Abstract
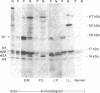
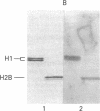
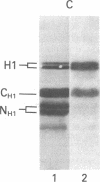
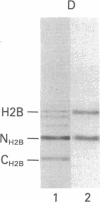
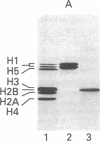
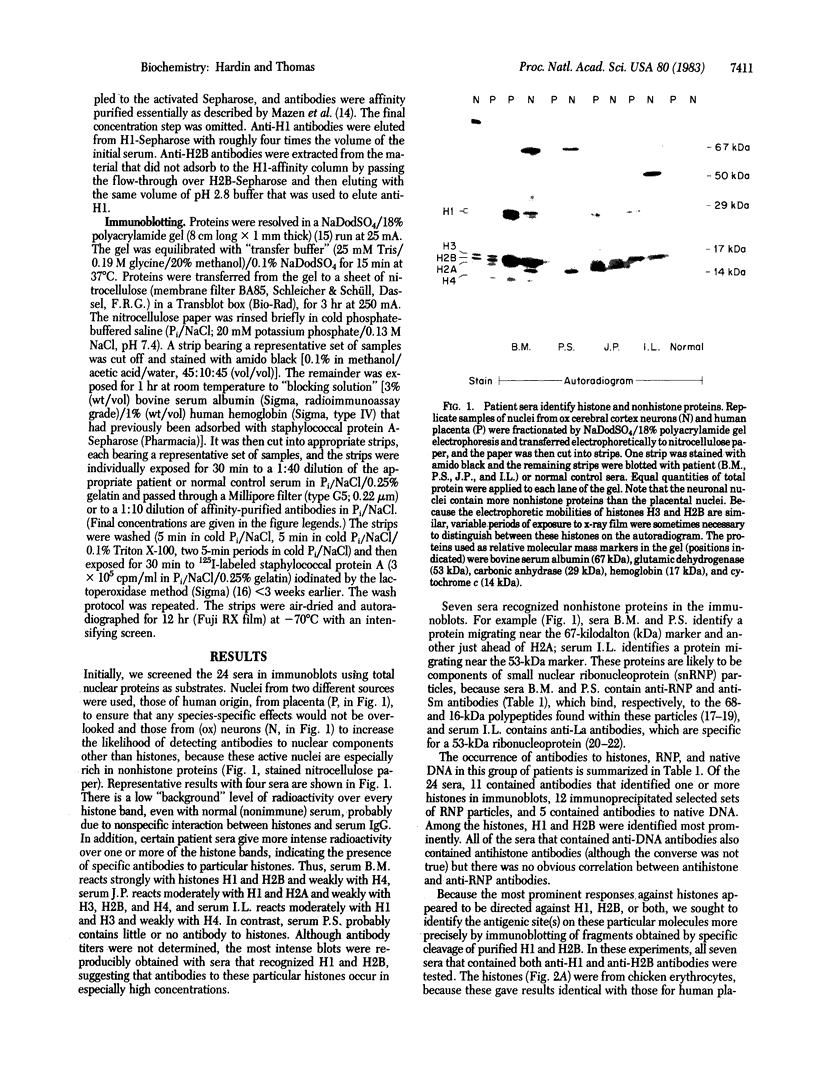
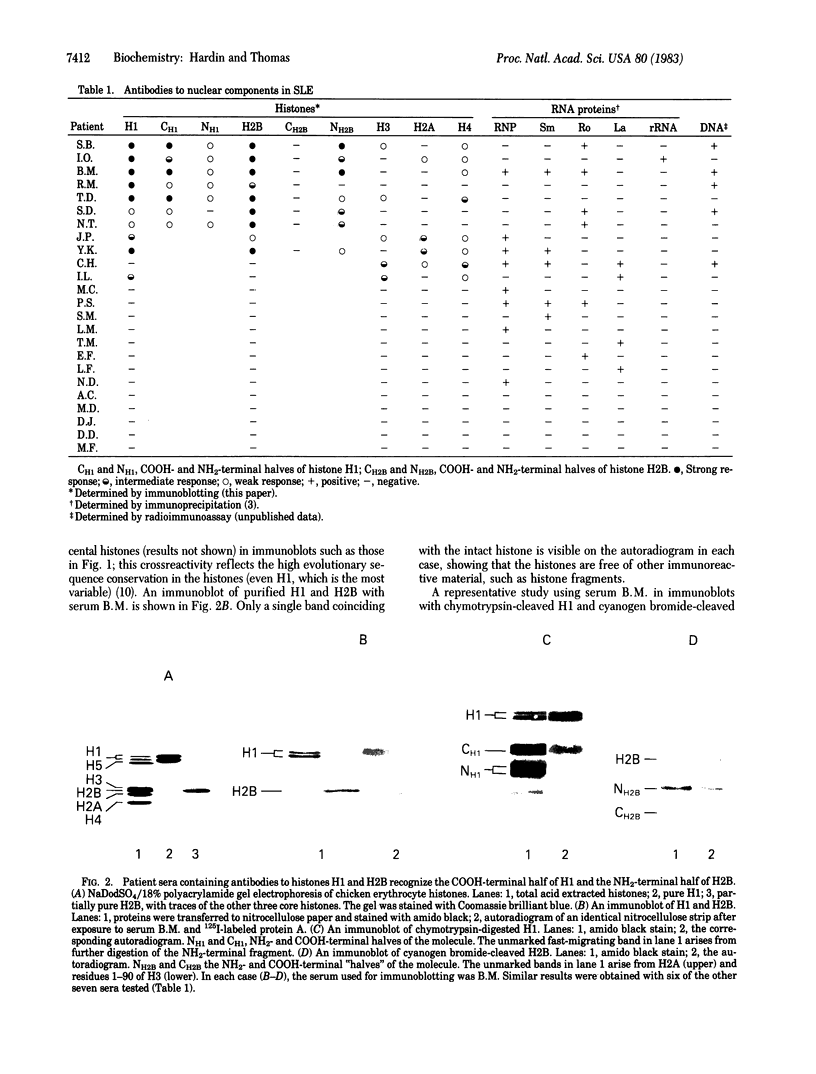
By the technique of immunoblotting we have assessed the ability of sera from 24 patients with systemic lupus erythematosus to bind nuclear proteins. Of the 11 patients who had antibodies to histones, 10 had antibodies to histone H1 and 9 of these also had antibodies to histone H2B. Antibodies to the other histones (H2A, H3, and H4) were less apparent. Five of the 11 patients (and two others in the remainder of the sample of 24) also had antibodies to a small number of nonhistone proteins that are probably components of ribonucleoprotein particles, but there was no obvious correlation between the presence of antihistone antibodies and the known antiribonucleoprotein activity of these sera. Separate determinants on H1 and H2B were demonstrated by immunoblotting with affinity-purified anti-H1 and anti-H2B antibodies derived from serum that showed both specificities. The localization of the determinants within the histone polypeptide chains was shown by immunoblotting with large fragments produced by specific proteolytic or chemical cleavage of the histones. The strongest determinant on H1 was located within the COOH-terminal half, with a weaker determinant being present within the NH2-terminal half; the H2B determinant(s) was located entirely within the NH2-terminal half of the molecule. The selectivity with which the antihistone antibodies in systemic lupus erythematosus are produced against the more exposed histones in the nucleosome (and perhaps against the most exposed regions of these histones) is consistent with the involvement of intact chromatin structures as immunogens in this disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Butler P. J., Pearson E. C., Thomas J. O. Stability of the higher-order structure of chicken-erythrocyte chromatin in solution. Eur J Biochem. 1981 Oct;119(3):469–476. doi: 10.1111/j.1432-1033.1981.tb05631.x. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Thomas J. O. Histones H1 and H5: one or two molecules per nucleosome? Nucleic Acids Res. 1981 Nov 25;9(22):5883–5894. doi: 10.1093/nar/9.22.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Chapman G. E., Danby S. E., Hartman P. G., Riches P. L. Studies on the role and mode of operation of the very-lysine-rich histone H1 (F1) in eukaryote chromatin. The properties of the N-terminal and C-terminal halves of histone H1. Eur J Biochem. 1975 Sep 15;57(2):521–528. doi: 10.1111/j.1432-1033.1975.tb02327.x. [DOI] [PubMed] [Google Scholar]
