Abstract
Background:
In patients with Ovarian Cancer (OvCa) exosomes released by tumor cells are present in the plasma and could be involved in tumor progression. This study examines the association between the exosome presence/protein content in plasma of OvCa patients and disease outcome, response to standard therapy and/or tumorresistance to therapies in patients studied at diagnosis and also serially during and after therapy.
Design and methods:
Exosomes were purified from OvCa patients’ plasma (n=22), patients with benign tumors (n=10) or (n=10) healthy controls (NC) using ultracentrifugation. Exosomes were visualized by scanning electron microscopy. Their protein content was measured. The presence of MAGE 3/6 and TGF-β1 in exosomes was evaluated in Western blots.
Results:
The OvCa patients’ plasma contained higher levels of exosomal proteins (p<0.05) compared to those isolated from plasma of patients with benign tumors or NC. Exosomes isolated from OvCa patients’s plasma carried TGF-β1 and MAGE3/6, which distinguished OvCa patients from those with benign tumors and NC. High protein levels of exosomes were seen in newly diagnosed patients; however in advanced stages of OvCa patients the protein content of isolated exosomes was significantly higher than that of early stages. The exosome levels variably changed during/after chemotherapy, and correlations between the changes in exosomal protein levels and clinical data suggested that the protein content of exosomes might be useful in predicting responses to therapy and prognosis in OvCa patients.
Conclusion:
Analysis of plasma exosomes levels offers a novel approach to diagnosis and monitoring response to therapies in OvCa patients.
Keywords: Exosomes, Ovarian carcinoma, Response to therapy, Prognosis
Introduction
The majority of women diagnosed with Ovarian Cancer (OvCa) already have advanced stage disease, limiting treatment options and contributing to a high risk of OvCa mortality. The incidence and mortality of OvCa has not appreciably changed in the past 50 years [1]. The lack of progress has been attributed mainly to local and regional recurrences, especially in patients with the stage III or IV disease. Moreover, there are currently no reliable, specific or sensitive markers for the detection and evaluation of OvCa progression.
Human tumors develop numerous and various mechanisms of suppression which target the host anti-tumor immune system and thus promote their own progression and survival [2,3]. Few years ago, it was observed that sera of patients with cancer, but not from normal controls (NC), suppressed functions of circulating CD8+ effector T cells and induced their apoptosis [4,5]. Subsequently, these suppressive effects were found to be mediated by small membraneous vesicles, exosomes, present in body fluids of cancer patients, which ranged in diameter from 50 to 100 nm and could be visualized by transmission electron microscopy [6].
Exosomes are virus-size, double-membrane bound vesicles that have a molecular composition resembling that of cell surface membranes in the mother cells [7]. All cells release exosomes either constitutively or upon activation/stress [8], and tumor cells are avid producers of exosomes. Exosomes are formed in the multivesicular bodies and are actively released through an exocytosis pathway normally used for elimination of unnecessary proteins [9]. Data indicate that Tumor-Derived Exosomes (TEX) differ from circulating exosomes found in the plasma of healthy individuals both in quantity and in the protein content [6]. The role, TEX play in cancer progression has been recently emerging, in large part due to the evidence that exosomes isolated from body fluids of cancer patients modulate functions of immune cells and thus interfere with the development of anti-tumor responses [10]. In addition to inducing apoptosis in activated CD8+ T cells, TEX inhibit NK cells functions [11], promote differentiation, expansion and suppressor function of regulatory T cells [12] impair myeloid precursor differentiation into dendritic cells [13]. Further, more recent evidence shows that TEX can mediate diverse biological functions, including the promotion of tumor growth and metastasis, enhancement of angiogenesis and alterations in functions of stromal cells in the Extracellular Matrix (ECM) thus promoting tumor invasiveness [14]. The exosomal cargo contains proteins, lipids, enzymes, cytokines and nucleic acids, including mRNA and miRNA, and exosomes serving as communication vehicles are able to deliver this cargo to distant recipient cells and alter their functions [14,15]. Taylor et al. [16] have reported that exosomes obtained from plasma of OvCa patients contained significant levels of mRNA and miRNA, providing an explanation for the ability of TEX to transfer genetic material between the cells. Another group has reported specific expression patterns of exosomal miRNA for lung cancer, colorectal cancer and diabetes, suggesting that miRNa in exosomes might serve as disease biomarkers [17]. Also, exosomal surviving was found to be highly enriched in TEX derived from plasma of patients with prostate cancer [18], while in another study of exosomes in melanoma, caveolin-1 was shown to be enriched [19], and in both cases, levels of these tumor-associated proteins were higher in patients with advanced than early disease or normal controls. These and other studies suggest that the abundance of TEX in plasma of patients with cancer could inform about the disease activity and potentially offer an opportunity for monitoring changes in response to therapy. If exosomes found in plasma or body fluids of cancer patients contain tumor cell-specific proteins and RNA, then their use as potential biomarkers of disease progression might be a reasonable option to consider.
