Abstract
Climate change is expected to affect the Alps by increasing the frequency and intensity of summer drought events with negative impacts on ecosystem water resources. The response of CO2 and H2O exchange of a mountain grassland to natural fluctuations of soil water content was evaluated during 2001-2009. In addition, the physiological performance of individual mountain forb and graminoid plant species under progressive soil water shortage was explored in a laboratory drought experiment. During the 9-year study period the natural occurrence of moderately to extremely dry periods did not lead to substantial reductions in net ecosystem CO2 exchange and evapotranspiration. Laboratory drought experiments confirmed that all the surveyed grassland plant species were insensitive to progressive soil drying until very low soil water contents (<0.01 m3 m−3) were reached after several days of drought. In field conditions, such a low threshold was never reached. Re-watering after a short-term drought event (5±1 days) resulted in a fast and complete recovery of the leaf CO2 and H2O gas exchange of the investigated plant species. We conclude that the present-day frequency and intensity of dry periods does not substantially affect the functioning of the investigated grassland ecosystem. During dry periods the observed “water spending” strategy employed by the investigated mountain grassland species is expected to provide a cooling feedback on climate warming, but may have negative consequences for down-stream water users.
Keywords: climate change, drought, photosynthesis, evapotranspiration, montane ecosystem
Introduction
Low water availability represents the main environmental constraint for plant growth and productivity worldwide (Luterbacher et al., 2004). Reduced precipitation coupled with increased temperatures, that enhance evapotranspiration, are likely to be the main drivers of more intense and longer dry periods over larger areas of the globe (Trenberth et al., 2007). Changes in hydroclimatic fields appear to have already started to lead to a progressive decline in global net primary productivity, ecosystem CO2 sink strength (Zhao and Running, 2010) and evapotranspiration (Jung et al., 2010). This trend is affecting particularly the Southern hemisphere, but involves also temperate regions of the Northern hemisphere, the Mediterranean area and the western US (Ciais et al., 2005; Trenberth et al., 2007).
In the greater Alpine region (GAR) a comprehensive analysis of 200 years of precipitation data (Brunetti et al., 2006) indicates a trend towards an increase in total precipitation in the northern part and a highly significant decrease in the southern part. For both regions, a shift in rainfall contribution, from summer and autumn to winter and spring is observed. While regional climate change scenarios regarding the GAR are still highly uncertain (Smiatek et al., 2009), projected precipitation trends suggest further reductions in summertime precipitation (Rotach et al., 1997; Smiatek et al., 2009). Exacerbated by increasing temperatures (Calanca et al., 2006), a limited soil water supply in the GAR may strongly influence rates of carbon assimilated by plants, as well as the flux of evapotranspired water (Lawlor and Cornic, 2002; Loreto and Centritto, 2004).
Different strategies have been developed by plants to cope with stressful dry conditions (Larcher, 2001): hydrolabile water-spending plants are resilient to variations in their leaf water potential that lead to temporary wilting and thus tend to keep their stomata open until the water balance deficit becomes severe. In contrast, hydrostable water-saving plants act conservatively and adjust stomatal apertures to keep the internal water deficit within narrow safety margins. Progressive stomata closure in response to incipient soil water limitations restricts CO2 diffusion from the atmosphere to the chloroplasts directly down-regulating photosynthesis (Brilli et al., 2007; Cornic, 2000; Evans and Loreto, 2000). Extreme soil water scarcity leads to the onset of metabolic constraints that impair the photosynthetic machinery more permanently (Flexas et al., 2008; Jones, 1985). However at ecosystem level, the occurrence of a severe drought, like the 2003 European heat wave, has triggered a controversial response, since either a decrease in total carbon assimilation and respiration (Ciais et al., 2005; Hussain et al., 2001) or an enhanced net carbon exchange (Schmitt et al., 2010) have been reported.
Likely due to the considerable hydrologic surplus of the Alps, also referred to as the “Water towers of Europe” (Viviroli et al., 2007), several field studies confirmed that current dry periods do not represent a limiting factor for CO2 (e.g. Schmitt et al., 2010; Wohlfahrt et al., 2008) and water vapour (e.g. Hammerle et al., 2008; Wieser et al., 2008) exchange in mountain grasslands. Given present and projected future trends of decreasing summer precipitation (Brunetti et al., 2006; Rotach et al., 1997; Smiatek et al., 2009), it is uncertain whether the occurrence of more severe dry periods will affect ecosystems in the Alps in the foreseeable future. Because precipitation that is not evapotranspired feeds runoff (Wieser et al., 2008), any future changes in evapotranspiration (or lack thereof) will not only be of local or regional importance, but affect downstream water users as well.
Assessing the effects of limiting soil water availability in the Alps requires determining the sensitivity of plant species to drier conditions and their ability to recover from extremely stressful events. However, to date there are only few reports providing information on individual mountain plant species sensitivity to soil water shortage (Brock and Galen, 2005; Johnson and Caldwell, 1975; Jolly et al., 2005; Peterson and Billings, 1982), and no study so far has investigated the ability of individual mountain plant species to recover after experiencing progressive soil drying, or any antecedent or co-occurring stressors (e.g. exposure to pollutants, wounding by mowing practice, etc.). Future changing environmental conditions in mountain grasslands may hypothetically modify plant-plant interactions resulting in altered plant population composition and dynamics (Kardol et al., 2010; Klanderund, 2005).
In this study we aim to assess (i) the extent to which the present-day frequency and magnitude of soil drying causes stress to mountain grasslands in the Alps, (ii) whether representative mountain forb and graminoid plant species exhibit different degrees of sensitivity to progressive soil water shortage and possess the ability to recover after experiencing short-term extremely dry conditions, and (iii) to define a threshold for the vulnerability of mountain grasslands to projected reductions in summertime precipitation in the Alps. To this end we carried out laboratory leaf gas exchange measurements of key mountain forb and graminoid plant species to support a 9-year dataset of eddy covariance net ecosystem CO2 and H2O flux measurements recorded in a mountain grassland in Austria.
Material and Methods
Field study site
The study site is located near Neustift (47°07′N, 11° 19′E) in the Stubai Valley (Austria) in the montane altitudinal zone at an elevation of 970 m a.s.l. in the middle of the flat valley bottom. The fetch of the micrometeorological flux measurements is homogenous up to 300 m to the east and 900 m to the west of the instrument tower, the dominant day and night time wind directions, respectively (Bamberger et al., 2010).
The climate is of continental humid type with alpine influences. The 1980-2000 average annual temperature is 6.50 °C (monthly minima and maxima of −2.20°C and 15.40°C, respectively). Average (1980-2000) annual precipitation amounts to 852 mm, of which 15-20 % falls as snow during December-February, when the site is typically covered by snow.
The vegetation has been classified as a Pastinaco-Arrhenatheretum and consists of ca. 20 plant species dominated by a few graminoid (Dactylis glomerata, Festuca pratensis, Alopecurus pratensis, Trisetum flavescens) and forb (Ranunculus acris, Taraxacum officinalis, Trifolium repens, Trifolium pratense, Carum carvi) species that make up on average 35 % and 42 % of the above-ground phytomass, respectively. The remainder of the above-ground phytomass (23 %) is composed by fruits, inflorescences, attached dead plant matter and cryptogams. The site is managed as a hay meadow and is cut three times per year, typically by early June, beginning of August and the end of September. Maximum canopy height varies between <0.05 m, after snowmelt, to 1.00-1.20 m before the cutting events. Maximum amounts of green area (GAI) reach values of 7-8 m2 m−2.
The soil, which is approximately 1 m deep, has been classified as a Fluvisol (FAO classification) and overlies bedrock that constitutes a mix of limestone and silicate rock. The soil profile comprises (from top to bottom): a 0.001 m superficial thin organic layer, a 0.02 m A horizon having an organic volume fraction of approximately 14 % followed by the B horizon described as a (sandy) loam.
