Abstract
Background:
An association of insulin use and risk of cancer has been reported but evidence is conflicting and methodological issues have been identified.
Objective:
To summarize results regarding insulin use and cancer risk by a systematic review and meta-analysis of cohort and case-control studies examining risk of cancer associated with insulin use in patients with diabetes.
Data Sources:
Systematic literature search in 5 databases: PubMed, Embase, Web of Science, Scopus and Cochrane Library.
Study Eligibility Criteria (PICOS):
Population: diabetes patients. Exposure: Users of any exogenous insulin. Comparison: Diabetes patients with or without use of antidiabetic drugs. Outcome: Any incident cancer. Study Design: Cohort and case-control studies.
Results:
42 eligible studies examined risk of any cancer and 27 site-specific cancers. Results of individual studies were heterogeneous. Meta-analyses were significant for: Insulin vs No Insulin: Increased risk for pancreas, liver, kidney, stomach and respiratory cancer, decreased risk for prostate cancer. Insulin vs Non-Insulin Antidiabetics: Increased risk for any, pancreatic and colorectal cancer. Glargine vs Non-Glargine Insulin: Increased risk for breast cancer, decreased risk for colon cancer.
Limitations:
Few studies available for most cancer sites and exposure contrasts, and few assess effect of dose and duration of exposure. Methodological issues in several studies. Availability of confounders.
Conclusions:
Insulin use was associated with risk of cancer at several sites. Cautious interpretation of results is warranted as methodological issues and limitations in several of the included studies have been identified. Choice of study design may have a profound effect on estimated cancer risk.
Keywords: Cancer risk, diabetes mellitus, insulin, neoplasm, meta-analysis, systematic review.
INTRODUCTION
Rationale
Associations between diabetes mellitus and increased risk of cancer at several sites have been established [1-3]. It remains unclear whether this relationship between diabetes and cancer is direct, e.g. because of hyperglycemia, or if it is mediated through underlying biologic factors like insulin resistance and hyperinsulinemia, or if it is indirectly linked through common risk factors such as obesity. Insulin is a growth factor, and it is biologically plausible that high levels of endogenous insulin or exposure to exogenous, administered insulin could stimulate neoplastic growth [4, 5]. In recent years, several studies have reported modification of cancer risk by use of specific antidiabetic drugs. A decreased risk associated with use of metformin has been reported in meta-analyses while results for thiazolidinedione are not conclusive [6-8]. Results from observational studies published in 2009 raised concerns of a link between insulin use and risk of cancer, but the results of these initial studies were inconclusive and conflicting [9-11]. Publication of many studies assessing risk of cancer at different sites from other data sources has ensued. Several of these observational studies have been hampered by methodological issues and did not take into account dose, duration and timing of insulin exposure or lacked information on important confounders [10, 12-14]. In addition, most studies have been too small for robust quantification of cancer risk, specially for examining cancer sites individually. The ability to study cancer at specific sites individually is important because cancer is not a homogenous disease and different pathways are involved in the aetiology for different subtypes of cancer [2].
Existing evidence from randomized controlled trials (RCT) is also limited. Two meta-analyses of RCT data published in the wake of the initial observational studies published in 2009 did not find an increased risk for insulin glargine and detemir [15, 16]. However, these studies were rather small for studying a rare event such as cancer and were of limited duration. A larger RCT study with 6 years duration that assessed insulin glargine exposure and had cancer incidence as a secondary outcome reported no increased risk of cancer overall and no significant results for site-specific cancers [17]. However, the general limitations of RCTs regarding representativeness of the study population apply [5], and this trial may have been too small to properly quantify risk of cancer at specific sites.
Clinical evidence suggests that there may be a link between use of exogenous insulin and risk of cancer at some sites but results are conflicting and inconclusive. The CAncer Risk and INsulin analogs (CARING) project aims to assess possible carcinogenic effects of insulin use combining data from health care databases in six European countries. As part of the CARING project, the present review and meta-analysis was undertaken to summarize published results on the topic.
Objective
To perform a systematic review and meta-analysis of published cohort and case-control studies that examined the risk of any type of cancer associated with use of exogenous human insulin or insulin analogs in patients with type 1 or type 2 diabetes.
METHODS
Protocol and Registration
The present study was developed according to the PRISMA guidelines [18], and supplemented by guidance from the Cochrane Collaboration Handbook [19]. The protocol was registered on Prospero (registration number CRD42012002428) [20].
Eligibility Criteria
The following PICOS eligibility criteria were applied:
Population: diabetes patients.
Exposure: diabetes patients using any exogenous human insulin or insulin analogues.
Comparison: diabetes patients, with or without use of antidiabetic drugs (i.e. use other types of insulin, non-insulin antidiabetic drugs, not use any insulin, or not use any antidiabetic drugs). Studies that only had persons without diabetes as comparator group were excluded.
Outcome: incident cancer at specific sites or cancer at any site as a composite outcome. Studies that only report the risk of cancer-related mortality are not included.
Study design: cohort and case-control studies.
The studies had to report sufficient data for proper evaluation of the study population, exposure, comparator and outcome to be considered for inclusion in the present review.
Information Sources
We performed a systematic literature search in 5 databases: Medline at PubMed, Embase, Scopus, Web of Science and The Cochrane Library. The last search was performed on 27 November 2012. The CARING project group concurrently performed a systematic review on risk of cancer in persons with diabetes compared to persons without diabetes [21]. Records from that review were assessed for inclusion in the present review.
Search Strategy
The specific search strategy for each database is presented in Supplementary Material 1 (440.2KB, pdf) . Search terms for diabetes, insulin and cancer (or similar terms) were applied in all searches, while terms for risk or incidence were added in free text searches. For Scopus and Web of Science, free-text searches were used. For Medline, Embase and Cochrane, we used thesaurus (MESH and Emtree terms). In addition, we performed a free text search in Medline, Embase and Cochrane Library limited to references published during the last year in order to identify references not yet indexed with MESH and Emtree terms. Except for limiting the free text search to publications from the last year, no restrictions were used on publication date, language or publication status.
Study Selection and Collection Process
ØK and VH developed the search strategy for each database in collaboration with a research librarian. ØK performed the final search in the databases, compiled a mutual reference list for all searches and removed duplicate references. ØK and JSL independently screened title and abstract of records for eligibility, and records identified by either of the reviewers as eligible for inclusion were retrieved in full text. If a conference abstract was deemed eligible for inclusion, a full text article was searched for in databases and included for full text reading if found. ØK and JSL independently assessed the full text records for inclusion and records that ØK and JSL agreed on were included in the review. Disagreements were resolved by discussion and by conferring with a third reviewer (PV).
Data Items
From each study, information was retrieved on risk of cancer, cancer site, definitions of exposure and comparator group (reference), covariates, study design, source population, data sources, and patient characteristics including diabetes type, age group and geographical location (country). Data was extracted by ØK and validated by JSL and disagreements were resolved by discussion.
Risk of Bias in Individual Studies
Risk of bias was assessed by the Newcastle Ottawa Scale (NOS) [22]. All studies were scored by two reviewers (ØK, JSL) and disagreement resolved by discussion and by conferring a third reviewer (PV). The user-defined items required in the NOS score were defined as follows (Supplementary Material 2 (440.2KB, pdf) ): age was the most important adjustment factor, the exposed in cohorts should be representative of the average “diabetic population using insulin”, minimum average exposure duration was 5 years, and loss to follow-up less than 10%. A conservative approach was chosen if information to score specific items were not available in the article, i.e. no points were given on an item if information was uncertain or missing.
Summary Measures and Synthesis of Results (Meta-Analysis)
Initially, the types of exposure-comparator contrasts and cancer sites examined in records included in the systematic review were assessed by inspecting the summary tables (Supplementary Material 3 (440.2KB, pdf) ). The contrasts can be categorized as: 1) insulin use versus no insulin use; 2) insulin use versus use of non-insulin antidiabetic drugs; 3) users of insulin A versus users of insulin B; and 4) users of insulin A versus users of insulin B or no insulin. Studies that examined contrast 1 and 2 were included in the pooled analyses while contrast 4 was omitted because of few populations. For contrast 3, glargine insulin users versus non-glargine insulin users was the most frequently used contrast and was included in pooled analyses.