The aim of this study was to investigate the protein levels and a partial molecular content of exosome isolated from plasma of OvCa patients with different clinical presentations at diagnosis and to retrospectively relate these findings with disease outcome.
In addition, changes in exosomal protein content during conventional chemotherapy were related to responses/tumor- cell resistance to therapy in patients serially studied for exosome protein levels.
Material and Methods
Patients and specimens
Plasma samples were collected from total of 22 OvCa patients and 10 patients diagnosed with serous cysts (benign conditions). Normal sera (n=10) obtained from age-matched volunteers were used as controls. For 12 OvCa patients plasma samples were obtained from newly diagnosed and later from the same patients during chemotherapy treatments (first-line chemotherapy and second-line chemotherapy). The use of patients’ samples was approved by the Ethics Committee of the University of Medical Sciences in Poznan, Poland. Histologic diagnoses, including tumor grade, were determined by the WHO criteria and were confirmed by reviews of the original H&E tissue sections. The Silverberg grading system for OvCa was used, and the grading was based on nuclear grade, architectural tumor grade and mitotic count. The clinical stage was assessed by using the International Federation of Gynecology and Obstetrics (FIGO) criteria for stage I to IV cancers. Venous blood samples were collected in green-top vacutainer tubes. Specimens were centrifuged at 2000 ×g for 15 minutes, and the plasma was removed and aliquoted for storage at −80°C until further use.
Exosome isolation from plasma
Plasma exosomes were isolated as previously described but with minor modification [20]. Briefly, thawed plasma samples were centrifuged at 500 × g for 30 min at 4 °C to remove cell debris and then 12,000 × g for 45 min to sediment larger micro vesicles. Next, plasma was filtered through a 0.22 µm bacterial filter (Costar, Corning Incorporated, USA) to remove particles larger than 200 nm. Aliquots (300 µl) of plasma were resuspended in Phosphate-Buffered Saline (PBS), transferred to ultracentrifuge tubes and ultra centrifuged at 120,000 × g for 90 min at 4°C, using a Beckman Ti70.1 rotor [21]. The exosome pellet was diluted in 50 µl of RIPA buffer with protease inhibitor (Sigma-Aldrich, St.Louis, USA). The protein level of the pellet was determined using the BCA protein assay kit (Sigma-Aldrich, St. Louis, USA). Freshly-isolated exosomes were immediately used for immunoblotting.
Scanning Electron Microscopy (SEM)
Pelleted exosomes were resuspended in PBS, spread on double-sided adhesive tape sticking to an aluminum table (SPI Supplies/Structure Probe, Inc., USA), evaporated by using a vacuum concentrator (Eppendorf, Hamburg, Germany) at 30°C and coated with gold by sputter coating (Balzer model SCP 50). Pictures were taken by Scanning Electron Microscope Evo 40 Series (Carl Zeiss, Oberkochen, Germany).
Western blot assays
Isolated exosomes were lysed using a RIPA buffer (Sigma-Aldrich Co.) containing 50 mM Tris-HCl, pH 8.0, 150 mM sodium chloride, 1.0% Igepal, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate and the protease inhibitor cocktail. The total protein concentration in exosomes extracts was determined by the Bradford assay. Subsequently, exosomes were resuspended in the loading buffer and boiled at 99°C for 5 min. Next, all samples were electrophoresed using SDS-PAGE, as previously described. Proteins were transferred to polyvinylidene fluoride membrane (Roche Diagnostics GmbH, Mannheim, Germany), which was blocked with 5% non-fat dry milk in Tris buffered saline/Tween 20. Immunodetection was performed with LAMP-1 Ab (Santa Cruz-sc-5570) at the dilution of 1:500, TGF-beta (Santa Cruz-sc-31608) (1:500) or MAGE 3/6 (Santa Cruz-sc-130810) (1:250) followed by incubation with appropriate HRP-conjugated secondary Ab (1:5000). Bands were revealed using Pierce ECL Plus Western Blotting Substrate (Thermo Fisher Scientific, Rockford, IL). The membranes were stripped and re-blotted with rabbit polyclonal anti-GAPDH Ab (FL-335) at a dilution of 1:2500, followed by goat anti-rabbit HRP-conjugated Ab (1:10000), to provide controls for equal protein loading.
Clinical evaluations
Patients gave targeted history and underwent physical exam, performance status evaluation and laboratory tests at the diagnosis and prior to each chemotherapy course. Ultrasound imaging was performed prior to each chemotherapy session and was reviewed by two independent gynecologists. Tumor recurrence was defined as identification of a new lesion of malignant disease on imaging studies using RECIST criteria (Response Evaluation Criteria in Solid Tumors) or through clinical examination.