The vertical distribution of root mass was determined in summer 2005 from three replicate soil cores taken randomly at the site. Soil cores were divided into seven layers (0-0.03 m, 0.03-0.08 m, 0.08-0.13 m, 0.13-0.23 m, 0.23-0.38 m, 0.38-0.53 m and >0.53 m). Roots within each layer were washed free from the soil under running water and sieved for classification into four diameter classes (0-1 mm, 1-2 mm, 2-5 mm and >5 mm). Roots (all diameter classes pooled) reach down to 0.50 m, but 80 % of them are concentrated in the upper 0.13 m of the soil.
Plant material and laboratory experiment of drought
Seeds of Trifolium pratense L., Dactylis glomerata L., Ranunculus acris L., Taraxacum officinalis Weber ex F.H.Wigg., four species that make up a major fraction of the local vegetation, were collected at the study site. All collected seeds were transferred to small pots (0.55 dm3), filled with commercial soil (resembling the structure and composition of the experimental site soil) and grown for four months to a height of 0.10-0.30 m in the greenhouse facility of the Botanical Garden of the University of Innsbruck (Innsbruck, Austria). Light was artificially supplied by Osram-Power Start 1000 HQT lamps (Osram®, Munich, Germany) set to provide a light intensity of ~ 700 μmol photons m−2 s−1 during a 12-hour photoperiod. Prior to the soil drying experiments plants were regularly watered to full pot water capacity. Progressive soil water shortage was induced by withholding water and soil water content (SWC) of every single pot (at 0.05 m depth) was routinely measured before leaf gas exchange measurements using a soil water sensor (WET-1, Eijkelkamp Agrisearch Equipment BV Giesbeek, The Netherlands). The time until plants reached the lowest SWC value (<0.01 m3 m−3), i.e. the length of the drought treatment, was 5 ± 1 days. After these minimum SWC values were reached, pots were re-watered to full capacity.
Leaf gas exchange
Leaf gas exchange measurements were collected from 5 ± 1 different individual plants for each of the investigated species in the laboratory. Leaves were enclosed in a 200 cm3 cuvette designed for conifer needles measurements (6400-05 Conifer Chamber; Li-Cor, Lincoln, NE, USA) and flushed with 0.5 l min−1 of synthetic air consisting of N2 (80%), O2 (20%) and CO2 (370 μmol mol−1). Before CO2 was added, the air stream was humidified by bubbling through water. The relative humidity of the air entering the cuvette was set to 40-50% by condensing the excess humidity in a water bath. The cuvette was connected to a portable infrared gas analyzer (LI-6400; Li-Cor, Lincoln, NE, USA). Leaf temperature was continuously monitored during all the measurements and maintained at 28 ± 2 °C, which is close to the optimum temperature of net photosynthesis of typical mountain grassland species (Bahn et al., 1999). The enclosed leaves were exposed to a light intensity of ~ 700 μmol photons m−2 s−1 provided by an external source (Osram-Power Start 1000 HQT), which enables light saturation of photosynthesis in typical mountain grassland forb and graminoid species (Bahn et al., 1999). All leaf gas exchange measurements were performed on intact leaves within 10–15 min to achieve steady-state conditions. Leaf area was determined by tracing leaves on paper of known area to mass ratio and weighing the traces at the end of the experiment. Net photosynthesis (A), transpiration (T), stomatal conductance (gs) and intercellular CO2 concentration (Ci) were calculated by the LI-6400 software according to von Caemmerer and Farquhar (1981), with modifications to account for instrumental losses and geometry. Instantaneous water use efficiency (WUE) was calculated as the ratio of net photosynthesis to transpiration.
Means of leaf gas exchange parameters before, during and after the laboratory drying experiment were compared with Tukey’s test using SigmaPlot software version 11.0 (Systat Software Inc., Chicago IL, USA).
In order to mathematically describe the response of leaf net photosynthesis and stomatal conductance to reductions in SWC we fitted the data to the following empirical model (from Aubinet et al., 2001):
| Eq. (1) |
Here Y refers to either net photosynthesis or stomatal conductance, Ymax to the corresponding maximum value under non-stressed conditions, and SWC50 to the SWC where Y reaches 50 % of Ymax. Equation (1) explained 32-59 % and 34-66 % of the variation in leaf net photosynthesis and stomatal conductance of the four investigated species, respectively.
Eddy covariance (EC)
EC flux measurements at this site began in 2001 and measurements continue as of this writing. Within this paper data from the growing seasons 2001-2009 are presented. Net ecosystem CO2, latent and sensible energy exchange were measured at 3 m above ground by the eddy covariance method (Baldocchi et al., 1988) using the same instrumentation and following the procedures of the EUROFLUX project (Aubinet et al., 2000). For details regarding instrumentation and flux calculation procedures we refer to our earlier papers (Hammerle et al., 2008; Haslwanter et al., 2009; Wohlfahrt et al., 2008). In the following and as customary in the field we employ the micrometeorological sign convention according to which negative fluxes represent transport from the atmosphere towards the surface, positive ones the reverse.
Half-hourly flux data were screened for validity by removal of time periods with (1) the H2O and CO2 signals outside physically plausible ranges, (2) the coefficient of variation for H2O and CO2 concentration and pressure within the IRGA outside plausible ranges, (3) the third rotation angle exceeding ± 10° (McMillen, 1988), (4) the stationarity test exceeding 60% (Foken and Wichura, 1996), (5) the deviation of the integral similarity characteristics larger than 60% (Foken and Wichura, 1996), and (6) the maximum of the footprint function (Hsieh et al., 2000) outside the boundaries of the meadow (cf. Novick et al., 2004). In the following only original data, i.e. without filling data gaps (Falge et al., 2001a,b), are presented.
Gross primary production (GPP) was calculated as GPP = NEE – RECO, where RECO refers to ecosystem respiration which was estimated from nighttime NEE measurements and extrapolated to daytime conditions using functional relationships with soil temperature as described in Wohlfahrt et al. (2008).
As a surrogate for canopy stomatal conductance, the ratio of evapotranspiration to the vapour pressure deficit (ET/VPD; mmol m−2 s−1 kPa−1), which assumes soil evaporation to be negligible and leaf temperatures to equal air temperature, was calculated.
After visual assessment of linearity, ecosystem flux parameters were linearly regressed against time or soil water content (see below) using SPSS software version 17.0 (IBM Corp., Somers NY, USA).
Ancillary data
Supporting meteorological measurements of relevance to this study included: photosynthetically active radiation (PAR) (BF3H, Delta-T, Cambridge, UK); air temperature (TA) and humidity, from which the vapour pressure deficit (VPD) was calculated, measured at 2 m height above ground by the means of a temperature/humidity sensor (RFT-2, UMS, Munich, Germany); soil temperature (TS) at 0.05 m depth measured with a thermocouple (TCAV, Campbell Scientific, Logan, UT, USA); volumetric soil water content (ML2x, Delta-T Devices, Cambridge, UK); precipitation (52202, R.M. Young, Traverse City, MI, USA). From 2006 onwards soil water content was measured also at 0.10 and 0.20 m depth using the same instrumentation as at 0.05 m depth. During 2005 and 2006 soil water potential at 0.05 m depth was measured episodically in the field using two tensiometers (2725ARL, Soilmoisture Equipment Corp., Santa Barbara, CA, USA) and used to calculate the soil water content at field capacity (matrix potential of −10kPa) and wilting point (matrix potential of −1500kPa).
Because green, photosynthetically active stems of forbs and graminoids make up an appreciable fraction of the total phytomass in mountain grassland ecosystems (Wohlfahrt et al., 2001; on average 29 % at this site), the green plant area index (GAI; m2 m−2) was employed to quantify the photosynthesising/transpiring plant area instead of the commonly used leaf area index (LAI). GAI was assessed (1) in a destructive fashion by clipping of square plots of 0.09 m2 (3-5 replicates) and subsequent plant area determination (Li-3100, Li-Cor, Lincoln, NE, USA) and (2) from measurements of maximum canopy height during the vegetation periods of 2000-2005 which was related (R2 = 0.89, n = 34) to destructively measured GAI using the following relationship (Wohlfahrt et al., 2008):
| Eq. (2) |
where h is the canopy height (m). Continuous time series of the GAI were derived by fitting sigmoid and quadratic functions to measured data separately for each growing phase before and after the third cut, respectively (Wohlfahrt et al., 2008).