Separate pooled analyses were performed for each combination of cancer site and exposure contrast (three selected) that had more than one study population available. One study could contribute more than one population to an analysis, e.g. if the presented risk estimate in the original study was stratified by gender. For studies that published several risk estimates for the same cancer site and exposure contrast (e.g. for different study designs), the following algorithm was applied for choosing which estimate to include (in order of importance): 1) estimates with prior cancer excluded was preferred over estimates adjusted for prior cancer: 2) intention-to-treat analysis preferred over other designs (e.g. as-treated analysis); 3) exposure categorized as exclusive use was preferred (monotherapy, e.g. “glargine only” preferred over “glargine and non-glargine”); 4) estimates without latency period preferred. If no decision could be made from this algorithm, reviewer 1 (ØK) made a final decision on which estimate to include. Estimates from statistical models adjusted for more covariates were preferred. Risk estimates stratified by dose or duration of insulin exposure were not included in pooled analyses.
Hazard ratio, incidence risk ratio, rate ratio and odds ratio as summary measures for the risk of incident cancer with 95% confidence intervals were retrieved from each study. These measures were weighted based on the inverse of the
standard error of the risk estimator from the individual studies. Chi square test were used to measure heterogeneity across studies. DerSimonian and Laird random effects models [23] was used in the main analyses regardless of the result of the test for heterogeneity. Additional pooled analyses with a fixed effect model were performed if studies did not exhibit statistically significant heterogeneity. Data were prepared in Microsoft Excel 2010 and analyzed in Stata version 8.
Risk of Bias Across Studies in Meta-Analysis
Risk of publication bias across studies was assessed by Egger’s regression analysis [24] in Stata version 8.
RESULTS
Study Selection
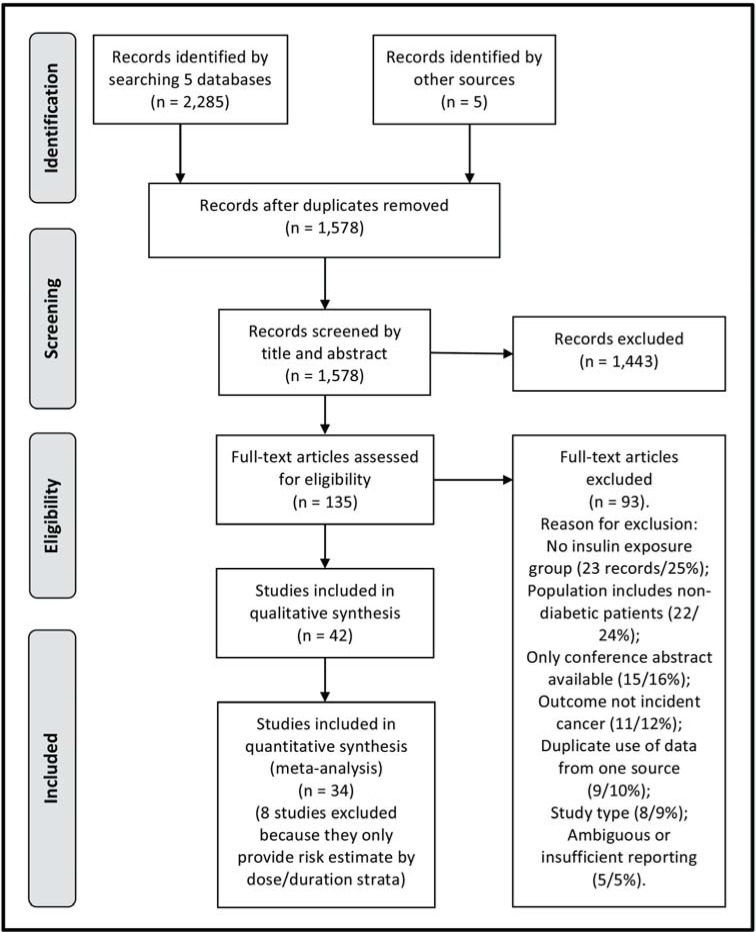
The selection process is shown in Fig. (1). Five databases were searched and 2,285 records were identified. After removal of duplicates and inclusion of 5 records from other sources, 1,578 records were screened. After screening of title and abstract by reviewer 1 (ØK) and reviewer 2 (JSL), 135 records were retrieved in full text. 42 records [25-66] were eligible for inclusion in the systematic review, while the remaining 93 records were excluded during full text reading for the following reasons: no insulin exposure group (25%), population includes non-diabetic patients (24%), only conference abstract available (16%), outcome was not incident cancer (12%), duplicate use of data from one source (10%), study type (9%), ambiguous or insufficient reporting of definitions (5%). For the category “duplicate use of data”, records were excluded as they were likely to be using the same data as one of the records included in the review and study the same cancer site and exposure contrast. These excluded records [67-75] and the overlapping records that are included are listed in Supplementary Material 7 (440.2KB, pdf) . The records [76-80] that were excluded because of insufficient reporting of definitions are likely to fulfill the criteria for inclusion in the present review but cannot be properly classified. The definition of the comparator group was not clearly defined, or contradicting information regarding the comparator group was found in tables and text of these studies.
Fig. (1).
Flow diagram for the study selection process (PRISMA).
Study Characteristics and Risk of Bias Within Studies
Tables 1 and 2 present the characteristics of the studies included in the systematic review for cohort and case-control studies, respectively. 27 cohort studies [25-51] and 15 case-control studies (9 nested case-control studies) [52-66] were included in the systematic review.
Table 1.
Characteristics of Cohort Studies Included in the Systematic Review (27 Records)
| Author (Country) | Study Design | Study Period | Data Source Population | Source Population | Diabetes Type | Data Source Exposure | New/ Prevalent Drug User | Data Source Outcome | Covariates | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Blin 2012 (France) [25] | cohort | 2003-2010 | insurance database | nationwide | DM2 | insurance database (claims) | new | insurance database | Medication possession ratio of insulin; age; sex; DM duration; DM type; ad drugs; comorbidities; all ATC codes (1st level); | 8 |
| Campbell 2010 (USA) [26] | cohort | 1992-2007 | Self-reported questionnaire | 21 states | DM2 | Self-reported questionnaire | prevalent | Self-reported questionnaire | sex (separate models); age; bmi; physical activity; NSAIDs; alcohol; family history colorectal cancer; endoscopy history; education; | 6 |
| Carstensen 2012 (Denmark) [27] | cohort | 1995-2009 | Diabetes register | nationwide | Unspecified | Diabetes register or prescription database | new | Cancer register | age; sex (separate models);calendar time; date of birth; | 9 |
| Chang 2011 (Taiwan) [28] | cohort | 2004-2007 | insurance database | nationwide | DM2 | insurance database (claims) | new | Cancer register | age; sex; dose of fast-acting insulin; metformin; sulfonylurea; alpha-glucosidase inhibitors; tzd; glinides; fast-acting insulin; premixed insulin; detemir; diabetes-related complications; comorbidities inpatients/outpatient; statins; aspirin; health service utilization; outpatient visits diabetes; outpatient visits non-diabetes; examinations various; physician characteristics; initiation year insulin; | 8 |
| Colhoun 2009 (Scotland) [29] | cohort | 2002/3-2005 | Diabetes register | nationwide | unspecified/ DM2/DM1 (varies by analysis) | Diabetes register | new/ prevalent (varies by analysis) | cancer register and causes of death register | varies by cancer site, design and model: prior cancer; age; sex; DM type; calendar year; bmi; hba1c; DM duration; smoking; diastolic bp; systolic bp; deprivation; metformin; sulfonlyurea; other oad; | 7/8* |
| Currie 2009 (UK) [30] | cohort | 2000-? | Physician database | nationwide | DM2 | Physician database (prescribed) | new | Physician database | age; sex; prior cancer; smoking; | 7/8* |
| Fagot 2012 (France) [31] | cohort | 2007-2010 | insurance database | nationwide | DM2 | insurance database (claims) | new | Hospital records database | age; sex; DM duration; metformin; pioglitazone; rosiglitazone; sulfonylurea; other niad; | 8 |
| Ferrara 2011 (USA) [32] | cohort | 1997-2005 | Diabetes register | Northern California | Unspecified | pharmacy database (dispensed) | prevalent | Cancer register | age; sex; HbA1c (baseline); DM duration; oad (pioglitazone, other tzd (almost exclusively troglitazone), metformin, insulin, sulfonylurea, and other oral agents (e.g. miglitol, acarbose, nataglinide, repaglinide)); year cohort entry; ethnicity; income; smoking; creatinine; congestive heart failure; new DM diagnosis; | 8 |