Statistical analysis
Descriptive statistics (mean ± SD) were used for assessments of continued variables and the frequency or percentages were used for categorical variables. Statistical analyses were performed with 22 OvCa patients compared to 10 NC and 10 benign tumor patients using Student’s t test. The value p<0.05 was considered to be significant.
Results
Clinical and pathological patients characteristics
Table 1 summarizes clinico-pathological characteristics of the OvCa patients enrolled in the study: age, stage, grade and histopathology subtype of tumor, surgical debulking, and chemotherapy treatments. Patients ranged in age from 49 to 67 years, with the median age of 55 years. Using the International Federation of Gynecology and Obstetrics (FIGO) criteria, eight patients were diagnosed with the III stage disease and four with stage Ic. Primary cytoreductive surgery was performed in nine patients. Tree patients underwent exploratory laparotomy due to extensive disease. All 12 patients were started on first-line chemotherapy three weeks after surgery. Nine patients received 6 courses of taxol and carboplatin chemotherapy and tree received second-line chemotherapy (topotecan). CA125 levels normalized by the third cycle of chemotherapy in 3 patients (Pt#4, Pt#8, Pt#9) and by the sixth cycle in 5 patients (Pt#3, Pt#7, Pt#10, Pt#11, Pt#12). Eight patients achieved a clinical response defined as a normal level of CA125/HE4 and no pathologic lesions by transvaginal ultrasound examinations. Four patients had an elevated CA125 after cycle 6 of first-line chemotherapy and measurable disease on both the ultrasound scan and physical examination (Pt#1, Pt#2, Pt#5, Pt#6).
Table 1.
Characteristics of twelve OvCa patients for whom follow up clinical data were available.
| Pt # | Age | Stage | Grade | Histopathology | Surgery | Therapy | Comment |
|---|---|---|---|---|---|---|---|
| 1 | 66 | III | 2 | Adenocaserous | EL | TC VI | No response |
| 2 | 54 | III | 3 |
Adenocarcinoma
undifferentiated |
EL | TCVI, Topotecan III | No response |
| 3 | 52 | IIIB | 3 | Adenocaserous | TAH, BSO, OM | TC III | Response |
| 4 | 51 | Ic | 3 | Adenocaserous | TAH, BSO, OM | TC III | Response |
| 5 | 58 | III | 3 | Adenocapapillaraeendometroides | TAH, BSO, OM | TC VI, Topotecan III | No response |
| 6 | 51 | IIIA | 3 | Carcinoma undifferentiated | TAH, BSO, OM | TC VI | No response |
| 7 | 67 | IIIB | 2 | Adenocaserous | TAH, BSO | TC VI Topotecan III | Response |
| 8 | 65 | III | 3 | Adenocaserous | EL | TC III | Response |
| 9 | 56 | Ic | 2 | Adenocaserous | TAH, BSO, OM | VI | Response |
| 10 | 49 | Ic | 3 | Adenocaendometroides | TAH, BSO, OM | TCVI | Response |
| 11 | 57 | Ic | 2 | Adenocaendometroides | TAH, BSO, OM | TCVI | Response |
| 12 | 53 | III | 2 | Adenocaserous | TAH, BSO, OM | TCVI | Response |
Abbreviations: EL: Exploratory Laparotomy; TAH: Total Abdominal Hysterectomy; BSO: Bilateral Salpingoophorectomy; OM: Omentectomy; TK: Taxol + carbopaltin
Plasma exosome levels in OvCa patients, benign tumor patients and NC
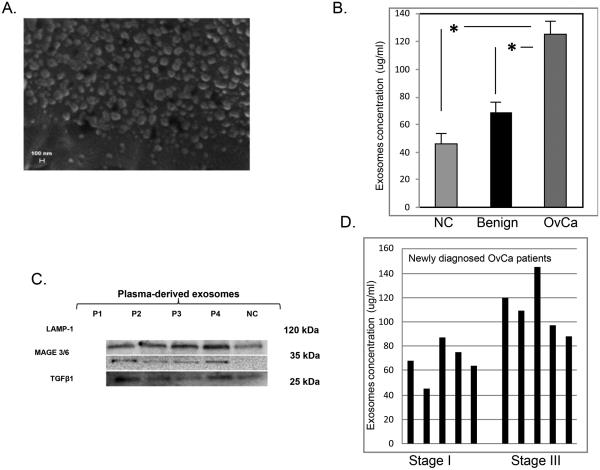
Exosomes were detectable in the plasma obtained from all OvCa patients, benign tumor patients and NC. Figure 1A shows a representative SEM (Scanning Electron Microscopy, Zeiss Evo 40) of an exosomal fraction isolated from plasma of a patient with OvCa. The exosomes we isolated were uniform in size (from 50-100 nM).The protein content of exosomes was higher in primary OvCa patients tested at diagnosis compared to exosomes in the plasma of NC (p<0.05). The mean plasma exosome level in OvCa patients was also significantly higher (125 µg/ml) than those in benign tumor patients (70 µg/ml; p<0.05; Figure 1B).