The Standardised Precipitation Index (SPI; McKee et al., 1993) is a standardising transform of the probability of observed precipitation. SPI values are positive (negative) for greater (lower) than median precipitation. The departure from zero is therefore a probability indication of the severity of wetness (positive SPI) or dryness (negative SPI). For example, SPI values of −1.00, −1.50 and −2.00 refer to a probability of exceedance of 15.87 %, 6.68 % and 2.28 %, respectively. Following McKee et al. (1993), SPI values in the range of −1.00 to −1.50, −1.50 to −2.00 and <−2.00 are referred to as moderately, severely and extremely dry conditions, respectively. SPI was calculated (using a software provided by the National Drought Mitigation Center, 2011) on a two-monthly basis using 1858-2009 monthly precipitation sums measured at the University of Innsbruck. A comparison between the 2001-2009 monthly precipitation sums (growing season only) from this data set and those measured at the study site showed no significant differences (slope and y-intercept of linear regression not significantly different from unity and zero, respectively, at p = 0.05; R2 = 0.67, n = 81) despite a 17 km distance.
Results
Observed periods of soil drying in the field
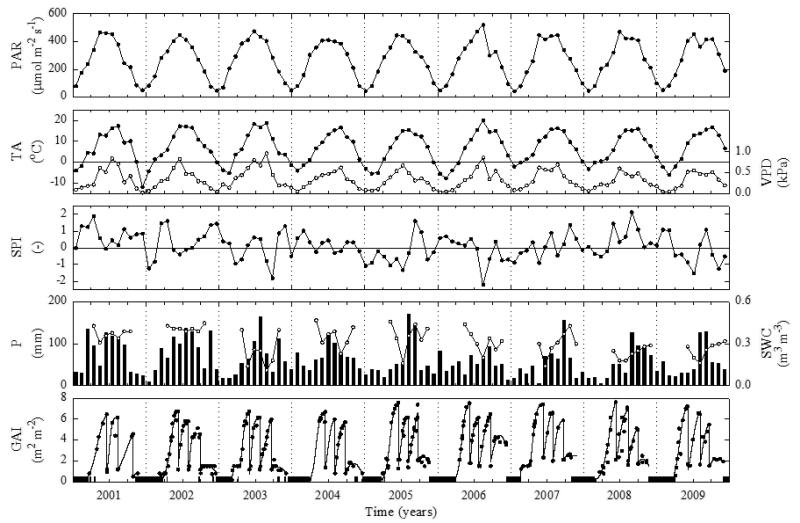
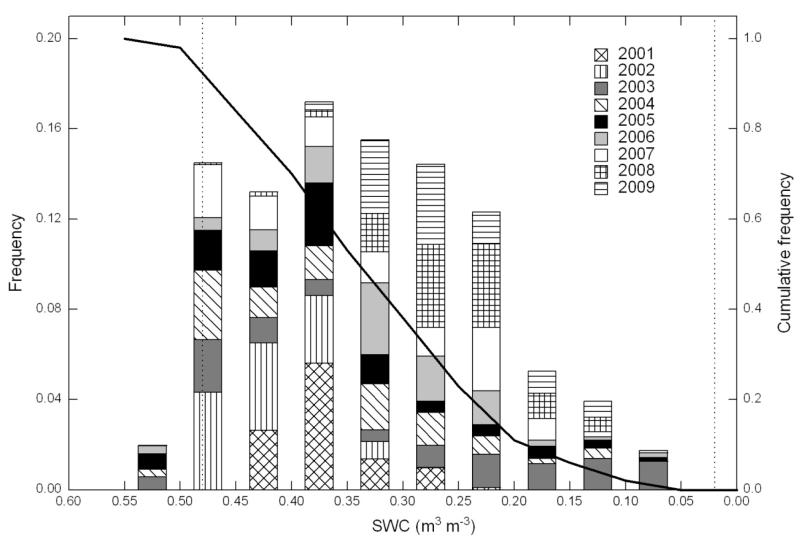
A general characterisation of the environmental conditions, in particular with regard to precipitation and soil water availability, during the 2001-2009 study period is presented in Fig. 1. Yearly total precipitation amounts were quite variable ranging from 582 mm (2006) to 984 mm (2002) with an average value of 731 mm, as opposed to the average value of 852 mm for the 1980-2000 period. During the main period of carbon uptake (April-September) rainfall occurred on average each second day, or every three days if days with < 1 mm precipitation are classified as dry. Employing the latter definition, a total of 9 periods with >10 consecutive dry days were encountered during the 9-year study period. The longest dry period occurred in 2003 and lasted for 15 days. The SPI fell below −1.00 several times during the 9-year study period indicating moderately dry conditions (McKee et al., 1993), once (2003) below −1.50 (severely dry) and once (2006) even somewhat below −2.00 (extremely dry). Around 92 % of the available half-hourly SWC measurements during the growing season were in the plant available water range (Fig. 2), i.e. between field capacity (0.48 m3 m−3) and wilting point (0.02 m3 m−3). Only 2% of the measurements fell into the lowest (0.10-0.05 m3 m−3) SWC class (Fig. 2). The wilting point was thus never reached under field conditions. The lowest measured SWCs occurred exclusively during the summers of 2003, 2005, 2006 and 2009 (Fig. 2) and were associated with moderately (2005), severely (2003, 2009) and extremely (2006) dry conditions identified by the SPI (Fig. 1). The seasonal development of the vegetation is illustrated in the lowermost panel of Fig. 1, showing rapid vegetation growth and re-growth in spring and after the three cutting events, respectively.
Figure 1.
Meteorological characterisation of the 2001-2009 study period, showing monthly averages of incident photosynthetically active radiation (PAR), air temperature (TA; closed symbols), vapour pressure deficit (VPD; open symbols), the Standardised Precipitation Index (SPI), volumetric soil water content (SWC; 0.05 m depth), monthly totals of precipitation (P), and daily average green area index (GAI). Black bars in the lowermost panel indicate snow cover duration, while symbols and lines indicate measurements of GAI and fits to measured GAI, respectively. Minimum GAI values during summertime correspond to meadow cuts occurring 3 times per year. SWC measurements during periods of soil frost may be unreliable due to frozen water and are thus not shown.
Figure 2.
Frequency distribution (bars) and cumulative frequency (solid line) of half-hourly volumetric soil water contents (SWC; 0.05 m depth) during the 2001-2009 study period. The dotted vertical lines refer to the SWC at field capacity (0.48 m3 m−3) and the wilting point (0.02 m3 m−3). SWC measurements during periods of soil frost may be unreliable due to frozen water and are thus not included. Note that the values on the x-axis are in descending order.