- Bustin M., Reisch J., Einck L., Klippel J. H. Autoantibodies to nucleosomal proteins: antibodies to HMG-17 in autoimmune diseases. Science. 1982 Mar 5;215(4537):1245–1247. doi: 10.1126/science.6460317. [DOI] [PubMed] [Google Scholar]
- Einck L., Dibble R., Frado L. Y., Woodcock C. L. Nucleosomes as antigens. Characterization of determinants and cross-reactivity. Exp Cell Res. 1982 May;139(1):101–110. doi: 10.1016/0014-4827(82)90323-8. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt D., Bustin M. Exposure of histone antigenic determinants in chromatin. Biochemistry. 1975 Apr 22;14(8):1689–1695. doi: 10.1021/bi00679a022. [DOI] [PubMed] [Google Scholar]
- Hardin J. A., Rahn D. R., Shen C., Lerner M. R., Wolin S. L., Rosa M. D., Steitz J. A. Antibodies from patients with connective tissue diseases bind specific subsets of cellular RNA-protein particles. J Clin Invest. 1982 Jul;70(1):141–147. doi: 10.1172/JCI110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusser C., Boesman M., Nordin J. H., Isliker H. Effect of chemical and enzymatic radioiodination on in vitro human Clq activities. J Immunol. 1973 Mar;110(3):820–828. [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter L., Schopfer K., Wilhelm J. A., Nyffenegger T., Parisot R. F., De Robertis E. M. Molecular characterization of ribonucleoprotein antigens bound by antinuclear antibodies. A diagnostic evaluation. Arthritis Rheum. 1982 Nov;25(11):1278–1283. doi: 10.1002/art.1780251102. [DOI] [PubMed] [Google Scholar]
- Mazen A., De Murcia G., Bernard S., Pouyet J., Champagne M. Localization of histone H5 in the subunit organization of chromatin using immunoelectron microscopy. Eur J Biochem. 1982 Sep;127(1):169–176. doi: 10.1111/j.1432-1033.1982.tb06852.x. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Oliver D., Sommer K. R., Panyim S., Spiker S., Chalkley R. A modified procedure for fractionating histones. Biochem J. 1972 Sep;129(2):349–353. doi: 10.1042/bj1290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson E. C., Butler P. J., Thomas J. O. Higher-order structure of nucleosome oligomers from short-repeat chromatin. EMBO J. 1983;2(8):1367–1372. doi: 10.1002/j.1460-2075.1983.tb01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekvig O. P., Hannestad K. The specificity of human autoantibodies that react with both cell nuclei and plasma membranes: the nuclear antigen is present on core mononucleosomes. J Immunol. 1979 Dec;123(6):2673–2681. [PubMed] [Google Scholar]
