SUMMARY
Interbacterial adhesion between streptococci and actinomyces promotes early dental plaque biofilm development. Recognition of coaggregation receptor polysaccharides (RPS) on strains of S. sanguinis, S. gordonii and S. oralis by Actinomyces spp. type 2 fimbriae is the principal mechanism of these interactions. Previous studies of genetic loci for synthesis of RPS (rps) and RPS precursors (rml, galE1 and galE2) in S. gordonii 38 and S. oralis 34 revealed differences between these strains. To determine whether these differences are strain- or species-specific, we identified and compared loci for polysaccharide biosynthesis in additional strains of these species and in several strains of the previously unstudied species, S. sanguinis. Genes for synthesis of RPS precursors distinguished the rps loci of different streptococci. Thus, rml genes for synthesis of TDP-L-Rha were in rps loci of S. oralis strains but at other loci in S. gordonii and S. sanguinis. Genes for two distinct galactose epimerases were also distributed differently. Thus, galE1 for epimerization of UDP-Glc and UDP-Gal was in galactose operons of S. gordonii and S. sanguinis strains but surprisingly, this gene was not present in S. oralis. Moreover, galE2 for epimerization of both UDP-Glc and UDP-Gal and UDP-GlcNAc and UDP-GalNAc was at a different locus in each species, including rps operons of S. sanguinis. The findings provide insight into cell surface properties that distinguish different RPS-producing streptococci and open an approach for identifying these bacteria based on the arrangement of genes for synthesis of polysaccharide precursors.
Keywords: biofilm, coaggregation, galactose epimerase, interbacterial adhesion, receptor polysaccharide, rps locus, Streptococcus sanguinis
INTRODUCTION
Early evidence for the important role of interbacterial adhesion in dental plaque biofilm formation came from the extensive network of coaggregations observed between strains of streptococci and actinomyces isolated from tooth surfaces (Cisar et al., 1979). The principle mechanism of these interactions is binding of specific streptococcal cell wall polysaccharides, referred to as coaggregation receptor polysaccharides (RPS) by adhesins of other bacteria such as Actinomyces spp. type 2 fimbriae. Seven structural types of RPS have been identified from over 25 coaggregating strains of S. sanguinis, S. gordonii, S. oralis (Cisar et al., 1997) and S. cristatus (Yang et al., 2011). Assembly of these polysaccharides depends on synthesis of specific hexa or heptasaccharide repeating units from nucleotide-linked sugars and subsequent polymerization of these units to form long polysaccharide molecules. RPS repeating units contain host-like disaccharide motifs, either GalNAcβ1-3Gal (Gn) or Galβ1-3GalNAc (G), that function as recognition sites for interbacterial adhesion (Cisar et al., 1995); other features determine RPS serotype. The seven known types of RPS are designated 1Gn, 2Gn, 2G, 3Gn, 3G, 4Gn and 5Gn to indicate their serotype (i.e. 1, 2, 3, 4 or 5) and receptor type (i.e. Gn or G). All RPS serotypes contain Galp, Galf and GalNAc. In addition, Glc and L-Rha occur in RPS serotypes 1, 2 and 3 and ribitol occurs in serotypes 4 and 5.
Molecular studies of the chromosomal locus (rps) for RPS biosynthesis in S. gordonii 38 (Xu et al., 2003, Yoshida et al., 2005) and in S. oralis 34 (Yoshida et al., 2006) revealed closely related genes for the transferases and polymerases that determine RPS structure along with glf for galactose furanose mutase, the enzyme that converts UDP-Galp to UDP-Galf (Fig. 1). The location however, of additional genes for synthesis of RPS precursors differed between strains. Thus, rml genes for synthesis of TDP-L-Rha were in the rps locus of S. oralis 34 but at other loci in S. gordonii 38. Further characterization of the latter strain revealed genes for two galactose epimerases; galE1 was downstream of galK and galT in the galactose operon and galE2 was downstream of rmlA, rmlC and rmlB in an operon for synthesis of polysaccharide precursors. Importantly, both galactose epimerases catalyzed interconversion of UDP-Glc and UDP-Gal for utilization of galactose via the Leloir pathway (Frey, 1996), but only GalE2 converted UDP-GlcNAc to UDP-GalNAc for RPS biosynthesis (Xu et al., 2003). The occurrence of galE1 and galE2 in S. oralis 34 was not determined.