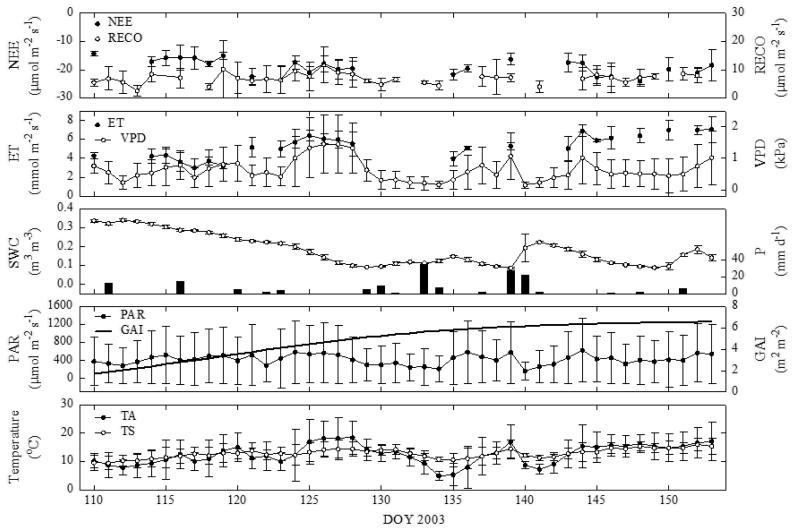
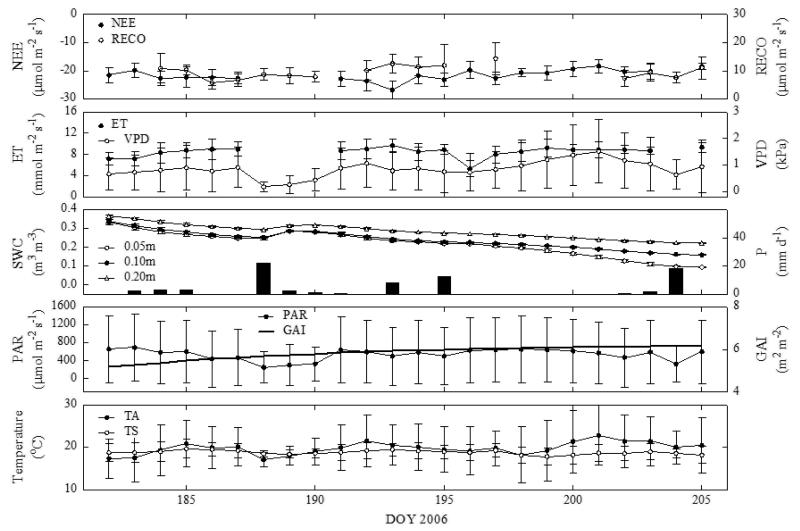
We illustrate the effects of decreasing SWC on the half-hourly net ecosystem CO2 exchange (NEE), nighttime NEE (RECO) and evapotranspiration (ET) by showing the two most severe dry periods during 2003 (SPI < −1.50) and 2006 (SPI < −2.00) in Fig. 3 and 4, respectively. In order to minimise confounding environmental effects, NEE and ET data have been filtered for dry sunny conditions with high evaporative demand, i.e. PAR > 1500 μmol m−2 s−1, VPD > 1 kPa and no precipitation, when restrictions due to soil drying would be expected to be most evident. RECO was filtered for PAR (< 50 μmol m−2 s−1) and no precipitation. Despite SWC dropping below 0.10 m3 m−3 during these two dry episodes, there was no significant reduction in observed NEE, RECO and ET (Fig. 3 and 4). In 2003 there even was a significant trend towards more negative NEE (i.e. larger net uptake of CO2; p < 0.05) and higher ET (p < 0.001) by the end of the dry period, which was due to a concurrent increase in GAI from 2 to 6 m2 m−2 (Fig. 3). Accounting for the change in GAI, NEE decreased and ET increased, on a unit green area basis. This reasoning is corroborated by the data from the 2006 drying period (Fig. 4), when due to the larger GAI and smaller increases in GAI over time (5.2-6.2 m2 m−2) no such trend in NEE and ET existed.
Figure 3.
Daily average net ecosystem CO2 exchange (NEE), night time ecosystem respiration (RECO), daytime evapotranspiration (ET), vapour pressure deficit (VPD), air (TA) and soil (TS; 0.05 m depth) temperature, incident photosynthetically active radiation (PAR), green area index (GAI), volumetric soil water content (SWC; 0.05 m depth) and daily totals of precipitation (P) during the first drying period in 2003. Error bars (± one standard deviation) show the dispersion in the filtered half-hourly data during each day.
Figure 4.
Same as Fig. 3, but for the 2006 drying period. Soil water contents at 0.1 and 0.2 m depth are shown in addition to 0.05 m. Note that soil drying continued until DOY 208, when a series of rain storms increased soil water content up to field capacity, but the grass was cut already on DOY 206 turning the site temporarily into a source of CO2 (Wohlfahrt et al., 2008).
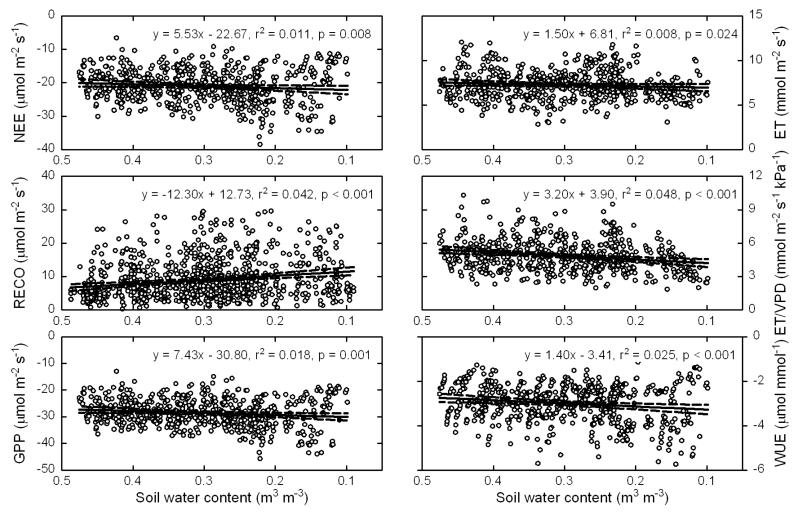
The pooled analysis of filtered data collected during the 2001-2009 study period (Fig. 5) confirms the findings of the two most pronounced periods of soil drying (Fig. 3 and 4). In order to minimise any above-mentioned confounding effects of inevitable changes in GAI over time, half-hourly data shown in Fig. 5 were filtered as described above for PAR, VPD and precipitation and in addition for GAI between 5-6 m2 m−2. These relatively high GAI values (Fig. 1) were selected because previous work (Hammerle et al., 2008; Wohlfahrt et al., 2008) showed that NEE and ET are relatively independent of GAI in this range, therefore isolating potential SWC effects on NEE and ET. Similar results were however obtained when data were filtered for lower GAI ranges (data not shown). Half-hourly NEE and GPP decreased (i.e. larger net uptake) with decreasing SWC (0.55 and 0.74 μmol m−2 s−1 for a 0.10 m3 m−3 change in SWC, respectively), while RECO increased (1.23 μmol m−2 s−1 for a 0.10 m3 m−3 change in SWC). Both ET and ET/VPD declined with progressively limiting SWC (Fig. 5), the decrease being more pronounced for ET/VPD, which suggests a reduction in surface conductance together with soil drying. WUE accordingly decreased (i.e. more net carbon gain per unit evapotranspired water) with decreasing SWC (Fig. 5). While the linear regressions shown in Fig. 5 were all significantly different from zero, changes in SWC explained less than 5 % of the variability in the data. We thus interpret Figure 5 to indicate that SWC is not a good predictor for other variables’ temporal variation.
Figure 5.
Daytime half-hourly measurements (symbols) from the period 2001-2009 of net ecosystem CO2 exchange (NEE), nighttime ecosystem respiration (RECO), gross primary production (GPP), evapotranspiration (ET), ET normalised by the vapour pressure deficit (VPD), and water use efficiency (WUE; NEE/ET) as a function of volumetric soil water content (SWC; 0.05 m depth). Solid and dashed lines represent linear regressions and associated 95% confidence intervals. Note that the values on the x-axis are in descending order.
Laboratory drought experiment
Under well-watered conditions, i.e. before soil drying was imposed, T. pratense and D. glomerata displayed the highest and the lowest A, respectively, with intermediate values recorded for the other two species (Table 1). Similarly, T. pratense showed the highest gs with R. acris, T. officinalis and D. glomerata exhibiting lower values (Table 1). However, on the basis of WUE, the investigated species ranked differently. T. officinalis exhibited the highest WUE, followed by T. pratense and with R. acris and D. glomerata showing lower values (Table 1).
Table 1.