| Hemkens 2009 (Germany) [33] | cohort | 2001-2005 | insurance database | nationwide | Unspecified | insurance database (claims) | new | insurance database | age; sex; dose; oad; federal state; year first insulin; drug use (gastrointestinal agents, ACE, antiarrhythmic, corticosteroids, parathyroid gland drugs, cytostatics for non-malignant disease); | 8 |
| Hense 2011 (Germany) [34] | cohort | 2003-2008 | insurance database | Munster district | DM2 | insurance database (claims) | prevalent | Cancer register | age; sex; DM duration; bmi; | 8 |
| Hsieh 2012 (Taiwan) [35] | cohort | 2000-2008 | insurance database | random sample of nationwide database | DM2 | insurance database (claims) | prevalent | insurance database | age; sex; | 9 |
| Kostev 2012 (Germany) [36] | cohort | 2000-2011 | Physician database | ns (IMS Disease Analyzer, covers 20 mill patients) | DM2 | Physician database (prescribed) | prevalent? | Physician database | age; sex; hba1c; cumulative duration exposure; private insurance status; urban location of practice; region; Charlson Comorbidity Index; | 7 |
| Lai 2012 (Taiwan) [37] | cohort | 2000-2008 | insurance database | random sample of nationwide database | Unspecified | insurance database (claims) | prevalent | insurance database | age; sex; | 8 |
| Lai 2012 (Taiwan) [38] | cohort | 2000-2008 | insurance database | random sample of nationwide database | Unspecified | insurance database (claims) | prevalent | insurance database | age; sex; obesity; pulmonary tuberculosis; copd; obesity; pneumoconiosis; asbestosis; tobacco use; | 8 |
| Lai 2012 (Taiwan) [39] | cohort | 2000-2008 | insurance database | random sample of nationwide database | Unspecified | insurance database (claims) | prevalent | insurance database | age; sex; comorbidities (cirrhosis, alcoholic liver damage, hepatitis B, hepatitis C); | 8 |
| Lind 2012 (Sweden) [40] | cohort | 1985-2007 | Hospital records database | ns (17 hospitals) | Unspecified | Hospital records database | prevalent? | Cancer register | age; bmi; time since start glargine; last insulin dose used; smoking | 9 |
| Ljung 2011 (Sweden) [41] | cohort | 2006/7-2008 | prescription database | nationwide | Unspecified/DM2 | pharmacy database (dispensed) | prevalent | Cancer register | age; sex. breast cancer: age at onset DM; bmi; smoking; cvd; age at first child; oestrogen; |
8 |
| Morden 2011 (USA) [42] | cohort | 2006-2008 | insurance database | nationwide | DM2 | insurance database (claims) | prevalent | insurance database | age; sex; obesity; insulin dose; metformin; ethnicity; diabetes complications; oestrogen; poverty; 14 Charlson comorbidities; tobacco; | 8 |
| Neumann 2012 (France) [43] | cohort | 2006-2009 | insurance database | nationwide | Unspecified | insurance database (claims) | prevalent | Hospital records database | age; sex; oad; | 8 |
| Newton 2012 (USA) [44] | cohort | 1992-2007 | Self-reported questionnaire | ns (CPS-II Nutrition Cohort participants, 1.2 million participants) | DM2 | Self-reported questionnaire | prevalent | questionnaire verified by medical records/ cancer register/ death index | age; sex; bmi; race; smoking; education; alcohol; | 7 |
| Oliveria 2008 (USA) [45] | cohort | 2000-2004 | insurance database | insured population (covers 42 million individuals) | Unspecified | insurance database (claims) | prevalent | insurance database (ICD-9) verified by pathology/medical records | age; sex. Colorectal cancer: history polyps; ulcerative colitis; Crohn's disease. Bladder cancer: schistosomiasis; pelvic radiation. Liver cancer: hepatitis B/C; cirrhosis; alcoholism. Pancreas cancer: partial gastrectomy; chronic pancreatitis; dvt; dermatomyositis/polymyositis; alcoholism; hepatitis B/C; history polyps; | 8 |
| Redaniel 2012 (UK) [46] | cohort | 1987-2007 | Physician database | nationwide | DM2 | Physician database (prescribed) | new | ns | cohort entry year; geography; | 9 |
| Ruiter 2012 (Netherlands) [47] | cohort | 2000-2008 | prescription database | Pharmo database from community pharmacies (covers 2.5 million individuals) | DM2 | pharmacy database (dispensed) | new | Hospital records database | age; sex; other insulin; calendar time; number hospitalisations; number of non- DM drugs used; | 8 |
| Suissa 2011 (UK) [48] | cohort matched | 2002-2009 | Physician database | nationwide | DM2 | Physician database (prescribed) | new/prevalent (varies by analysis) | Physician database | Matching on: birth year; calendar time; duration prior insulin use. Adjust for: age; bmi; HbA1c; DM duration; duration insulin use; history of cancer other than breast and nmsc cancer; metformin; sulfonylurea; tzd; smoking; alcohol; oophorectomy; hrt; statin; |
8 |
| Tseng 2012 (Taiwan) [49] | cohort | 2005 | insurance database | random sample of nationwide register | DM2 | insurance database (claims) | prevalent | insurance database | age; sex; occupation; geography; | 8 |
| Van Staa 2012 (UK) [50] | cohort matched | 1997-2006 | Physician database | nationwide (GPRD) | DM2 | Physician database (prescribed) | new | Physician database | Matching on: age; sex; calendar year. Adjust for: age; sex; bmi; HbA1c; oad; ses; smoking; alcohol; coronary heart disease; coronary revascularization; hyperlipidaemia; hypertension; peripheral vascular disease; renal impairment; angina; ARB; antiplatelet; beta-blockers; calcium- channel blockers; diuretics; nitrates; NSAIDs; aspirin; statins; calendar year; (some variables only for subset of patients) |
8 |
| Yang 2010 (Hong Kong) [51] | cohort matched | 1996-2005 | Diabetes register | nationwide (all public hospitals) | DM2 | hospital inpatient and outpatient database | new | Hospital records database | Matching on: age; smoking; propensity score. Adjust for: Specific cancer sites: only adjust for hba1c? Any cancer: age; DM duration; HbA1c; spot urinary albumin-to-creatinine ratio (Ln ACR 1); retinopathy; metformin; smoking; hdl; triglycerides; estimated glomerular filtration rate (eGFR); |
9 |
Abbreviations: ACE, ACE inhibitor; Ad, antidiabetic drugs; ARB, Angiotensin II receptor blocker; ATC, Anatomical Therapeutic Chemical (ATC) classification system for drugs; Bmi, body mass index; Bp, blood pressure; Copd, chronic obstructive pulmonary disease; Cvd, cardiovascular disease; DM, diabetes mellitus; DM1, diabetes type 1; DM2, diabetes type 2; Dvt, Deep venous thrombosis; Hdl, High-density lipoprotein; Hrt, hormone replacement therapy; Niad, non-insulin antidiabetics; Nmsc, non-melanoma skin cancer; NOS, Newcastle Ottawa Scale; ns, not specified; Oad, oral antidiabetics; Ses, socioeconomic status; tzd, thiazolidinedione.
NOS vary in analyses depending on whether prior cancer is adjusted or excluded.
Table 2.