Figure 1.
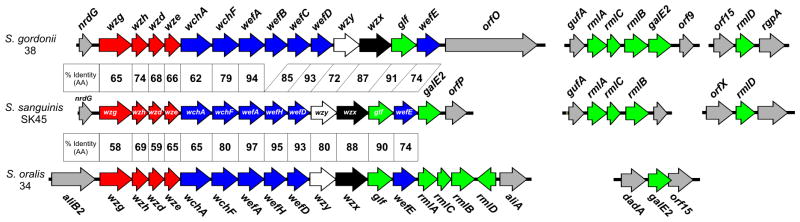
Comparison of rps locus of S. sanguinis SK45 (GenBank HM046485.1) with those of S. gordonii 38 (GenBank AY147914.1) and S. oralis 34 (GenBank AB181234.2) showing percent identity between encoded regulatory proteins (red), glycosyl transferases (blue), polysaccharide polymerases (white), flippases (black), enzymes for synthesis of nucleotide-linked sugars (green) and flanking genes (gray). Synthesis of TDP-L-Rha and UDP-GalNAc depend on additional loci in S. gordonii 38 (GenBank AY147913.1), S. sanguinis SK45 (GenBank JX407104.1, JX407105.1) and S. oralis 34 (GenBank KC620451). The presence of wefB and wefC in the rps2Gn operon of S. gordonii 38 verses wefH in the rps1Gn operons of S. sanguinis SK45 and S. oralis 34 is associated with the structural difference between RPS2Gn and RPS1Gn.
We have now compared genetic loci for RPS biosynthesis in additional strains of S. gordonii and S. oralis and have identified the corresponding loci in several strains of S. sanguinis. The results suggest species-specific differences in genetic loci for carbohydrate metabolism and cell surface polysaccharide biosynthesis between the three major species of RPS-producing mitis group streptococci.
METHODS
Streptococcal strains and culture conditions
Table S1 lists all strains of S. gordonii, S. oralis and S. sanguinis considered in the present study. To the best of our knowledge, these strains represent different clones with the possible exception of S. gordonii SK120 and SK121, which are colonial variants (Kilian et al., 1989). Whole or partial genome sequences are available for many of these at http://www.ncbi.nlm.nih.gov/ and http://www.homd.org/. Strains from our collection (Hsu et al., 1994) were grown at 37°C in Todd-Hewitt Broth (THB) (Difco Laboratories).
DNA sequencing
Overlapping PCR products were amplified from streptococcal genomic DNA templates using primers designed from gene sequences of S. gordonii 38 (Xu et al., 2003) or S. oralis 34 (Yoshida et al., 2006) or genomes of S. sanguinis SK36, S. sanguinis SK355 or S. oralis Uo5. Reaction products were purified using QIAquick spin columns (Qiagen) and sequenced at either the NIDCR Sequencing Core Facility or Sequetech (Mountain View, CA, USA). Sequences were assembled and annotated using Vector NTI software (Invitrogen) and compared by BLAST (National Center for Biotechnology).
Comparative PCR studies
PCR with genomic DNA templates from strains of S. sanguinis or S. gordonii was performed using Platinum PCR SuperMix (Invitrogen) and primers (Table S2) for adjacent genes of S. sanguinis SK45 (nrdG-wzg, wefE-galE2-orfP, gufA-rmlA or orfX-rmlD) or S. gordonii 38 (nrdG-wzg, gufA-rmlA, rmlB-galE2-orf9 or orf15-rmlD-rgpA). Reaction products were separated by electrophoresis in 0.8% agarose and stained with ethidium bromide.