Laboratory drought experiment
Net photosynthetic CO2 assimilation rate, stomatal conductance to H2O, intercellular CO2 concentration and water use efficiency measured in well-watered plants (W), under severe drought stress conditions (SWC < 0.01 m3m−3) (D) and one and three days after relief of water stress (1,3 DAR). Values represent means of five to six plants ± 1 standard deviation of the mean; different lowercase letters indicate significant differences at P < 0.05 among means within each plant species before and after imposing drought conditions and during the recovery period, while different capital letters indicate significant differences among means at P < 0.05 between well-watered plant species.
| Species | Net CO2 Assimilation (μmol m-2s-1) |
Stomatal conductance (mol m-2s-1) |
Intercellular [CO2] (μmol mol-1) |
WUE (μmol CO2 / mmol H2O) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W | D | 1DAR | 3 DAR | W | D | 1DAR | 3 DAR | W | D | 1DAR | 3 DAR | W | D | 1DAR | 3 DAR | |
| Trifolium pratense |
B16.82 ± 1.55a |
4.48 ± 2.08b |
16.53 ± 2.35a |
15.18 ± 1.69a |
A0.208 ± 0.020a |
0.041 ± 0.012b |
0.191 ± 0.020a |
0.220 ± 0.055a |
A378 ± 6a |
347 ± 15b |
366 ± 1ab |
368 ± 7ab |
B3.46 ± 0.10a |
4.42 ± 1.10a |
3.79 ± 0.45a |
3.14 ± 0.40a |
| Ranunculus acris |
A11.40 ± 1.98a |
1.95 ± 1.45b |
6.59 ± 3.99ab |
10.10 ± 0.88a |
B0.156 ± 0.033a |
0.048 ± 0.003b |
0.123 ± 0.086ab |
0.165 ± 0.047a |
A378 ± 6a |
380 ± 22a |
378 ± 6a |
381 ± 6a |
A2.41 ± 0.35a |
1.86 ± 1.52a |
2.29 ± 0.61a |
2.46 ± 0.62a |
|
Dactlylis
glomerata |
C6.52 ± 1.09a |
1.71 ± 1.63b |
7.36 ± 2.19a |
6.93 ± 1.18a |
B0.100 ± 0.021a |
0.022 ± 0.011b |
0.123 ± 0.045a |
0.122 ± 0.018a |
A369 ± 6a |
373 ± 20a |
369 ± 4a |
364 ± 11a |
A2.57 ± 0.37a |
2.69 ± 1.36a |
2.55 ± 0.28a |
2.38 ± 0.36a |
|
Taraxacum
officinalis |
A12.94 ± 1.41a |
2.26 ± 1.62b |
5.41 ± 2.00b |
10.53 ± 1.26a |
B0.128 ± 0.027a |
0.013 ± 0.008b |
0.060 ± 0.022b |
0.143 ± 0.035a |
B331 ± 6a |
342 ± 40a |
325 ± 5a |
332 ± 5a |
C4.47 ± 0.65a |
4.04 ± 1.76a |
3.76 ± 0.37ab |
3.26 ± 0.50b |
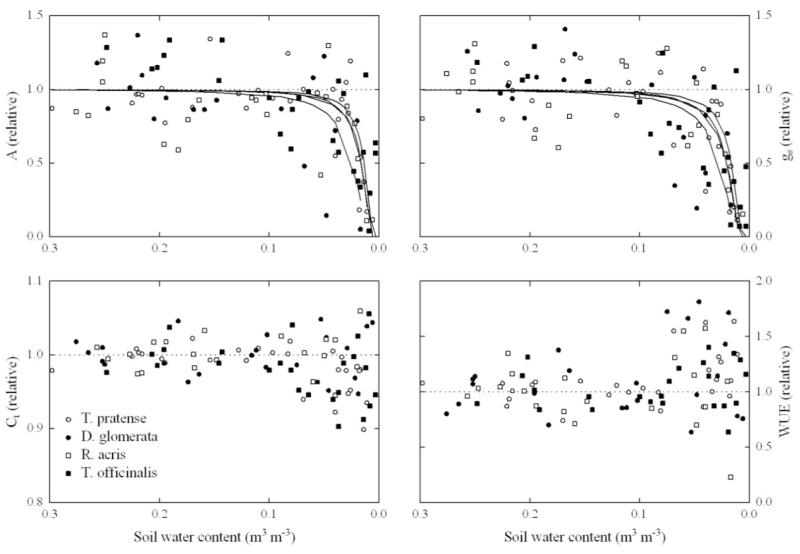
All the investigated plant species were insensitive to developing drought stress conditions until SWC approached very low values (< 0.10 m3 m−3), which resulted in a sudden drop of gs (Fig. 6). The fast decline in gs matched the decrease in A, since the calculated values of Ci remained fairly constant under limiting soil water conditions with a slight tendency towards higher values at the lowest SWCs recorded (Fig. 6), indicating a relatively larger reduction in gs as compared to A.
Figure 6.
Instantaneous leaf net photosynthetic CO2 assimilation rate (A), stomatal conductance to H2O (gs), intercellular CO2 concentration (Ci) and water use efficiency (WUE; A/transpiration) as a function of volumetric soil water content (SWC). Data have been normalised with their maximum values (Ymax) determined by fitting Eq. (1) to the data. Lines in the two upper panels represent fits to data using Eq. (1). Note that the values on the x-axis are in descending order.
Interestingly, our measurements revealed only minor differences between plant species when comparing the kinetic of A and gs responses to decreasing SWC. The SWC50 values, (i.e. the SWC at which A and gs reach 50% of their maximum values under un-stressed conditions) ranged between 0.014-0.024 and 0.017-0.021 m3 m−3 for A and gs, respectively (values not significantly different between species, p > 0.05). Only under conditions of very limited SWC (< 0.10 m3 m−3) did WUE increase in all the investigated plant species (Fig. 6). As soon as SWC approached the minimum values recorded (< 0.01 m3 m−3), plants were fully re-watered to the initial soil water levels. Already 24 hours after re-watering all physiological parameters recovered to their pre-stress values, except for T. officinalis, which completely recovered 3 days after re-watering (Table 1).
Discussion
In the present paper we have first evaluated the CO2 and water vapour exchange of a mountain grassland ecosystem in response to natural soil water fluctuations and then we examined ecosystem level CO2 and water vapour fluxes with respect of those measured at leaf level in four different key grassland plant species during a controlled laboratory drought experiment.
Our results highlight that the natural occurrence of moderately to extremely dry periods identified by the SPI (McKee et al., 1993) and ensuing low SWCs during the past 9 years (2001-2009) did not lead to substantial reductions of the net ecosystem CO2 and H2O exchange in the investigated mountain grassland ecosystem, confirming earlier studies at the same site based on a shorter data record (Hammerle et al., 2008; Wohlfahrt et al., 2008) and several differently managed sub-alpine grassland ecosystems in the same area (Schmitt et al., 2010). Laboratory measurements confirmed that the four investigated key grassland plant species are insensitive to progressive drought conditions until an extremely low SWC is achieved, a threshold that according to our measurements was hardly reached at our field site during the study period.
We do not exclude that the observed lack of trends (r2 < 0.042) in NEE, GPP and RECO with regard to SWC may have been caused by correlated changes in variables creating more favourable conditions for carbon assimilation and respiration other than lower soil water availability (e.g. higher temperatures). However, increased biomass productivity of mountain grasslands subject to imposed dry conditions has been reported also by Gilgen and Buchmann (2009) and was explained by improved soil oxygen availability. A higher soil oxygen concentration is expected to increase soil mineralisation rates and consequently nutrient availability, thus leading to enhanced root metabolic activity and microbial respiration, which in turn contributes to higher RECO and productivity (Gilgen and Buchmann, 2009). Our investigation of ecosystem-level CO2 fluxes may appear not consistent with Ciais et al. (2005), who reported significant reductions in both GPP and RECO for many investigated European ecosystems during the 2003 heat wave. However, two out of the 14 sites studied by Ciais et al. (2005), an evergreen coniferous forest in France and an open shrubland in Italy, likewise exhibited an increase in both GPP and RECO consistent with our results showing a limited sensitivity of CO2 and H2O fluxes to low SWC values. In contrast, both Ciais et al. (2005) and Hussain et al. (2011) report a decrease in GPP and RECO in 2003 for a grassland in France and Germany, respectively. Support for our results derives from a closely related study by Schmitt et al. (2010), who reported increased biomass and decreased NEE (i.e. more net uptake of CO2) in 2003 for several sub-alpine Austrian grasslands differing in land use.