- Rubin R. L., Joslin F. G., Tan E. M. Specificity of anti-histone antibodies in systemic lupus erythematosus. Arthritis Rheum. 1982 Jul;25(7):779–782. doi: 10.1002/art.1780250712. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Wolin S. L., Rinke J., Pettersson I., Mount S. M., Lerner E. A., Hinterberger M., Gottlieb E. Small ribonucleoproteins from eukaryotes: structures and roles in RNA biogenesis. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):893–900. doi: 10.1101/sqb.1983.047.01.103. [DOI] [PubMed] [Google Scholar]
- Stollar B. D., Ward M. Rabbit antibodies to histone fractions as specific reagents for preparative and comparative studies. J Biol Chem. 1970 Mar 25;245(6):1261–1266. [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables P. J., Charles P. J., Buchanan R. R., Yi T., Mumford P. A., Schrieber L., Room G. R., Maini R. N. Quantitation and detection of isotypes of anti-SS-B antibodies by ELISA and Farr assays using affinity purified antigens. An approach to the investigation of Sjögren's syndrome and systemic lupus erythematosus. Arthritis Rheum. 1983 Feb;26(2):146–155. doi: 10.1002/art.1780260205. [DOI] [PubMed] [Google Scholar]
- Von Holt C., Strickland W. N., Brandt W. F., Strickland M. S. More histone structures. FEBS Lett. 1979 Apr 15;100(2):201–218. doi: 10.1016/0014-5793(79)80337-3. [DOI] [PubMed] [Google Scholar]
- White P. J., Billings P. B., Hoch S. O. Assays for the Sm and RNP autoantigens: the requirement for RNA and influence of the tissue source. J Immunol. 1982 Jun;128(6):2751–2756. [PubMed] [Google Scholar]
- di Padua Mathieu D., Mura C. V., Frado L. L., Woodcock C. L., Stollar B. D. Differing accessibility in chromatin of the antigenic sites of regions 1-58 and 63-125 of histone H2B. J Cell Biol. 1981 Oct;91(1):135–141. doi: 10.1083/jcb.91.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden P. D., Strickland W. N., Strickland M., von Holt C. The complete amino-acid sequence of histone H2B from erythrocytes of the adult domestic fowl Gallus domesticus. Biochim Biophys Acta. 1982 Apr 21;703(1):17–20. doi: 10.1016/0167-4838(82)90004-8. [DOI] [PubMed] [Google Scholar]