Plasmid-based genetic complementation
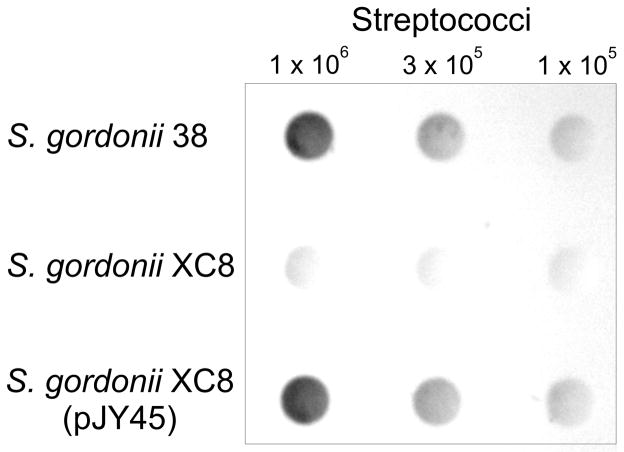
Procedures used for PCR amplification and cloning of S. sanguinis SK45 galE2 into Escherichia coli-streptococcus shuttle vector pJY, and transformation of the resulting plasmid (pJY-45) into S. gordonii XC8 have been described (Yang et al., 2009) as have those for dot immunoblotting (Yang et al., 2010). Briefly, nitrocellulose membranes were spotted with serial 3-fold dilutions of equivalent S. gordonii 38, S. gordonii XC8 and S. gordonii XC8(pJY-45) suspensions, incubated with affinity-purified rabbit anti-RPS2Gn IgG followed by peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad) and developed with a Metal Enhanced DAB substrate Kit (Thermo scientific).
RESULTS
Arrangement rml genes, galE1 and galE2 in S. gordonii and S. oralis
To determine whether the arrangement of genes for RPS biosynthesis in S. gordonii 38 (Fig. 1) is characteristic of this species, we performed comparative PCR studies with primers for adjacent genes of strain 38 and genomic DNA templates from this strain and RPS3G-producing S. gordonii strains SK120 and SK121. The PCR products from these strains were indistinguishable by gel electrophoresis (results not shown), thereby indicating that as in strain 38 (Fig. 1), the rps locus of both SK120 and SK121 were downstream of nrdG and that rmlA, rmlC, rmlB and galE1 were between gufA and orf9 with rmlD between orf15 and rgpA. A galE1-containing galactose operon, comparable to that of strain 38 (Xu et al., 2003), was also identified in each RPS3G-producing strain of S. gordonii.
We also searched the sequenced genome of S. gordonii Challis for genes comparable to those present in strain 38. Although strain Challis does not produce a G- or Gn type of RPS, it does have an uncharacterized operon (bp 2,093,657 to 2,082,323) for wzy-dependent polysaccharide biosynthesis immediately downstream of nrdG. In addition, rmlA, rmlC, rmlB, and galE2 are at a separate locus (bp 1,058,910 to 1,062,652) with rmlD further downstream (bp 1,070,475 to 1,071,326). A galactose operon containing galK, galT and galE1 (bp 967,706 to 971,632) was also identified. Thus, genetic loci for the synthesis of TDP-L-Rha, UDP-Gal and UDP-GalNAc were comparable between S. gordonii Challis and three RPS-producing strains of this species.
Examination of nine available whole or partial S. oralis genomes revealed a typical rps operon between dexB and aliA in each of six strains, including an rps2Gn locus (Xu et al., 2003) in strain SK100, an rps2G locus (Yoshida et al., 2005) in strain SK313, an rps3G locus (Yoshida et al., 2008) in strains ATCC35037 (SK23) and SK10 and, an rps4Gn locus (Yang et al., 2009) in strains SK610 and Uo5. Different operons for synthesis of other polysaccharides were present in the remaining three strains (SK255, SK304 and SK1074). All S. oralis strains had a full complement of rml genes except SK255 and RPS4Gn-producing SK610 and Uo5. These genes, when present, were always at the 3′-end of polysaccharide gene clusters and arranged as in S. oralis 34 (Fig. 1), with rmlD transcribed from the opposite strand. We also searched each genome for homologues of S. gordonii GalK, GalT, GalE1, and GalE2 to identify genes involved in UDP-Gal and UDP-GalNAc biosynthesis. All S. oralis genomes contained closely linked galK and galT but surprisingly, none contained galE1. This species did however contain galE2. Eight of nine S. oralis genomes had a single copy of this gene at a locus like the one in S. oralis 34 (Fig. 1). The remaining strain (SK304) contained two copies of galE2, one at a locus like that in other S. oralis strains and the other at a different locus (ctg120008662248, nucleotides 236395 to 237405) like one present in certain strains of S. mitis and S. pneumoniae.