Our laboratory measurements illustrate that under suitable environmental conditions, the key plant species constituting most of the investigated mountain ecosystem display significant differences in the absolute rates of photosynthesis and stomatal conductance (cf. Bahn et al., 1999). Under progressive soil water shortage, all the surveyed forb and graminoid plant species did not down-regulate stomatal conductance, in order to reduce internal water loss, as soil water became limiting until extremely low SWCs. Acting as “water spending” plants (Levitt, 1980), these mountain grassland plant species kept transpiration at maximum rates despite considerable reductions in soil water availability. Only when SWC approached critical levels < 0.10 m3 m−3, corresponding to a relative extractable soil water content of 0.17 (relative extractable soil water being defined as SWC scaled between field capacity and wilting point), did stomata suddenly close. Such a lack of gradual stomatal regulation to progressive soil water reduction likely represents the result of the evolutionary adaptation to an environment rarely subjected to scarce soil water availability.
The investigated grassland species demonstrated a different adaptation to soil drying as compared to the tree species in the 2003 European drought study by Granier et al. (2007). The plants investigated by Granier et al. (2007) apparently used water more conservatively, restricting ET, GPP and RECO already at relative extractable water contents of 0.20-0.40. Hammerle et al. (2008) speculated that the observed insensitivity of ET to low SWC at our site might be related to the fact that, while more than 80 % of the roots are in the upper 0.12 m of the soil volume, plant species present at the site may be able to take up water from greater depths (Miller et al., 2007). Notwithstanding this possibility, the present laboratory measurements enable us to demonstrate that relevant limitations to leaf gas exchange only occur at extremely low SWC levels (< 0.10 m3 m−3) hardly reached in natural field conditions, even at 0.05 m depth. Clearly, field conditions differ considerably from those created in laboratory experiments, in particular soil drying proceeded much faster with the potted plants due to the smaller exploitable soil volume and due to the lack of occasional convective re-wetting events (Fig. 3 and 4). Whether differences between in situ and laboratory soil drying affect the physiological behaviour of plant species remains to be investigated, preferably by manipulating soil moisture under field conditions (e.g. Gilgen and Buchmann, 2009). However, we reason that, in general, the conditions reproduced by laboratory drought studies are more stressful than in the field (Sinclair et al. 1986; Brilli et al. 2007; Centritto et al. 2011). Thus, our results confirming the insensitivity of the CO2 and H2O exchange to reductions in SWC in the field and in laboratory conditions are interpreted to show that the grassland plants that were used in our experiment are particularly resistant to stress. By exploring extremely lower SWCs which never observed in the field, our laboratory experiment not only supported field data, but also identified a possible threshold for gas exchange impairment, and showed that all the investigated plant species do not rapidly regulate stomatal conductance until SWC approaches the wilting point.
A fast and complete recovery in net photosynthesis and stomatal conductance was observed already one day after re-watering following a short term drying event. This indicates an excellent resilience of the photosynthetic machinery and the absence of biochemical or photochemical damage in the investigated mountain grassland species. Recovery from biochemical and photochemical photosynthetic limitations is generally slower (Lawlor and Cornic, 2002; Flexas et al., 2004a). The observed fast recovery rather indicates that the photosynthetic inhibition under fast-developing limitation of soil water conditions is solely related to diffusive limitations that are rapidly reverted. In fact, T. officinalis, the plant species showing the lowest Ci and the highest WUE even under control conditions, was the only species that did not recover promptly from the imposed dry event. In all cases, it is likely that we have only assessed part of the diffusive limitation and that the actual concentration of CO2 at the site of carboxylation was much lower in stressed leaves, because of the simultaneous reduction of mesophyll conductance (Galmes et al., 2007; Loreto et al., 1992). It has been shown that any reduction in mesophyll conductance can be also recovered very quickly, and may play an important role in temporarily reducing photosynthesis of stressed leaves (Centritto et al., 2003). However further investigations are required to estimate the mesophyll conductance of mountain grassland plant species and thus to discern the relative contribution of stomatal and mesophyll limitations to photosynthetic carbon assimilation under severe and prolonged dry conditions (Flexas et al., 2004b; Grassi and Magnani, 2005).
Ultimately, our results indicate that the productivity of the investigated mountain grassland ecosystem is resilient to present-day magnitudes and durations of soil drying, but may be vulnerable to the projected increased frequency and severity of summertime dry periods (Rotach et al., 1997; Smiatek et al., 2009). Assuming typical average daily evapotranspiration rates on the order of 3 mm d−1 (Wieser et al., 2008) and given an active soil volume down to a depth of 0.40 m (where > 99 % of the roots are located), it can be estimated that once soil water content reaches a threshold of 0.10 m3 m−3, at least 10 additional rainless days are required before SWC50 is approached and major limitations to net photosynthesis and stomatal conductance occur. This simple calculation is likely to represent an underestimation as it does not account for vertical water redistribution due to capillary rise and for higher SWCs at lower depths and may be put into perspective with the longest observed consecutive dry periods (i.e. 15 days in 2003). It is thus reasonable to speculate that soil water shortage is not going to impair the productivity of the investigated mountain grassland in the foreseeable future. As a consequence, mountain grasslands are expected to provide a negative (cooling) feedback effect on air temperature under present-day combined dry and heat episodes (Teuling et al., 2010). However, once critical low SWCs are reached, our results suggest a rapid and dramatic decline in both ecosystem CO2 assimilation and evapotranspiration.
As none of the investigated plant species showed a superior physiological performance and a distinct water saving strategy under limiting SWC, we expect minor consequences of dry period occurrence on species interactions within the existing plant community. Once critically low soil water contents are reached, other (non-native or invasive) plant species with a more conservative water use strategy and different suites of physiological and morphological traits conferring persistence under dry conditions may however be expected to gain competitive advantage over the existing plant association (Brock and Galen, 2005; Kardol et al., 2010; Klanderund, 2005).
The aggressive use of water resources by the investigated grassland plant species may have important consequences for the future role of the Alps as the “water towers of Europe” (Viviroli et al., 2007). As shown by Wieser et al. (2008), grassland mountain ecosystems evapotranspire around 50 % of precipitation during wet, but up to 90 % during dry years. In other words, the amount of water available for deep drainage and run-off is dramatically reduced during dry years, partially due to the observed low water use efficiency exhibited by the grassland vegetation under present-day conditions. Although river discharge from the Alps is also fuelled by run-off of water from non-grassland areas (in particular from melting glaciers; Pelliccioti et al., 2010), the “water spending” strategy employed by the plant species of the investigated mountain grassland ecosystem may contribute to the recession of stream flow during dry periods and thus affect the water supply to the low-land areas surrounding the Alps (Viviroli et al., 2007; Vanham et al., 2009).
Acknowledgements
This study was financially supported by the Austrian National Science Fund under contracts P17560-B16 and P19849-B16, the Tyrolean Science Fund under contract UNI-0404/33 and UNI-0404/486, the EU FP5 project CarboMont (EVK2-CT2001-00125) and the EU Marie Curie IAPP project 218065. Family Hofer (Neustift, Austria) is thanked for granting us access to the study site, the staff of the Botanical Garden of the Institute of Botany of the University of Innsbruck for raising the experimental plants. Long-term temperature and precipitation data have been generously provided by the Austrian Hydrographic Service and the HISTALP project (http://www.zamg.ac.at/histalp/).