Characteristics of Case-Control Studies Included in the Systematic Review (15 Records)
| Author (Country) | Study Design | Study Period | Data Source Population | Source Population | Source for Controls | Age Group | Matching Variables | Diabetes Type | Data Source Exposure | New/Prevalent Drug User | Data Source Outcome | Covariates | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bodmer 2010 (UK) [52] | case-control nested | 1994-2005 | physician database (GPRD) | nationwide | population (GPRD) | 30-79 | index date; age; sex; general practice; | DM2 | Physician database (prescribed) | prevalent | Physician database | bmi; DM duration; HbA1c; metformin; sulfonylurea; tzd; prandial glucose regulators; acarbose; oestrogen; smoking; | 9 |
| Bodmer 2011 (UK) [53] | case-control nested | 1995-2009? | physician database (GPRD) | nationwide | population (GPRD) | <90 | index date; age; sex; general practice; years of history in database | Unspecified | Physician database (prescribed) | prevalent | Physician database | bmi; HbA1c; DM duration; metformin; sulfonylurea; smoking; oestrogens; oral contraceptives; history of hysterectomy/endometriosis/polycystic ovaries; | 9 |
| Bodmer 2012 (UK) [54] | case-control nested | 1995-2009 | physician database (GPRD) | nationwide | population (GPRD) | <90 | index date; age; sex; general practice; years of history in database | Unspecified | Physician database (prescribed) | prevalent | Physician database | bmi; DM duration; HbA1c; metformin; sulfonylurea; smoking; aspirin; NSAIDs; statin; | 9 |
| Bodmer 2012 (UK) [55] | case-control nested | 1995-2009 | physician database (GPRD) | nationwide | population (GPRD) | <90 | index date; age; sex; general practice; years of history in database | Unspecified | Physician database (prescribed) | prevalent | Physician database | bmi; metformin; sulfonylurea; smoking; | 9 |
| Bonelli 2003 (Italy) [56] | case-control | 1992-1996 | hospital records | ns (patients from 7 gastroenterology and endoscopy hospital units in Northern Italy) | hospital | 18-75 | ns | Unspecified | Interview | prevalent | hospital | age; sex; hospital; education; occupation; alcohol; smoking; | 5 |
| Chang 2012 (Taiwan) [57] | case-control nested | 2000-2007 | insurance database | nationwide | population | 30-100 | calendar time; age; gender; follow-up duration; (treatment duration) | DM2 | insurance database (claims) | prevalent | Cancer register | Glitazones; metformin; sulfonylurea; glinides. varies by cancer site (stepwise selection): number of oad; statins; aspirin; beta-blockers; calcium-channel blockers; ACE; ARB; alpha-glucosidase inhibitors; chronic liver disease; chronic kidney disease; nephropathy; neuropathy; retinopathy; peripheral vascular disease; cerebrovascular disease; cvd; depression; chronic lung disease; | 8 |
| Chang 2012 (Taiwan) [58] | case-control nested | 2000-2007 | insurance database | nationwide | population | 30-100 | index date; age; sex; dm duration | DM2 | insurance database (claims) | prevalent | Cancer register | sulfonylurea; glinides; metformin; tzd; alpha-glucosidase inhibitors; statin; aspirin; beta-blockers; calcium-channel blockers; ACE; chronic liver disease; chronic kidney disease; nephropathy; cerebrovascular disease; | 8 |
| Cleveland 2012 (USA) [59] | case-control | 1996-1997 | rapid reporting system for cancer, interview | population (Nassau and Suffolk counties of Long Island) | population | all | age | DM2 | Interview | prevalent | hospital, confirmed by physician records | bmi; metformin; insulin secretagogues (sulfonylurea); menopausal status; race; | 5 |
| Fortuny 2005 (Spain) [60] | case-control | 1998-2002 | hospital records | ns ("centres" in 4 cities (Barcelona, Tortosa, Reus and Madrid)) | hospital | all | age; sex; centre; | DM2 | interview | prevalent | hospital clinical data, verified by histology, immunohistochemistry test, flow cytometry | age; sex; bmi; ad drugs; ses; study centre; | 5 |
| Kawaguchi 2010 (Japan) [61] | case-control nested | 2004-2008 | hospital (hepatitis C patients) | ns (patients from 3 hospitals specialized for liver diseases) | hospital | 40+ | no | DM2 | ns | prevalent | hospital biopsy | age; sex; bmi; HbA1c; prior metastatic liver tumour; cholangiocellular carcinoma; history of pancreatic tumour; sulfonylurea (gliclazide or glibenclamide);cirrhosis; albumin; alcohol?; AST; lactate dehydrogenase (LDH); alkaline phosphatase (ALP); platelet count; gamma-glutamyl transpeptidase? | 7 |
| Koro 2007 (USA) [62] | case-control nested | 1997-2004 | insurance database | ns (9 census regions, 30 different healthcare plans, 38 million patients (IHCIS)) | population (insurance database) | 18+ | age; sex; index date; duration follow-up in database | DM2 | insurance database (claims) | prevalent | insurance database | age | 9 |
| Li 2011 (USA) [63] | case-control (Pooled 3 case-control studies: MDACC; SFBA; NCI) | MDACC: 2001-2008; SFBA: 1995-1999; NCI: 1986-1989. | MDACC: outpatient clinic; SFBA: cancer register(?); NCI: cancer register. | MDACC: ns (one tertiary referral hospital); SFBA: population-based; NCI: population-based. | MDACC: hospital; SFBA, NCI: population. | MDACC: all; SFBA: 21-85; NCI: 21-79 | age; sex; race (MDACC, NCI); geography (NCI); | Unspecified | Interview | prevalent | MDACC: hospital data with pathological confirmation. SFBA, NCI: cancer register. | age; sex; bmi; oad; race; education; smoking; alcohol; study site; | 6 |
| Mizuno 2013 (Japan) [64] | case-control | 1999-2011 | hospital records | ns (DM patients treated at specialized DM institute) | hospital | all | no | Unspecified | ns | prevalent | hospital data, verified by histology or clinical course | sulfonylurea; glinides; metformin; tzd; alpha-glucosidase inhibitors; family history with DM; statin; | 4 |
| Vinikoor 2009 (USA) [65] | case-control | 2001-2006 | rapid reporting system for cancer, interview | population-based (33 counties in North Carolina) | population | 40-80 | age; sex; race; | Unspecified | Interview | prevalent | Cancer register | age; sex; bmi; race; family history of colorectal cancer; NSAIDs; calcium intake; education; | 7 |
| Yang 2004 (UK) [66] | case-control nested | 1990-2002 | Physician database (GPRD) | nationwide | population (GPRD) | all | age; calendar period; duration follow-up in database | DM2 | Physician database (prescribed) | prevalent | computerized medical records | sex; bmi; DM2 duration; metformin; sulfonylurea; cholecystectomy history; smoking; NSAIDs/aspirin; | 9 |
Abbreviations: ACE, ACE inhibitor; Ad, antidiabetic drugs; ARB, Angiotensin II receptor blocker; Bmi, body mass index; Cvd, cardiovascular disease; DM, diabetes mellitus; DM1, diabetes type 1; DM2, diabetes type 2; NOS, Newcastle Ottawa Scale; ns, not specified; Oad, oral antidiabetics; Ses, socioeconomic status; tzd, thiazolidinedione.
Risk of Bias Within Studies
The NOS score for each study is presented in Tables 1 and 2. The highest NOS score was 9 and the lowest score was 4 (attainable score was 0-9). Among 27 cohort studies, 1 had NOS 6 and the other 26 studies had NOS score 7-9, i.e. of fair quality according to NOS. Among the 15 case-control studies, 5 studies had NOS 4-6 and all of these were “traditional” case-control studies (i.e. not nested). The other case-control studies had NOS score 7-9.
RESULTS of Individual Studies
In the summary tables all cancer sites are presented together (Supplementary Material 3 (440.2KB, pdf) ). Several studies have more than one risk estimate presented for each cancer site and exposure contrast because the study reported results for several study designs (e.g. with or without latency period, intention-to-treat and as-treated analyses), or reported both an overall risk estimate as well as risk by strata of dose/duration of insulin exposure. Results of individual studies are presented in Supplementary Material 4 (440.2KB, pdf) separately for the site-specific cancers examined and for any cancer as a composite outcome. Only the preferred risk estimate for each combination of cancer site and exposure contrast according to the algorithm given in Methods is presented.
Cancer at any site and at the following 13 specific sites was examined in more than one study per exposure contrast and was eligible for inclusion in pooled analyses: breast, prostate, stomach, pancreatic, liver, colorectal, colon, rectal, respiratory, bladder, kidney, melanoma, and non-Hodgkin’s lymphoma (NHL). The results for these cancer sites (Supplementary Material 4 (440.2KB, pdf) ) reveals substantial heterogeneity of results, as point estimates for risk were spread both above and below unity (RR=1) for most cancer sites and exposure contrasts. More consistent results (point estimates) may be present for the exposure contrast insulin versus no insulin for any cancer (3 of 4 populations had point estimate above unity, and with statistical significance), pancreas (7 of 8 populations above unity, 6 significant), liver (5 of 6 populations above unity, 4 significant), stomach (3 of 3 populations above unity, 3 significant), respiratory (5 of 6 populations above unity, 4 significant), bladder (4 of 5 populations above unity, 1 significant), kidney (4 of 4 populations above unity, 2 significant), and prostate cancer (3 of 3 populations below unity, 2significant). For the exposure contrast glargine versus non-glargine insulin use, 6 of 6 populations had risk estimate above unity for prostate cancer but none of the individual risk estimates were statistically significant.