Genetic basis of RPS biosynthesis in S. sanguinis
We then turned our attention to S. sanguinis, a previously unstudied species. The rps1Gn locus identified from S. sanguinis SK45 and that of S. oralis 34 were syntenous from wzg for a transcriptional regulator to wefE for the last glycosyltransferase (Fig. 1). The gene downstream of wefE was however, rmlA in S. oralis 34 versus a galE homologue in S. sanguinis SK45. The encoded S. sanguinis protein was 58% identical to GalE2 of S. gordonii 38 (and S. oralis 34) and 43% identical to GalE1 of S. gordonii 38. Identification of the S. sanguinis SK45 gene as galE2 was confirmed by its ability, when expressed from pJY-45, to restore RPS production by a galE2 mutant (strain XC8) of S. gordonii 38 (Fig. 2). Further sequencing of S. sanguinis SK45 revealed rmlA, rmlC and rmlB at one locus and rmlD at another (Fig. 1) as well as a galactose operon (not shown in Fig. 1) containing galK, galT and galE1 (GalE1 of S. sanguinis SK45 and S. gordonii 38 are 96% identical). Thus, the only significant difference noted between S. sanguinis SK45 and S. gordonii involved the location of galE2.
Figure 2.
Detection of cell surface RPS on S. gordonii 38, S. gordonii XC8 and S. gordonii XC8(pJY-45) by dot immunoblotting with rabbit anti-RPS2Gn antibody. Expression of S. sanguinis SK45 galE2 from pJY45 restored RPS production in S. gordonii XC8, a galE2 mutant of wild type strain 38.
To determine whether findings with S. sanguinis SK45 were typical of this species, we performed comparative PCR studies with primers for adjacent genes of strain SK45 and genomic DNA templates from this and six other RPS1Gn-producing S. sanguinis strains (Table S1, 16 to 21). The PCR products observed from all strains were indistinguishable by gel electrophoresis (results not shown). Related genes were also identified in 21 whole or partial S. sanguinis genomes. Twenty of these had galE1-containing galactose operons and rml loci like those of strain SK45. The only exception was S. sanguinis ATCC49296. This strain, which lacked galE1 and rml genes but contained an rps4Gn locus and galE2, was not like any other strain of S. sanguinis. Instead, it resembled S. oralis SK610 and Uo5 (Table S1), which supports earlier suggestions (Narikawa et al., 1995, Zahner et al., 2011) that strain ATCC 49296 has been misclassified as S. sanguinis. Six typical S. sanguinis strains (Table S1, 21 to 26) had rps1Gn loci that included galE2, as in strain SK45 (Fig. 1). Additional copies of galE2 were not identified in genomes of these strains. The remaining 14 strains (Table S1, 27 to 40) lacked rps1Gn loci but instead had other operons for polysaccharide biosynthesis downstream of nrdG. The nucleotide sequences of these operons, although similar between strains (~90% identical), were distinguished by the presence of galE2, which was the last gene in the operons of eight strains (Table S1, 27 to 34) but was not present in the other six strains (Table S1, 35 to 40). Thus, when present, the location of galE2 at the 3′-end of a polysaccharide gene cluster distinguished S. sanguinis from S. gordonii and S. oralis.