References
- Aubinet M, Grelle A, Ibrom A, Rannik Ü, Moncrieff J, Foken T, Kowalski AS, Martin PH, Berbigier P, Bernhofer Ch., Clement R, Elbers J, Granier A, Grünwarld T, Morgenstern K, Pilegaard K, Rebmann C, Snijders W, Valentini R, Vesala T. Estimates of the annual net carbon and water exchange of forest: the EUROFLUX methodology. Adv. Ecol. Res. 2000;30:113–175. [Google Scholar]
- Aubinet M, Chermanne B, Vandenhaute M, Longdoz B, Yernaux M, Laitat E. Long term carbon dioxide exchange above a mixed forest in the Belgian Ardennes. Agric. For. Meteorol. 2001;108(4):293–315. [Google Scholar]
- Bahn M, Wohlfahrt G, Haubner E, Horak I, Michaeler W, Rottmar K, Tappeiner U, Cernusca A. Leaf photosynthesis, nitrogen contents and specific leaf area of grassland species in mountain ecosystems under different land-use. In: Cernusca A, Tappeiner U, Bayfield N, editors. Land-use changes in European mountain ecosystems ECOMONT – concept and results. Blackwell Wissenschaftsverlag; Berlin: 1999. pp. 247–255. [Google Scholar]
- Baldocchi DD, Hicks BB, Meyers TP. Measuring biosphere-atmosphere exchanges of biologically related gases with micrometeorological methods. Ecology. 1988;69:1331–1340. [Google Scholar]
- Bamberger I, Hörtnagl L, Schnitzhofer R, Graus M, Ruuskanen TM, Müller M, Dunkl J, Wohlfahrt G, Hansel A. BVOC fluxes above mountain grassland. Biogeosciences. 2010;7:1413–1424. doi: 10.5194/bg-7-1413-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Barta C, Fortunati A, Lerdau M, Loreto F, Centritto M. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007;175:244–254. doi: 10.1111/j.1469-8137.2007.02094.x. [DOI] [PubMed] [Google Scholar]
- Brock M, Galen C. Drought tolerance in the alpine dandelion, Taraxacum ceratophorum (Asteraceae), its exotic congener T. officinale, and interspecific hybrids under natural and experimental conditions. Am. J. Bot. 2005;92:1311–1321. doi: 10.3732/ajb.92.8.1311. [DOI] [PubMed] [Google Scholar]
- Brunetti M, Maugeri M, Nanni T, Auer I, Böhm R, Schöner W. Precipitation variability and changes in the greater Alpine region over the 1800-2003 period. J. Geophys. Res. 2006;111:D11107. doi:10.1029/2005JD006674. [Google Scholar]
- Calanca P, Roesch A, Jasper K, Wild M. Global warming and the summertime evapotranspiration regime of the Alpine region. Clim. Change. 2006;79:65–78. [Google Scholar]
- Ciais P, Reichstein M, Viovy N, Granier A, Ogée J, Allard V, Aubinet M, Buchmann N, Bernhofer C, Carrara A, Chevallier F, De Noblet N, Friend AD, Friedlingstein P, Grünwald T, Heinesch B, Keronen P, Knohl A, Krinner G, Loustau D, Manca G, Matteucci G, Miglietta FJ, Ourcival M, Papale D, Pilegaard K, Rambal S, Seufert G, Soussana JF, Sanz MJ, Schulze ED, Vesala T, Valentini R. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437:529–533. doi: 10.1038/nature03972. [DOI] [PubMed] [Google Scholar]
- Centritto M, Loreto F, Chartzoulakis K. The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ. 2003;26:585–594. [Google Scholar]
- Centritto M, Brilli F, Fodale R, Loreto F. Different sensitivity of isoprene emission, respiration and photosynthesis to high growth temperature coupled with drought stress in black poplar (Populus nigra) saplings. Tree Physiology. 2011;31:275–286. doi: 10.1093/treephys/tpq112. [DOI] [PubMed] [Google Scholar]
- Cornic G. Drought stress inhibits photosynthesis by decreasing stomatal apertures – not by affecting ATP synthesis. Trends Plant Sci. 2000;5:187–188. [Google Scholar]
- Evans JR, Loreto F. Acquisition and diffusion of CO2 in higher plant leaves. In: Leegood RC, Sharkey TD, von Caemmerer S, editors. Photosynthesis: Physiology and Metabolism. Kluwer Academic Publishers; Dordrecht: 2000. pp. 321–351. [Google Scholar]
- Falge E, Baldocchi D, Olson R, Anthoni P, Aubinet M, Bernhofer Ch., Burba G, Ceulemans R, Clement R, Dolman H, Granier A, Gross P, Grünwald T, Hollinger D, Jenson N-O, Katul G, Keronen P, Kowalski A, Lai CT, Law B, Meyers T, Moncrieff J, Moors EJ, Munger W, Pilegaard K, Rannik Ü, Rebmann C, Sukyer A, Tenhunen J, Tu K, Verma S, Vesala T, Wilson K, Wofsy S. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 2001a;107:43–69. [Google Scholar]
- Falge E, Baldocchi D, Olson R, Anthoni P, Aubinet M, Bernhofer Ch., Burba G, Ceulemans R, Clement R, Dolman H, Granier A, Gross P, Grünwald T, Hollinger D, Jenson N-O, Katul G, Keronen P, Kowalski A, Lai CT, Law B, Meyers T, Moncrieff J, Moors EJ, Munger W, Pilegaard K, Rannik Ü, Rebmann C, Sukyer A, Tenhunen J, Tu K, Verma S, Vesala T, Wilson K, Wofsy S. Gap filling strategies for long-term energy flux data sets, a short communication. Agric. For. Meteorol. 2001b;107:71–77. [Google Scholar]
- Flexas J, Ortuño MF, Ribas-Carbo M, Diaz-Espejo A, Flórez-Sarasa ID, Medrano H. Mesophyll conductance to CO2 in Arabidopsis thaliana. New Phytol. 2004a;175:501–511. doi: 10.1111/j.1469-8137.2007.02111.x. [DOI] [PubMed] [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004b;6:269–279. doi: 10.1055/s-2004-820867. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ. 2008;31:602–621. doi: 10.1111/j.1365-3040.2007.01757.x. [DOI] [PubMed] [Google Scholar]
- Foken T, Wichura B. Tools for quality assessment of surface-based flux measurements. Agric. For. Meteorol. 1996;78:83–105. [Google Scholar]
- Galmes J, Medrano H, Flexas J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007;175:81–93. doi: 10.1111/j.1469-8137.2007.02087.x. [DOI] [PubMed] [Google Scholar]
- Gilgen AK, Buchmann N. Response of temperate grasslands at different altitudes to simulated summer drought differed but scaled with annual precipitation. Biogeosciences. 2009;6:2525–2539. [Google Scholar]
- Granier A, Reichstein M, Bréda N, Janssens IA, Falge E, Ciais P, Grünwald T, Aubinet M, Berbigier P, Bernhofer Ch., Buchmann N, Facini O, Grassi G, Heinesch B, Ilvesniemi H, Keronen P, Knohl A, Köstner B, Lagergren F, Lindroth A, Longdoz B, Loustau D, Mateus J, Montagnani L, Nys C, Moors E, Papale D, Peiffer M, Pilegaard K, Pita G, Pumpanen J, Rambal S, Rebmann C, Rodrigues A, Seufert G, Tenhunen J, Vesala T, Wang Q. Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year: 2003. Agric. For. Meteorol. 2007;143:123–145. [Google Scholar]
- Grassi G, Magnani F. Stomatal mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005;28:834–849. [Google Scholar]
- Hammerle A, Haslwanter A, Tappeiner U, Cernusca A, Wohlfahrt G. Leaf area controls on energy partitioning of a temperate mountain grassland. Biogeosciences. 2008;5:421–431. doi: 10.5194/bg-5-421-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslwanter A, Hammerle A, Wohlfahrt G. Open- vs. closed-path eddy covariance measurements of the net ecosystem carbon dioxide and water vapour exchange: a long-term perspective. Agric. For. Meteorol. 2009;149:291–302. doi: 10.1016/j.agrformet.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CI, Katul G, Chi TZ. An approximate analytical model for footprint estimation of scalar fluxes in thermally stratified atmospheric flows. Adv. Water Resour. 2000;23:765–772. [Google Scholar]
- Hussain MZ, Grünwald T, Tenhunen JD, Li YL, Mirzae H, Bernhofer C, Otieno D, Dinh NQ, Schmidt M, Wartinger M, Owen K. Summer drought influence on CO2 and water fluxes of extensively managed grassland in Germany. Agric. Ecosyst. Environ. 2011;141:67–76. [Google Scholar]
- Jones HG. Partitioning stomatal and non-stomatal limitations to photosynthesis. Plant Cell Environ. 1985;8:95–104. [Google Scholar]
- Jolly WM, Dobbertin M, Zimmermann NE. Divergent vegetation growth responses to the 2003 heat wave in the Swiss Alps. Geophys. Res. Lett. 2005;32:L18409. doi: 10.1029/2005GL023252. [Google Scholar]
- Johnson DA, Caldwell MM. Gas exchange of four arctic and alpine tundra plant species in relation to atmospheric and soil moisture stress. Oecologia. 1975;21:91–108. doi: 10.1007/BF00345552. [DOI] [PubMed] [Google Scholar]
- Jung M, Reichstein M, Ciais P, Seneviratne SI, Sheffield J, Goulden ML, Bonan G, Cescatti A, Chen J, de Jeu R, Dolman AJ, Eugster W, Gerten D, Gianelle D, Gobron N, Heinke J, Kimball J, Law BE, Montagnani L, Mu Q, Mueller B, Oleson K, Papale D, Richardson AD, Roupsard O, Running S, Tomelleri E, Viovy N, Weber U, Williams C, Wood E, Zaehle S, Zhang K. Recent decline in the global land evapotranspiration trend due to limited moisture supply. Nature. 2010;467:951–954. doi: 10.1038/nature09396. [DOI] [PubMed] [Google Scholar]
- Kardol P, Campany CE, Souza L, Norby RJ, Weltzin JF, Classen AT. Climate change effects on plant biomass alter dominance patterns and community evenness in an experimental old-field ecosystem. Global Change Biol. 2010;16:2676–2687. [Google Scholar]
- Klanderund K. Climate change effects on species interactions in an alpine plant community. J. Ecol. 2005;93:127–137. [Google Scholar]
- Larcher W. Ökophysiologie der Pflanzen. Ulmer-Verlag Stuttgart. 2001.
