stands of the inland northwest. Ecology 87, 2511–2522 (2006). [DOI] [PubMed] [Google Scholar]
- Pietikäinen J., Hiukka R. & Fritze H. Does short-term heating of forest humus change its properties as a substrate for microbes? Soil Biol. Biochem. 32, 277–288 (2000). [Google Scholar]
- Wardle D. A., Zackrisson O. & Nilsson M. C. The charcoal effect in Boreal forests: mechanisms and ecological consequences. Oecologia 115, 419–426 (1998). [DOI] [PubMed] [Google Scholar]
- DeBano L. F. The role of fire and soil heating on water repellency in wildland environments: a review. J. Hydrol. 231, 195–206 (2000). [Google Scholar]
- Hamman S. T., Burke I. C. & Knapp E. E. Soil nutrients and microbial activity after early and late season prescribed burns in a Sierra Nevada mixed conifer forest. Forest Ecol. Manag. 256, 367–374 (2008). [Google Scholar]
- Isobe K., Otsuka S., Sudiana I., Nurkanto A. & Senoo K. Community composition of soil bacteria nearly a decade after a fire in a tropical rainforest in East Kalimantan, Indonesia. J. Gen. Appl. Microbiol 55, 329–337 (2009). [DOI] [PubMed] [Google Scholar]
- Liu Z. H., Yang J., Chang Y., Weisberg P. J. & He H. S. Spatial patterns and drivers of fire occurrence and its future trend under climate change in a boreal forest of Northeast China. Global Change Biol. 18, 2041–2056 (2012). [Google Scholar]
- Chu H. Y. et al. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ. Microbiol 12, 2998–3006 (2010). [DOI] [PubMed] [Google Scholar]
- Shen C. C. et al. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 57, 204–211 (2013). [Google Scholar]
- Ferrenberg S. et al. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. Isme J. 7, 1102–1111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron Y. & Dubuc M. Succession in the Southern Part of the Canadian Boreal Forest. Vegetatio. 79, 51–63 (1989). [Google Scholar]
- Rees D. C. & Juday G. P. Plant species diversity on logged versus burned sites in central Alaska. Forest Ecol. Manag. 155, 291–302 (2002). [Google Scholar]
- Griffiths R. I. et al. The bacterial biogeography of British soils. Environ. Microbiol 13, 1642–1654 (2011). [DOI] [PubMed] [Google Scholar]
- Rousk J. et al. Soil bacterial and fungal communities across a pH gradient in an arable soil. Isme J. 4, 1340–1351 (2010). [DOI] [PubMed] [Google Scholar]
- Fierer N. & Jackson R. B. The diversity and biogeography of soil bacterial communities. P. Natl. Acad. Sci. USA 103, 626–631 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber C. L., Hamady M., Knight R. & Fierer N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microb. 75, 5111–5120 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. L. et al. Environmental and spatial characterisation of bacterial community composition in soil to inform sampling strategies. Soil Biol. Biochem. 41, 2292–2298 (2009). [Google Scholar]
- Cai W. H., Yang J., Liu Z. H., Hu Y. M. & Weisberg P. J. Post-fire tree recruitment of a boreal larch forest in Northeast China. Forest Ecol. Manag. 307, 20–29 (2013). [Google Scholar]
- Chang Y. et al. Long-term forest landscape responses to fire exclusion in the Great Xing'an Mountains, China. Int. J. Wildland Fire 16, 34–44 (2007). [Google Scholar]
- Wang C. K. et al. The influence of fire on carbon distribution and net primary production of boreal Larix gmelinii forests in north-eastern China. Global Change Biol. 7, 719–730 (2001). [Google Scholar]
- Soverel N. O., Coops N. C., Perrakis D. D. B., Daniels L. D. & Gergel S. E. The transferability of a dNBR-derived model to predict burn severity across 10 wildland fires in western Canada. Int. J. Wildland Fire 20, 518–531 (2011). [Google Scholar]
- Hoy E. E., French N. H. F., Turetsky M. R., Trigg S. N. & Kasischke E. S. Evaluating the potential of Landsat TM/ETM+ imagery for assessing fire severity in Alaskan black spruce forests. Int. J. Wildland Fire 17, 500–514 (2008). [Google Scholar]
- Walkley A. & Black I. A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38 (1934). [Google Scholar]
- Brookes P. C., Landman A., Pruden G. & Jenkinson D. S. Chloroform Fumigation and the Release of Soil-Nitrogen - a Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 17, 837–842 (1985). [Google Scholar]
- Jonasson S., Michelsen A., Schmidt I. K., Nielsen E. V. & Callaghan T. V. Microbial biomass C, N and P in two arctic soils and responses to addition of NPK fertilizer and sugar: Implications for plant nutrient uptake. Oecologia 106, 507–515 (1996). [DOI] [PubMed] [Google Scholar]
- Biddle J. F., Fitz-Gibbon S., Schuster S. C., Brenchley J. E. & House C. H. Metagenomic signatures of the Peru Margin subseafloor biosphere show a genetically distinct environment. P. Natl. Acad. Sci. USA 105, 10583–10588 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder J. & Knight R. Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat. Methods 7, 668–669 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010). [DOI] [PubMed] [Google Scholar]
- Caporaso J. G. et al. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26, 266–267 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis T. Z. et al. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 34, W394–W399 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M. & Cole J. R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 73, 5261–5267 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith D. P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 61, 1–10 (1992). [Google Scholar]
- Luo Y. Q., Hui D. F. & Zhang D. Q. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta-analysis. Ecology 87, 53–63 (2006). [DOI] [PubMed] [Google Scholar]
- Smouse P. E., Long J. C. & Sokal R. R. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Syst. Zool. 35, 727–732 (1986). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supporting information