14 cancer sites were only examined in one study per exposure contrast and were not included in pooled analyses: leukemia, Hodgkin’s lymphoma (HL), multiple myeloma, brain, head-neck, skin, testis, ovarian, uterus, cervical, thyroid, oesophagus, gastrointestinal, and lymphoma. Results of these studies are presented in Supplementary Material 5 (440.2KB, pdf) .
Synthesis of Results (Meta-Analysis)
In total, 34 studies were included in pooled analyses. Table 3 presents the results of pooled analyses by random effects model for the 14 cancer sites and exposure contrasts with sufficient number of studies (populations). Significant increased risk of cancer for the exposure contrast insulin versus no insulin was found for cancer in pancreas, liver, kidney and the respiratory system, and a marginal
Table 3.
Results of Pooled Analyses for Cancer Sites and Exposure Contrasts Examined in More than One Study. DerSimonian and Laird Random Effects Model and Fixed Effects Model
| Cancer Site | Exposure Contrast | Number of Populations* | Random Effects Model | Fixed Effects Model‡ | Heterogeneity† | ||
|---|---|---|---|---|---|---|---|
| RR | [95% CI] | RR | [95% CI] | p | |||
| Any | insulin vs no insulin | 4 | 1.04 | [0.75 , 1.45] | <0.001 | ||
| insulin vs niad | 2 | 1.52 | [1.16 , 2.00] | 0.043 | |||
| glargine vs non-glargine | 7 | 0.96 | [0.83 , 1.10] | <0.001 | |||
| stomach | insulin vs no insulin | 3 | 1.65 | [1.02 , 2.68] | 0.002 | ||
| insulin vs niad | 1 | na | - | - | |||
| glargine vs non-glargine | 1 | na | - | - | |||
| pancreatic | insulin vs no insulin | 8 | 2.58 | [2.05 , 3.25] | <0.001 | ||
| insulin vs niad | 3 | 3.83 | [1.43 , 10.23] | 4.37 | [2.62 , 5.67] | 0.167 | |
| glargine vs non-glargine | 3 | 1.17 | [0.78 , 1.77] | 1.12 | [0.86 , 1.46] | 0.128 | |
| Liver | insulin vs no insulin | 6 | 1.84 | [1.32 , 2.58] | <0.001 | ||
| insulin vs niad | 1 | na | - | - | |||
| glargine vs non-glargine | 2 | 0.89 | [0.64 , 1.24] | 0.88 | [0.68 , 1.14] | 0.203 | |
| kidney | insulin vs no insulin | 4 | 1.38 | [1.06 , 1.79] | 0.002 | ||
| insulin vs niad | 0 | na | - | - | |||
| glargine vs non-glargine | 1 | na | - | - | |||
| bladder | insulin vs no insulin | 5 | 1.09 | [0.93 , 1.28] | 1.07 | [0.98 , 1.17] | 0.096 |
| insulin vs niad | 0 | na | - | - | |||
| glargine vs non-glargine | 2 | 1.34 | [0.81 , 2.22] | 1.32 | [0.93 , 1.86] | 0.150 | |
| colorectal | insulin vs no insulin | 7 | 1.16 | [0.87 , 1.55] | <0.001 | ||
| insulin vs niad | 2 | 1.79 | [1.36 , 2.36] | 1.79 | [1.36 , 2.36] | 0.474 | |
| glargine vs non-glargine | 4 | 0.92 | [0.75 , 1.13] | 0.92 | [0.75 , 1.13] | 0.742 | |
| Colon | insulin vs no insulin | 5 | 1.02 | [0.92 , 1.13] | 1.02 | [0.92 , 1.13] | 0.675 |
| insulin vs niad | 1 | na | - | - | |||
| glargine vs non-glargine | 2 | 0.71 | [0.56 , 0.91] | 0.72 | [0.58 , 0.89] | 0.265 | |
| rectal | insulin vs no insulin | 6 | 1.00 | [0.85 , 1.17] | 1.00 | [0.85 , 1.17] | 0.565 |
| insulin vs niad | 0 | na | - | - | |||
| glargine vs non-glargine | 0 | na | - | - | |||
| respiratory | insulin vs no insulin | 6 | 1.30 | [1.14 , 1.47] | <0.001 | ||
| insulin vs niad | 1 | na | - | - | |||
| glargine vs non-glargine | 4 | 0.99 | [0.83 , 1.17] | 0.99 | [0.83 , 1.17] | 0.733 | |
| NHL | insulin vs no insulin | 4 | 1.16 | [0.83 , 1.62] | 0.020 | ||
| insulin vs niad | 0 | na | - | - | |||
| glargine vs non-glargine | 0 | na | - | - | |||
| melanoma | insulin vs no insulin | 3 | 0.99 | [0.80 , 1.22] | 0.99 | [0.81 , 1.20] | 0.322 |
| insulin vs niad | 0 | na | - | - | |||
| glargine vs non-glargine | 0 | na | - | - | |||
| prostate | insulin vs no insulin | 3 | 0.80 | [0.73 , 0.88] | 0.80 | [0.73 , 0.88] | 0.825 |
| insulin vs niad | 3 | 1.15 | [0.86 , 1.54] | 1.15 | [0.86 , 1.54] | 0.477 | |
| glargine vs non-glargine | 6 | 1.13 | [0.98 , 1.32] | 1.13 | [0.98 , 1.32] | 0.726 | |
| breast | insulin vs no insulin | 7 | 0.90 | [0.81 , 1.00] | 0.033 | ||
| insulin vs niad | 4 | 1.13 | [0.88 , 1.45] | 1.13 | [0.88 , 1.45] | 0.862 | |
| glargine vs non-glargine | 9 | 1.14 | [1.01 , 1.29] | 1.14 | [1.01 , 1.29] | 0.059 | |
Abbreviations: na, not applicable. NHL, non-Hodgkin's lymphoma. niad, non-insulin antidiabetic drugs. NOS, Newcastle Ottawa Scale. Underlined estimates indicate statistical significance at 5% level.
Some studies contribute more than one population in one analysis, e.g. if results in the original study is only presented stratified by gender.
Only run for heterogeneous studies (test for heterogeneity p>0.05).
Chi square test for heterogeneity.
significance for stomach cancer. A decreased risk was observed for prostate cancer. Non-significant results were observed for any cancer, bladder, colorectal, colon, rectal, non-Hodgkin’s lymphoma, melanoma and breast cancer. For the exposure contrast insulin versus non-insulin antidiabetic drugs, significant increased risk of any cancer, pancreatic and colorectal cancer was observed, while results for prostate and breast cancer were not significant. Glargine use was associated with a significantly decreased risk of colon cancer compared to non-glargine use breast cancer were marginally significant, while any cancer, pancreatic, liver, bladder, colorectal, respiratory and prostate cancer was not statistically significant.
Additional fixed effects models were run for studies that did not exhibit significant heterogeneity (p>0.05, Table 3). These analyses gave similar results as the random effects model except for an even higher risk for pancreatic cancer.
8 studies only provided risk estimates by dose or duration of exposure [33, 50, 52-55, 60, 66] while other studies provided dose or duration risk estimates in addition to average risk estimates. However, pooled analyses by dose or duration was assessed as not feasible because these risk estimates were reported for different cancer sites, exposure contrasts and exposure definitions (e.g. mean or cumulative dose, duration since start exposure or cumulative duration. Dose and duration risk estimates were identified for any cancer, breast, pancreatic, prostate, liver, colorectal, ovarian, lung cancer and lymphoma (Supplementary Material 6 (440.2KB, pdf) ).
Risk of Bias Across Studies
Egger’s regression test did not reveal any significant (p <0.05) publication bias for any cancer site.
DISCUSSION
Summary of Evidence
In the present meta-analysis, insulin exposure seems to be associated with an increased risk of cancer in pancreas, liver, kidney, stomach and respiratory system and decreased risk of prostate cancer, when compared to no insulin use. Compared to use of non-insulin antidiabetic drugs, insulin was associated with increased risk of any cancer, pancreatic and colorectal cancer. For users of glargine insulin compared to users of non-glargine insulin, a decreased risk of colon cancer as well as a marginally significant increased risk of breast cancer was observed. However, the results from individual studies reveal substantial variation in the reported cancer risk for most cancer sites. For 11 cancer sites results were only available in one population per exposure contrast.