- Lasslop G, Reichstein M, Papale D, Richardson A, Arneth A, Barr A, Stoy P, Wohlfahrt G. Separation of net ecosystem CO2 exchange into assimilation and respiration using a light response curve approach: critical issues and global evaluation. Global Change Biol. 2010;16:187–208. [Google Scholar]
- Lawlor DW, Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficit in higher plants. Plant Cell Environ. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- Levitt J. Responses of plants to environmental stresses. Academic Press; New York: 1980. [Google Scholar]
- Loreto F, Harley PC, Di Marco G, Sharkey TD. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiol. 1992;98:1437–1443. doi: 10.1104/pp.98.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Centritto M. Photosynthesis in a changing world. Plant Biol. 2004;6:239–241. doi: 10.1055/s-2004-820885. [DOI] [PubMed] [Google Scholar]
- Luterbacher J, Dietrich D, Xoplaki E, Grosjean M, Wanner H. European seasonal and annual temperature variability, trends and extremes since 1500. Science. 2004;303:1499–1503. doi: 10.1126/science.1093877. [DOI] [PubMed] [Google Scholar]
- McKee TB, Doesken NJ, Kleist J. The relation of drought frequency and duration to time scales. Proceedings of the Eighth Conference on Applied Climatology; Boston. American Meteorological Society; 1993. pp. 179–184. [Google Scholar]
- McMillen RT. An eddy correlation technique with extended applicability to non-simple terrain. Boundary-Layer Meteorol. 1988;43:231–245. [Google Scholar]
- Miller GR, Baldocchi DD, Law BE, Meyers T. An analysis of soil moisture dynamics using multi-year data from a network of micrometeorological observation sites. Adv. Water Resour. 2007;30:1065–1081. [Google Scholar]
- National Drought Mitigation Center 2011 http://drought.unl.edu/monitor/spi/program/spi_program.htm.
- Novick KA, Stoy PC, Katul GG, Ellsworth DS, Siqueira MBS, Juang J, Oren R. Carbon dioxide and water vapor exchange in a warm temperate grassland. Oecologia. 2004;138:259–274. doi: 10.1007/s00442-003-1388-z. [DOI] [PubMed] [Google Scholar]
- Pelliccioti F, Bauder A, Parola M. Effect of glaciers on streamflow trends in the Swiss Alps. Water Resour. Res. 2010;46:W10522. doi:10.1029/2009WR009039. [Google Scholar]
- Peterson KM, Billings WD. Growth of Alpine plants under controlled drought. Arctic Alpine Res. 1982;14:189–194. [Google Scholar]
- Rotach MW, Marinucci MR, Wild M, Tschuck P, Ohmura A, Beniston M. Nested regional simulation of climate change over the Alps for the scenario of a doubled greenhouse forcing. Theor. Appl. Climatol. 1997;57:209–227. [Google Scholar]
- Schmitt M, Bahn M, Wohlfahrt G, Tappeiner U, Cernusca A. Land use affects the net ecosystem CO2 exchange and its components in mountain grasslands. Biogeosciences. 2010;7:2297–2309. doi: 10.5194/bg-7-2297-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Ludlow MM. Influence of soil water supply on the plant water balance of four tropical grains legumes. Australian Journal of Plant Physiology. 1986;13:329–341. [Google Scholar]
- Smiatek G, Kunstmann H, Knoche R, Marx A. Precipitation and temperature statistics in high-resolution regional climate models: Evaluation for the European Alps. J. Geophys. Res. 2009;114:D19107. doi:10.1029/2008JD011353. [Google Scholar]
- Teuling AJ, Seneviratne SI, Stöckli R, Reichstein M, Moors E, Ciais P, Luyssaert S, van den Hurk B, Ammann C, Bernhofer C, Dellwik E, Gianelle D, Gielen B, Grünwald T, Klumpp K, Montagnani L, Moureaux C, Sottocornola M, Wohlfahrt G. Contrasting response of European forest and grassland energy exchange to heat waves. Nature Geoscience. 2010;3:722–727. [Google Scholar]
- Trenberth KE, Josey PD, Ambenje P, Bojariu R, Easterling D, Klein Tank A, Parker D, Rahimzadeh F, Renwick JA, Rusticucci M, Soden B, Zhai P. Observations: surface and atmospheric climate change. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2007. pp. 235–336. [Google Scholar]
- Vanham D, Fleischhacker E, Rauch W. Impact of an extreme dry and hot summer on water supply security in an alpine region. Water Sci. Technol. 2009;59:469–477. doi: 10.2166/wst.2009.887. [DOI] [PubMed] [Google Scholar]
- Viviroli D, Dürr HD, Messerli B, Meybeck M, Weingartner R. Mountains of the world, water towers for humanity: typology, mapping and global significance. Water Resour. Res. 2007;43 doi:10.2029/2006WR005653. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Wieser G, Hammerle A, Wohlfahrt G. The water balance of grassland ecosystems in the Austrian Alps. Arctic Antarctic Alpine Res. 2008;40:439–445. doi: 10.1657/1523-0430(07-039)[WIESER]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfahrt G, Sapinsky S, Tappeiner U, Cernusca A. Estimation of plant area index of grasslands from measurements of canopy radiation profiles. Agric. For. Meteorol. 2001;109:1–12. [Google Scholar]
- Wohlfahrt G, Hammerle A, Haslwanter A, Bahn M, Tappeiner U, Cernusca A. Seasonal and inter-annual variability of the net ecosystem CO2 exchange of a temperate mountain grassland: Effects of weather and management. J. Geophys. Res. 2008;113:D08110. doi: 10.1029/2007jd009286. doi:10.1029/2007JD009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Running SW. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science. 2010;329:940–943. doi: 10.1126/science.1192666. [DOI] [PubMed] [Google Scholar]