DISCUSSION
Differences in the genetic loci for synthesis of polysaccharide precursors between RPS-producing strains of S. gordonii, S. sanguinis and S. oralis provide insight into cell-surface properties that distinguish these streptococci. Synthesis of complex polysaccharides depends on an array of genes, some that contribute only to polymer synthesis and other so-called housekeeping genes that have broader metabolic roles (Bentley et al., 2006). The former genes typically occur in large operons, such as those for RPS biosynthesis, with the latter at other loci. Thus, the location of rml genes in different streptococci (Fig. 1) suggests that the requirement for TDP-L-Rha is limited to RPS biosynthesis in S. oralis, but extends to other pathways in S. sanguinis and S. gordonii. Support for this interpretation comes from production of an L-Rha-containing polysaccharide of the type described from S. mutans (Yamashita et al., 1998) by S. sanguinis and S. gordonii (Cisar et al., 1997). Genes for synthesis of these polysaccharides, which include rgpA, are downstream of rmlD in S. sanguinis and S. gordonii (Fig. 1) but not present in genomes of S. oralis.
In previous studies of S. gordonii 38 (Xu et al., 2003), we showed that galE2 is essential for RPS biosynthesis and attributed this to the unique ability of GalE2 to supply UDP-GalNAc. In the present study, we identified a galE homologue in the rps1Gn operon of S. sanguinis SK45 based on its ability to restore RPS production in a galE2 mutant of strain 38 (Fig. 2). Similar galE homologues occur in rps1Gn operons as well as other operons for polysaccharide biosynthesis in many but not all sequenced S. sanguinis strains (Table S1). With the exception of atypical ATCC49296, these strains also harbor galactose operons that contain homologues of S. gordonii 38 galE1. The only other homologous genes in S. sanguinis are rmlB and rmlC, which have well established roles in L-Rha biosynthesis. From this, we suspect that the supply of UDP-GalNAc for polysaccharide biosynthesis in this species depends solely on expression of galE2 from rps1Gn operons (and other polysaccharide gene clusters). The location of galE2 in rps1Gn operons of S. sanguinis (Fig. 1) but outside similar operons in S. oralis can be explained by the presence or absence of galE1 in these species, respectively. Thus, the presence of galE1 in S. sanguinis may limit the role of galE2 to RPS biosynthesis while the absence of galE1 in S. oralis may make galE2 essential for RPS biosynthesis and galactose metabolism. The broader role of galE2 in S. oralis is consistent with the ability of this gene to fulfill both roles in galE1 mutants of S. gordonii (Xu et al., 2003).
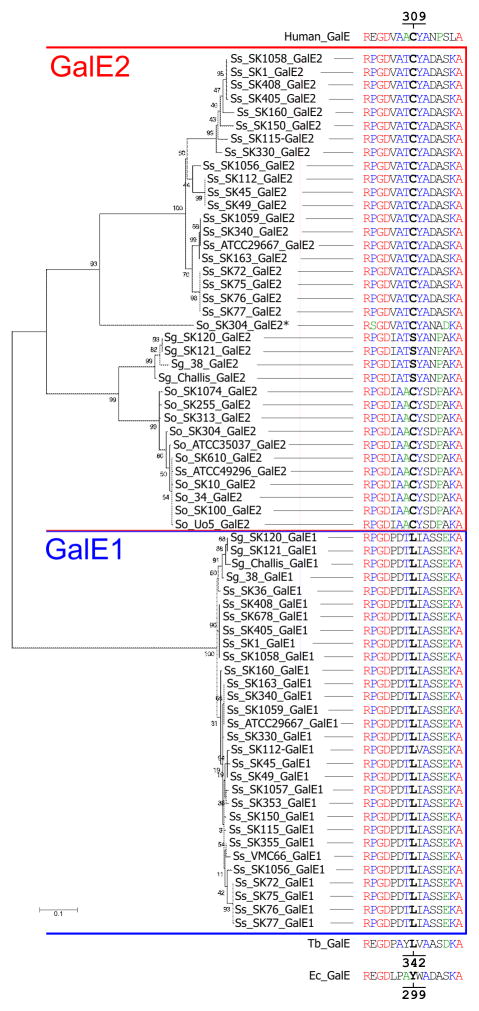
Molecular phylogenetic analysis of encoded protein sequences offered additional support for the present identification of streptococcal galE homologues as galE1 or galE2. Thus, the deep separation of GalE1 and GalE2 sequences (Fig. 3) is consistent with previous phylogenetic and structural studies of the UDP-hexose 4-epimerase family (Ishiyama et al., 2004), which suggest that differences in substrate preference appeared early in the evolution of these enzymes. Further evidence for this difference was obtained by comparing these sequences with those of E. coli K12 GalE (Thoden et al., 2002), Trypanosoma brucei GalE (Roper et al., 2005) and human GalE (Thoden et al., 2002). The human protein has substrate specificity like S. gordonii 38 GalE2 whereas the other two have substrate specificity like S. gordonii 38 GalE1. The different substrate preference of E. coli