The importance of assessing dose and duration of insulin use in addition to the average risk has been revealed in several studies observing an increased risk of cancer at different sites even in the initial period after treatment initiation or switch in therapy [27, 40, 50], and the exposure duration may be too short to be a causal factor for the occurrence of cancer. In particular, a substantial increased risk of pancreas cancer is observed and reverse casualty is important to consider for this cancer site. Analyses by duration of insulin exposure reveal specially high risk with shorter durations compared to longer durations [27, 63, 64, 68]. A similar increased risk is observed in the early period after diagnosis of diabetes [63, 81]. This could be a result of diabetes as an early sign of pancreatic cancer (protopathic bias) or ascertainment bias after diabetes diagnosis.
Confounding by severity or indication is a concern in pharmacoepidemiological studies, and could be more pronounced when comparing a third-line therapy like insulin to first line therapies like metformin in patients with type 2 diabetes [14]. Characteristics of populations receiving these two therapies can be substantially different concerning diabetes duration, obesity and other factors. This effect may be less pronounced for use of specific insulin types compared to users of other insulin types, although physician preference for specific insulin types cannot be excluded. Furthermore, a protective effect from metformin use has been reported [6] and this is important to consider when insulin is compared to metformin or other oral antidiabetic drugs.
A few studies presented several results for the same comparison but from different study designs, e.g. intention-to-treat and as-treated analysis, with or without latency period, new user design or “prevalent users design”. This enable assessment of the impact the choice of study design has on results. As an example, Colhoun et al. [29] reported results for use of “glargine only” and breast cancer risk that were substantially different by study design (range 1.47 to 3.65). Thus, if a different algorithm for selection of estimate to include in the present meta-analysis had been applied, the marginally significant results for glargine use and breast cancer could have been different. This is likely to apply for other comparisons as well.
During screening, only 2 randomized controlled trials (RCT) that assessed the risk of cancer in diabetes patients allocated to receive insulins were identified. The Origin trial [17] included 12,537 people with impaired glucose tolerance or diabetes type 2 for an average follow-up time of 6.2 years to study cardiovascular events as primary outcome. Participants were randomly allocated to receive insulin glargine or standard care and risk of new or recurrent cancer was a secondary outcome. There was no difference in risk of any cancer for the glargine group compared to the standard care group (Hazard Ratio 1.00 [95% CI, 0.88-1.13]). No significant difference in risk was reported for specific cancer sites: breast (1.01 [0.60-1.71]), lung (1.21 [0.87-1.67]), colon (1.09 [0.79-1.51]), prostate (0.94 [0.70-1.26]), melanoma [0.88 [0.44-1.75]) or cancer at other sites [0.95 [0.80-1.14]). A long-term safety study designed to assess ocular complications followed 1,017 persons with type 2 diabetes (82). Participants were randomly assigned to insulin glargine or NPH insulin with a mean cumulative exposure of 4 years. As an additional outcome, malignant neoplasms reported as serious adverse events were assessed and occurred in 51 patients and with relative risk 0.63 [0.36-1.09] for glargine. Risk of benign and malignant neoplasms was 0.90 0.64-1.26.
Two meta-analyses of RCT data from manufacturer’s pharmacovigilance databases were also identified. Home et al. [15] analysed data from 12 phase 2-4 RCTs conducted by Sanofi-Aventis on insulin glargine versus any active comparator (insulin or oral antidiabetics) in type 1 and type 2 diabetes patients. Included studies were between 4 and 52 weeks duration except for the study by Rosenstock et al. [82] mentioned above, and data in the meta-analysis were primarily driven by those data. 10,880 patients were included and incident malignant cancer occurred in 91 patients with relative risk 0.90 [0.60-1.36] for glargine. Dejgaard et al. [16] performed a meta-analysis of 21 Novo Nordisk-sponsored RCTs of insulin detemir compared to NPH insulin (16 trials) or insulin glargine (5 trials) in patients with type 1 or type 2 diabetes. RCTs of at least 12 weeks duration were included, with median exposure to insulin of 24 weeks (max 115 weeks) in trials of detemir versus NPH insulin, and 51 weeks (max 64 weeks) in trials of detemir versus glargine. Malignant cancer occurred in 21 of 6,644 patients with Odds Ratio 2.44 [1.01-5.89] for NPH insulin versus detemir, and 16 events in 2,049 patients with Odds Ratio 1.47 [0.55-3.94] for glargine versus detemir.
Limitations
Potential flaws in observational studies of insulin use and risk of cancer have been extensively debated, and the quality of studies included in the present systematic review is a concern. As a measure of the quality of each study, we used the NOS score and most studies could be considered as fair to high quality. However, it can be argued that NOS score is a crude quality measure. Generally, NOS takes into account the quality of the underlying data sources but does not fully account for important issues in pharmacoepidemiological studies, such as definition of drug exposure and time-related biases. For instance, the study by Yang et al. [51] reported a substantial decreased risk of cancer for insulin users compared to nonusers (HR 0.17 [0.09-0.32]). Potentially serious flaws in the study design have been pointed out [13, 83] but the study was nevertheless scored as NOS 9. Potential time-related and other biases of other studies included in the present systematic review have been discussed [10, 12, 14] and these studies also received high NOS scores [30, 33, 52]. Thus, the NOS do not seem to fully reflect important aspects of quality of the studies of the present review and has low granularity to distinguish studies of higher and lower quality.
The availability of covariates to adjust for confounding varied substantially in included studies (Table 1 and 2). The NOS score does to some extent take into account confounder adjustment, however, adjustment for age and one other factor gave full score on this NOS item. The most important cofounders to adjust for may vary by cancer site and a more thorough assessment of confounder adjustment is desirable. Included studies examined a wide variety of exposures and comparators and this is useful for assessing consistency of the association of insulin and cancer. However, there were too few studies (populations) for most combinations of cancer site and exposure contrast to perform pooled analyses, and additional subgroup or meta-regression analyses could not be performed to assess possible determinants of cancer risk such as diabetes type, gender, age, incident or prevalent insulin use and study design. Egger’s regression test did not reveal any significant publication bias for any cancer site. However, the number of studies in each analysis was low and the test may not have sufficient power to distinguish chance from real asymmetry [19]. Selective reporting was observed within some published studies as only the analyses with significant results were reported [44, 64, 79].
CONCLUsions
The results from individual studies in the present review revealed substantial variation in reported risk of cancer associated with use of insulin, and varied by type of comparison group for the insulin users. Many studies are too small to make any firm conclusions. The pooled analyses revealed significantly increased or decreased risk of cancer at several sites for insulin users. However, there were few available studies in each pooled analysis, and subgroup analyses of possible determinants of cancer risk like diabetes type was not feasible. It is imperative to consider the data quality and conduct of individual studies when interpreting these results and the choice of study design in individual studies may have an effect on the estimated cancer risk. Extensive review of the quality of methods, design and conduct of studies was not the aim of the present review. A fit-for-purpose system for evaluating the quality of pharmacoepidemiological studies would be useful in any further evaluation of whether the observed associations can be attributed to issues with study design, analysis and low quality of data.
ACKNOWLEDGEMENTS
Heleen Bronsveld is acknowledged for valuable input on identifying references and discussion of results. Research librarian Edith Clausen is acknowledged for her help to develop the search strategy.
CONFLICT OF INTEREST
Marloes T Bazelier and Frank de Vries are employed by Utrecht University and are conducting research under the umbrella of the Centre for Research Methods. This Centre has received unrestricted funding from the Netherlands Organisation for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), the Royal Dutch Pharmacists Association (KNMP), the private-public funded Top Institute Pharma (www.tipharma.nl, includes co-funding from universities, government, and industry), the EU Innovative Medicines Initiative (IMI), the EU 7th Framework Program (FP7), the Dutch Ministry of Health and industry (including GlaxoSmithKline, Pfizer, and others). Marie L De Bruin is employed by Utrecht University and is conducting research under the umbrella of the WHO Collaborating Centre for pharmaceutical policy and regulation. This Centre receives no direct funding or donations from private parties, including pharma industry. Research funding from public-private partnerships, e.g. IMI, TI Pharma (www.tipharma.nl) is accepted under the condition that no company-specific product or company related study is conducted. The Centre has received unrestricted research funding from public sources, e.g. Netherlands Organisation for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), EU 7th Framework Program (FP7), Dutch Medicines Evaluation Board (MEB), and Dutch Ministry of Health. None of the abovementioned companies was involved in the preparation of this manuscript.
The other Co-authors do not have any conflict of interest to declare.
Funding
The research leading to the results of this study has received funding from the European Community’s Seventh Framework Programme (FP-7) under grant agreement number 282526, the CARING project. The funding source had no role in study design, data collection, data analysis, data interpretation or writing of the report.
PATIENT CONSENT
Declared none.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–23. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and Cancer: A consensus report. Diabetes Care. 2010;33:1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010;47:87–95. doi: 10.1007/s00592-010-0187-3. [DOI] [PubMed] [Google Scholar]
- 4.Pollak M, Russell-Jones D. Insulin analogs and cancer risk: cause for concern or cause célèbre?. Int J Clin Pract. 2010;64:628–36. doi: 10.1111/j.1742-1241.2010.02354.x. [DOI] [PubMed] [Google Scholar]
- 5.Home P. Insulin Therapy and Cancer. Diabetes Care. 2013;36:S240–S244. doi: 10.2337/dcS13-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCensi A, Puntoni M, Goodwin P, et al. Metformin and Cancer Risk in Diabetic Patients: A Systematic Review and Meta-analysis. Cancer Prevent Res. 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 7.Colmers IN, Bowker SL, Johnson JA. Thiazolidinedione use and cancer incidence in type-2 diabetes: A systematic review and meta-analysis. Diabet & Metabol. 2012;38:475–84. doi: 10.1016/j.diabet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Soranna D, Scotti L, Zambon A, et al. Cancer Risk Associated with Use of Metformin and Sulfonylurea in Type 2 Diabetes: A Meta-Analysis. Oncologist. 2012;17:813–22. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith U, Gale EAM. Does diabetes therapy influence the risk of cancer?. Diabetologia. 2009;52:1699–708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- 10.Pocock SJ, Smeeth L. Insulin glargine and malignancy: an unwarranted alarm. Lancet. 2009;374:511–3. doi: 10.1016/S0140-6736(09)61307-6. [DOI] [PubMed] [Google Scholar]
- 11.Gale EA. Insulin glargine and cancer: another side to the story?. Lancet. 2009;374:521. doi: 10.1016/S0140-6736(09)61477-X. [DOI] [PubMed] [Google Scholar]
- 12.Hernández-Díaz S, Adami HO. Diabetes therapy and cancer risk: causal effects and other plausible explanations. Diabetologia. 2010;53:802–8. doi: 10.1007/s00125-010-1675-2. [DOI] [PubMed] [Google Scholar]
- 13.Renehan AG. Insulin analogs and cancer risk: the emergence of second-generation studies. Diabetologia. 2012;55:7–9. doi: 10.1007/s00125-011-2352-9. [DOI] [PubMed] [Google Scholar]
- 14.Suissa S, Azoulay L. Metformin and the Risk of Cancer: Time-related biases in observational studies. Diabetes Care. 2012;35:2665–73. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Home PD, Lagarenne P. Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia. 2009;52:2499–506. doi: 10.1007/s00125-009-1530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dejgaard A, Lynggaard H, Rastam J, Krogsgaard TM. No evidence of increased risk of malignancies in patients with diabetes treated with insulin detemir: a meta-analysis. Diabetologia. 2009;52:2507–12. doi: 10.1007/s00125-009-1568-4. [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. New Engl J Med. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Available from www.cochrane-handbook.org. 2011. The Cochrane Collaboration.Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. [Google Scholar]
- 20.Karlstad , Hjellvik V, Furu K, et al. Use of insulin and insulin analogs and risk of cancer.PROSPERO 2012. CRD42012002428. PROSPERO International prospective register of systematic reviews cited 2013 Oct 20: Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?.ID=CRD42012002428. 2013 [Google Scholar]
- 21.Starup-Linde J, Karlstad O, Aistrup Eriksen S, et al. CARING (CAncer Risk and INsulin analoGues): The Association of Diabetes Mellitus and Cancer Risk with Focus on Possible Determinants- a Systematic Review and a Meta-Analysis. Curr Drug Saf. 2013;8:000–000. doi: 10.2174/15748863113086660071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute (OHRI) Available from: http://www.ohri.ca/Programs/clinical_epidemiolog y/oxford.asp. 2013 [Google Scholar]
- 23.Böhning D, Boca Raton FL. Computer-assisted analysis of mixtures and applications: meta-analysis. disease mapping and others Monographs on statistics and applied probability. Chapman & Hall/CRC. 2000:1–232. [Google Scholar]
- 24.Matthias E, George DS, Martin S, Christoph M. Bias in meta-analysis detected by a simple. graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blin P, Lassalle R, Dureau-Pournin C, Ambrosino B, Bernard MA, Abouelfath A, et al. Insulin glargine and risk of cancer: A cohort study in the French National Healthcare Insurance Database. Diabetologia. 2012;55:644–53. doi: 10.1007/s00125-011-2429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell PT, Deka A, Jacobs EJ, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology. 2010;139:1138–46. doi: 10.1053/j.gastro.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 27.Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia. 2012;55:948–58. doi: 10.1007/s00125-011-2381-4. [DOI] [PubMed] [Google Scholar]
- 28.Chang CH, Toh S, Lin JW, et al. Cancer risk associated with insulin Glargine among adult type 2 diabetes patients - a nationwide Cohort study. PLoS ONE. 2011;6:e21368. doi: 10.1371/journal.pone.0021368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colhoun HM. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755–65. doi: 10.1007/s00125-009-1453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 31.Fagot JP, Blotiere PO, Ricordeau P, Weill A, Alla F, Allemand H. Does Insulin Glargine Increase the Risk of Cancer Compared With Other Basal Insulins?.A French nationwide cohort study based on national administrative databases. Diabetes Care. 2012;10 doi: 10.2337/dc12-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrara A, Lewis JD, Quesenberry CP, et al. Cohort Study of Pioglitazone and Cancer Incidence in Patients With Diabetes. Diabetes Care. 2011;34:923–9. doi: 10.2337/dc10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–44. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hense HW, Kajuter H, Wellmann J, Batzler W. Cancer incidence in type 2 diabetes patients - first results from a feasibility study of the D2C cohort. Diabetol Metab Syndr. 2011;3:15. doi: 10.1186/1758-5996-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Dia Res. 2012;2012:413782. doi: 10.1155/2012/413782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostev K. Risk of breast cancer in patients on long-acting insulin analogs in comparison with those on human insulin. Diabetologia. 2012;55:1554–5. doi: 10.1007/s00125-012-2497-1. [DOI] [PubMed] [Google Scholar]
- 37.Lai HC, Tsai IJ, Chen PC, et al. Gallstones a cholecystectomy chronic pancreatitis and the risk of subsequent pancreatic cancer in diabetic patients: a population-based cohort study. J Gastroenterol. 2012;48(6):721–7. doi: 10.1007/s00535-012-0674-0. [DOI] [PubMed] [Google Scholar]
- 38.Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DPH, Chen CC. Antidiabetes Drugs Correlate With Decreased Risk of Lung Cancer: A Population-Based Observation in Taiwan. Clin Lung Cancer. 2012;13:143–8. doi: 10.1016/j.cllc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: A population-based cohort study. Am J Gastroenterol. 2012;107:46–52. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 40.Lind M, Fahlen M, Eliasson B, Oden A. The relationship between the exposure time of insulin glargine and risk of breast and prostate cancer: an observational study of the time-dependent effects of antidiabetic treatments in patients with diabetes. Prim Care Diabetes. 2012;6:53–9. doi: 10.1016/j.pcd.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Ljung R, TalbAck M, Haglund B, Jonasson JM, Gudbjornsdottir S, Steineck G. Insulin glargine use and short-term incidence of malignancies - A three-year population-based observation. Acta Oncol. 2011;50:685–92. doi: 10.3109/0284186X.2011.558913. [DOI] [PubMed] [Google Scholar]
- 42.Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, Korc M. Further exploration of the relationship between insulin glargine and incident cancer: A retrospective cohort study of older medicare patients. Diabetes Care. 2011;34:1965–71. doi: 10.2337/dc11-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: A population-based cohort study. Diabetologia. 2012;55:1953–62. doi: 10.1007/s00125-012-2538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newton CC, Gapstur SM, Campbell PT, Jacobs EJ. Type 2 diabetes mellitus insulin-use and risk of bladder cancer in a large cohort study. Int J Cancer. 2013;132:2186–91. doi: 10.1002/ijc.27878. [DOI] [PubMed] [Google Scholar]