K12 and human GalE depends to a significant extent on specific amino acid residues that function as gatekeepers for entry of acetylated substrates into the catalytic sites of these enzymes (Thoden et al., 2001). Thus, the bulky side chain of Tyr299 in E. coli GalE allows entry of smaller unacetylated substrates but blocks entry of larger acetylated substrates whereas the smaller side chain of Cys309 in human GalE allows entry of both unacetylated and acetylated substrates. In T. brucei GalE, which is more closely related to streptococcal GalE1 than E. coli K12 GalE (Ishiyama et al., 2004), the equivalent residue is Leu342 (Shaw et al., 2003). Significantly, Leu also occurs at the corresponding position in all streptococcal GalE1 sequences. In contrast, Cys as in human GalE, is present in all streptococcal GalE2 sequences except those of S. gordonii GalE2, which have Ser, a residue of similar size. These findings strongly support identification of both galE1 and galE2 based on their location in the streptococcal chromosome, and thereby strengthen the conclusion that the presence of galE1 in S. sanguinis and S. gordonii distinguishes these species from S. oralis.
Figure 3.
Molecular phylogenetic analysis of full length GalE protein sequences as deposited in GenBank (Table S1) from strains of S. gordonii (Sg), S. oralis (So) and S. sanguinis (Ss) showing separation of GalE1 and GalE2 in an unrooted tree constructed by Maximum Likelihood method using MEGA5 (Tamura et al., 2011). Bootstrap values (%) are based on 1500 replications; the scale bar refers to genetic divergence as calculated by the MEGA software. The ability of human GalE and inability of E. coli (Ec) GalE to act on acetylated substrates depends in part on C309 in the former protein and Y299 in the latter. C or S occurs at the equivalent position in all streptococcal GalE2 sequences. In contrast, L occurs in all GalE1 sequences and in T. brucei (Tb) GalE (L342), which has substrate specificity like that of E. coli GalE.
RPS-bearing streptococci are prominent early colonizes of the tooth surface where they appear in micro-colonies along with other cell types including type 2 fimbriated Actinomyces spp. (Palmer et al., 2003) and non-RPS producing strains of S. gordonii that bind Gn-types of RPS (Chalmers et al., 2008). While S. oralis is the major RPS-producing species (Hsu et al., 1994), strains identified as S. sanguinis, S. gordonii, S. cristatus (Yang et al., 2011), and S. australis (J. Cisar, unpubl. obs.) also produce these polysaccharides. Three RPS-producing strains of S. mitis (i.e. strains J22, ATCC15914 and H127) were also reported previously (Cisar et al., 1997). Subsequent taxonomic studies have however identified strain J22 as S. oralis (Kilian et al., 2008) and strains ATCC15914 and H127 as S. oralis and S. australis, respectively, (M. Kilian, personal communication). Production of Gn- or G-types of RPS by strains of S. oralis but not S. mitis is consistent with the presence of identifiable rps operons in six of the nine available S. oralis genomes (Table S1, 6 to 14) but not in any of 12 S. mitis genomes (J. Cisar unpubl. obs.). Related operons for cell-surface polysaccharide biosynthesis do however occur in many strains of S. mitis (Kilian et al., 2008). These operons may well direct synthesis of polysaccharides that represent a novel type of RPS, such as one identified from S. mitis SK137 (Bergstrom et al., 2000). Accordingly, evolution of S. oralis and S. mitis could reflect the association of these closely related streptococci with different members of the plaque biofilm community.
Supplementary Material
Polysaccharides and galactose epimerases identified from strains of S. gordonii, S. oralis and S. sanguinis.
Oligonucleotide primers for comparative PCR studies of S. sanguinis and S. gordonii strains.
Acknowledgments
We thank Medha Bhagwat for phylogenetic analysis of GalE sequences and both Robert Palmer, and John Thompson for helpful reviews of the manuscript. Support for this work was from the Intramural Research Program of NIDCR.
References
- Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, Samuel G, Skovsted IC, Kaltoft MS, Barrell B, Reeves PR, Parkhill J, Spratt BG. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. Plos Genet. 2006;2:262–269. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom N, Jansson PE, Kilian M, Skov Sorensen UB. Structures of two cell wall-associated polysaccharides of a Streptococcus mitis biovar 1 strain. A unique teichoic acid-like polysaccharide and the group O antigen which is a C-polysaccharide in common with pneumococci. Eur J Biochem. 2000;267:7147–7157. doi: 10.1046/j.1432-1327.2000.01821.x-i2. [DOI] [PubMed] [Google Scholar]
- Chalmers NI, Palmer RJ, Cisar JO, Kolenbrander PE. Characterization of a Streptococcus sp.-Veillonella sp. Community Micromanipulated from Dental Plaque. J Bacteriol. 2008;190:8145–8154. doi: 10.1128/JB.00983-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar JO, Kolenbrander PE, McIntire FC. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979;24:742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar JO, Sandberg AL, Abeygunawardana C, Reddy GP, Bush CA. Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology. 1995;5:655–662. doi: 10.1093/glycob/5.7.655. [DOI] [PubMed] [Google Scholar]
- Cisar JO, Sandberg AL, Reddy GP, Abeygunawardana C, Bush CA. Structural and antigenic types of cell wall polysaccharides from viridans group streptococci with receptors for oral actinomyces and streptococcal lectins. Infect Immun. 1997;65:5035–5041. doi: 10.1128/iai.65.12.5035-5041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey PA. The Leloir pathway: a mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. FASEB J. 1996;10:461–470. [PubMed] [Google Scholar]
- Hsu SD, Cisar JO, Sandberg AL, Kilian M. Adhesive properties of viridans group streptococcal species. Microb Ecol Health Dis. 1994;7:125–137. [Google Scholar]
- Ishiyama N, Creuzenet C, Lam JS, Berghuis AM. Crystal structure of WbpP, a genuine UDP-N-acetylglucosamine 4-epimerase from Pseudomonas aeruginosa: substrate specificity in UDP-hexose 4-epimerases. J Biol Chem. 2004;279:22635–22642. doi: 10.1074/jbc.M401642200. [DOI] [PubMed] [Google Scholar]
- Kilian M, Mikkelsen L, Henrichsen J. Taxonomic study of viridans streptococci: description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Niven 1946), Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrewes and Horder 1906) Int J Syst Bacteriol. 1989;39:471–484. [Google Scholar]
- Kilian M, Poulsen K, Blomqvist T, Havarstein LS, Bek-Thomsen M, Tettelin H, Sorensen UBS. Evolution of Streptococcus pneumoniae and Its Close Commensal Relatives. Plos One. 2008;3:1–11. doi: 10.1371/journal.pone.0002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narikawa S, Suzuki Y, Takahashi M, Furukawa A, Sakane T, Mizushima Y. Streptococcus oralis Previously Identified as Uncommon ‘Streptococcus sanguis’ in Behcets-Disease. Archs Oral Biol. 1995;40:685–690. doi: 10.1016/0003-9969(95)00042-n. [DOI] [PubMed] [Google Scholar]