- 45.Oliveria SA, Koro CE, Ulcickas YM, Sowell M. Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes Metab Syndr Clin Res Rev. 2008;2:47–57. [Google Scholar]
- 46.Redaniel MT, Jeffreys M, May MT, Ben-Shlomo Y, Martin RM. Associations of type 2 diabetes and diabetes treatment with breast cancer risk and mortality: a population-based cohort study among British women. Cancer Causes Control. 2012;23:1785–95. doi: 10.1007/s10552-012-0057-0. [DOI] [PubMed] [Google Scholar]
- 47.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Risk of cancer in patients on insulin glargine and other insulin analogs in comparison with those on human insulin: results from a large population-based follow-up study. Diabetologia. 2012;55:51–62. doi: 10.1007/s00125-011-2312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suissa S, Azoulay L, Dell'Aniello S, Evans M, Vora J, Pollak M. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia. 2011;54:2254–62. doi: 10.1007/s00125-011-2190-9. [DOI] [PubMed] [Google Scholar]
- 49.Tseng CH. Diabetes and non-Hodgkin's lymphoma: analyses of prevalence and annual incidence in 2005 using the National Health Insurance database in Taiwan. Ann Oncol. 2012;23:153–8. doi: 10.1093/annonc/mdr334. [DOI] [PubMed] [Google Scholar]
- 50.Van Staa TP, Patel D, Gallagher AM, De Bruin ML. Glucose-lowering agents and the patterns of risk for cancer: A study with the General Practice Research Database and secondary care data. Diabetologia. 2012;55:654–65. doi: 10.1007/s00125-011-2390-3. [DOI] [PubMed] [Google Scholar]
- 51.Yang X, Ko GTC, So WY, et al. Associations of hyperglycemia and insulin usage with the risk of cancer in type 2 diabetes: The Hong Kong diabetes registry. Diabetes. 2010;59:1254–60. doi: 10.2337/db09-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–8. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin and the risk of ovarian cancer: A case-control analysis. Gynecol Oncol. 2011;123:200–4. doi: 10.1016/j.ygyno.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 54.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin is not associated with a decreased risk of colorectal cancer: A case-control analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:280–6. doi: 10.1158/1055-9965.EPI-11-0992-T. [DOI] [PubMed] [Google Scholar]
- 55.Bodmer M, Becker C, Jick SS, Meier CR. Metformin does not alter the risk of lung cancer: A case-control analysis. Lung Cancer. 2012;78:133–7. doi: 10.1016/j.lungcan.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Bonelli L, Aste H, Bovo P, et al., editors. A case control study in Northern Italy. Northern Italy Pancreas: Exocrine pancreatic cancer. cigarette smoing.and diabetes mellitus . [DOI] [PubMed] [Google Scholar]
- 57.Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM, Arnold CK. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology. 2012;55:1462–72. doi: 10.1002/hep.25509. [DOI] [PubMed] [Google Scholar]
- 58.Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM. Oral insulin secretagogues insulin and cancer risk in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:E1170–E1175. doi: 10.1210/jc.2012-1162. [DOI] [PubMed] [Google Scholar]
- 59.Cleveland RJ, North KE, Stevens J, Teitelbaum SL, Neugut AI, Gammon MD. The association of diabetes with breast cancer incidence and mortality in the Long Island Breast Cancer Study Project. Cancer Causes Control. 2012;23:1193–203. doi: 10.1007/s10552-012-9989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fortuny J, Benavente Y, Bosch R, Garcia-Villanueva M, de Sevilla AF, de SS. Type 2 diabetes mellitus its treatment and risk for lymphoma. Eur J Cancer. 2005;41:1782–7. doi: 10.1016/j.ejca.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 61.Kawaguchi T, Taniguchi E, Morita Y, et al. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479–86. doi: 10.1111/j.1478-3231.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 62.Koro C, Barrett S, Qizilbash N. Cancer risks in thiazolidinedione users compared to other anti-diabetic agents. Pharmacoepidemiol Drug Saf. 2007;16:485–92. doi: 10.1002/pds.1352. [DOI] [PubMed] [Google Scholar]
- 63.Li D, Tang H, Hassan M, Holly E, Bracci P, Silverman D. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22:189–97. doi: 10.1007/s10552-010-9686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizuno S, Nakai Y, Isayama H, et al. Risk factors and early signs of pancreatic cancer in diabetes: screening strategy based on diabetes onset age. J Gastroenterol. 2013;48:238–46. doi: 10.1007/s00535-012-0622-z. [DOI] [PubMed] [Google Scholar]
- 65.Vinikoor LC, Long MD, Keku TO, Martin CF, Galanko JA, Sandler RS. The association between diabetes. insulin use.and colorectal cancer among Whites and African Americans. Cancer Epidemiol Biomarkers Prev . 2009;18:1239–42. doi: 10.1158/1055-9965.EPI-08-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–50. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Blin P, Lassalle R, Dureau-Pournin C, et al. Insulin glargine and risk of cancer: A cohort study in the french national healthcare insurance database. Fund Clin Pharmacol. 2012;26:1–122. doi: 10.1007/s00125-011-2429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silverman DT, Schiffman M, Everhart J, et al. Diabetes mellitus other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80:1830–7. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjornsdottir S, Steineck G. Insulin glargine use and short-term incidence of malignancies a population-based follow-up study in Sweden. Diabetologia. 2009;52:1745–54. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- 71.Ljung R, Talback M, Haglund B, Jonasson JM, Gudbjornsdottir S, Steineck G. Insulin glargine use and short-term incidence of breast cancer - a four-year population-based observation. Acta Oncol. 2012;51:400–2. doi: 10.3109/0284186X.2011.624118. [DOI] [PubMed] [Google Scholar]
- 72.Liao KF, Lai SW, Li CI, Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: A population-based cohort study in Taiwan. J Gastroenterol Hepatol. 2012;27:709–13. doi: 10.1111/j.1440-1746.2011.06938.x. [DOI] [PubMed] [Google Scholar]
- 73.Liao KF, Lai SW, Li CI. The impact of anti-diabetic drugs on colorectal cancer risk in a large cohort of women with diabetes. Libyan J Med. 2012;7 doi: 10.3402/ljm.v7i0.17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monami M, Lamanna C, Pala L, et al. Treatment with insulin secretagogues and cancer-related mortality in type 2 diabetic patients a retrospective cohort study. Exp Clin Endocrinol Diabetes. 2008;116:184–9. doi: 10.1055/s-2007-992157. [DOI] [PubMed] [Google Scholar]
- 75.Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol. 2009;46:279–84. doi: 10.1007/s00592-008-0083-2. [DOI] [PubMed] [Google Scholar]
- 76.Buchs AE, Silverman BG. Incidence of malignancies in patients with diabetes mellitus and correlation with treatment modalities in a large Israeli health maintenance organization: A historical cohort study. Metabolism. 2011;60:1379–85. doi: 10.1016/j.metabol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 77.Chuang TY, Lewis DA, Spandau DF. Decreased incidence of nonmelanoma skin cancer in patients with type 2 diabetes mellitus using insulin: a pilot study. Brit J Dermatol. 2005;153:552–7. doi: 10.1111/j.1365-2133.2005.06738.x. [DOI] [PubMed] [Google Scholar]
- 78.Donadon V, Balbi M, Valent F, Avogaro A. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J Gastroenterol. 2010;16:3025–32. doi: 10.3748/wjg.v16.i24.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of Antidiabetic Agents and the Risk of Pancreatic Cancer: A Case-Control Analysis. Am J Gastroenterol. 2012;107:620–6. doi: 10.1038/ajg.2011.483. [DOI] [PubMed] [Google Scholar]
- 80.Mannucci E, Monami M, Balzi D, et al. Doses of insulin and its analogs and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. 2010;33:1997–2003. doi: 10.2337/dc10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia. 2011;54:2263–71. doi: 10.1007/s00125-011-2242-1. [DOI] [PubMed] [Google Scholar]
- 82.Rosenstock J, Fonseca V, McGill JB, et al. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised open-label study. Diabetologia. 2009;52:1971–3. doi: 10.1007/s00125-009-1452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carstensen B. Associations of Hyperglycemia and Insulin Usage With the Risk of Cancer in Type 2 Diabetes. The Hong Kong Diabetes Registry Diabetes. 2010;59:e17–e18. doi: 10.2337/db10-0777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.