- Palmer RJ, Gordon SM, Cisar JO, Kolenbrander PE. Coaggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J Bacteriol. 2003;185:3400–3409. doi: 10.1128/JB.185.11.3400-3409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper JR, Guther MLS, MacRae JI, Prescott AR, Hallyburton I, Acosta-Serrano A, Ferguson MAJ. The suppression of galactose metabolism in procylic form Trypanosoma brucei causes cessation of cell growth and alters procyclin glycoprotein structure and copy number. J Biol Chem. 2005;280:19728–19736. doi: 10.1074/jbc.M502370200. [DOI] [PubMed] [Google Scholar]
- Shaw MP, Bond CS, Roper JR, Gourley DG, Ferguson MA, Hunter WN. High-resolution crystal structure of Trypanosoma brucei UDP-galactose 4′-epimerase: a potential target for structure-based development of novel trypanocides. Mol Biochem Parasitol. 2003;126:173–180. doi: 10.1016/s0166-6851(02)00243-8. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoden JB, Henderson JM, Fridovich-Keil JL, Holden HM. Structural analysis of the Y299C mutant of Escherichia coli UDP-galactose 4-epimerase. Teaching an old dog new tricks. J Biol Chem. 2002;277:27528–27534. doi: 10.1074/jbc.M204413200. [DOI] [PubMed] [Google Scholar]
- Thoden JB, Wohlers TM, Fridovich-Keil JL, Holden HM. Human UDP-galactose 4-epimerase. Accommodation of UDP-N-acetylglucosamine within the active site. J Biol Chem. 2001;276:15131–15136. doi: 10.1074/jbc.M100220200. [DOI] [PubMed] [Google Scholar]
- Xu DQ, Thompson J, Cisar JO. The gene cluster for receptor polysaccharide biosynthesis in Streptococcus gordonii 38. J Bacteriol. 2003;185:5419–5430. doi: 10.1128/JB.185.18.5419-5430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Tsukioka Y, Tomihisa K, Nakano Y, Koga T. Genes involved in cell wall localization and side chain formation of rhamnose-glucose polysaccharide in Streptococcus mutans. J Bacteriol. 1998;180:5803–5807. doi: 10.1128/jb.180.21.5803-5807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Cisar JO, Bush CA. Structure of type 3Gn coaggregation receptor polysaccharide from Streptococcus cristatus LS4. Carbohydr Res. 2011;346:1342–1346. doi: 10.1016/j.carres.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ritchey M, Yoshida Y, Bush CA, Cisar JO. Comparative structural and molecular characterization of ribitol-5-phosphate-containing Streptococcus oralis coaggregation receptor polysaccharides. J Bacteriol. 2009;191:1891–1900. doi: 10.1128/JB.01532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shelat NY, Bush CA, Cisar JO. Structure and molecular characterization of Streptococcus pneumoniae capsular polysaccharide 10F by carbohydrate engineering in Streptococcus oralis. J Biol Chem. 2010;285:24217–24227. doi: 10.1074/jbc.M110.123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Ganguly S, Bush CA, Cisar JO. Carbohydrate engineering of the recognition motifs in streptococcal co-aggregation receptor polysaccharides. Mol Microbiol. 2005;58:244–256. doi: 10.1111/j.1365-2958.2005.04820.x. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Ganguly S, Bush CA, Cisar JO. Molecular basis of L-rhamnose branch formation in streptococcal coaggregation receptor polysaccharides. J Bacteriol. 2006;188:4125–4130. doi: 10.1128/JB.01843-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Yang J, Peaker PE, Kato H, Bush CA, Cisar JO. Molecular and antigenic characterization of a Streptococcus oralis coaggregation receptor polysaccharide by carbohydrate engineering in Streptococcus gordonii. J Biol Chem. 2008;283:12654–12664. doi: 10.1074/jbc.M801412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner D, Gandhi AR, Yi H, Stephens DS. Mitis Group Streptococci Express Variable Pilus Islet 2 Pili. Plos One. 2011;6:e25124. doi: 10.1371/journal.pone.0025124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Polysaccharides and galactose epimerases identified from strains of S. gordonii, S. oralis and S. sanguinis.
Oligonucleotide primers for comparative PCR studies of S. sanguinis and S. gordonii strains.