Figure 1.
Quantification and characterization of plasma-derived exosomes in OvCa patients, benign tumor patients and NCA.
Plasma-derived exosomes in OvCa patients contain TGF-β1 and MAGE 3/6
Exosomes isolated from plasma of all subjects were characterized by immunoblotting for the presence of Lysosome-Associated Membrane Protein-1 (LAMP-1) to confirm their endosomal origin. Western blot analysis showed little exosomal TGF-beta in plasma samples collected from benign tumor patients and NC without any previous diagnosis of cancer. In contrast, exosomes isolated from OvCa subjects (primary and chemotherapy treated) contained high levels of TGF-beta. The MAGE3/6 protein was present on exosomes from all OvCa patients but was undetectable on exosomes from benign tumors or NC. Thus, exosomes isolated from plasma of OvCa patients carried TGF-beta and MAGE3/6, and this cargo distinguished OvCa patients from those with benign tumors or NC (Figure 1C).
Quantitative analysis of exosome proteins in OvCa patients
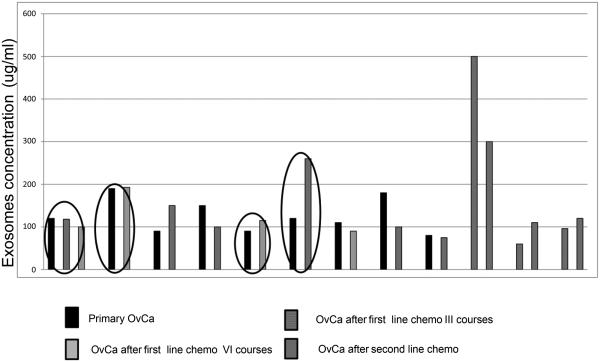
High levels of exosomal proteins were seen in newly diagnosed OvCa patients both in less (FIGO IC) and more advanced stages (FIGO III) of the disease. However, the protein content of exosome fraction was higher in stage III FIGO than in stage I FIGO OvCa patients (Figure 1D). The exosome levels variably changed during chemotherapy of OvCa patients (Figure 2). In patients 1, 2 and 5 there was no detectable clinical response to chemotherapy, as evidenced by high levels of CA125 or HE4 and by pathologic lesions visible on the patients’ ultrasound scans. In patients #1 and #2, the level of exosomes did not change in the course of chemotherapy. In patient #5, only a slight increase of exosome proteins was observed during the second-line chemotherapy. Patients #3 and #6 also showed an increase in exosomal proteins after chemotherapy, while in patients #8 and #10, chemotherapy was associated with a dramatic drop in levels of exosomal proteins. Correlations between the exosomal protein levels and clinical data suggested that these protein levels reflect responses to therapy received, which is an important prognostic factor in OvCa [22]. All patients are being followed to evaluate their long-term clinical responses. In addition, changes in the content of exosomalTGF-β1 and MAGE3/6 were studied by Western blots in selected patients to determine how these changes correlate with responses to chemotherapy.
Figure 2.
Quantative analysis of exosomal proteins in exosomes isolated from plasma of different OvCa patients.
Discussion
The role of Tumor-derived Exosomes (TEX) in mediating tumor-induced suppression of immune cells in the tumor microenviroment and the periphery has been recently recognized, and TEX are considered to represent one mechanism of tumor escape from the host immune system [6]. In addition, the possibility that TEX is involved in the delivery to non-immune cells of signals responsible for the promotion of tumor growth and metastasis is being considered [14]. While all activated cells can produce exosomes, we and others have reported that their levels in body fluids of patients with cancer are significantly and sometimes dramatically higher than those in normal plasma [9]. Further, these exosomes and can be isolated, measured and molecularly defined [14,15]. Accumulating data suggest that exosomes present in cancer patients’ body fluids might serve as new diagnostic and prognostic biomarkers [23]. For example, in melanoma patients with stage 4 disease, the high protein content of exosomes isolated from plasma (>50 µg/mL) correlated with poor survival [14]. Several previous studies have reported high levels of exosomes in body fluids of patients with OvCa [16]. The present study confirms these earlier findings and in addition, it demonstrates that OvCa exosome fractions contain a molecular profile distinguishable from that of exosomes from plasma of individuals with benign tumors or NC. Expression of OvCa exosomes in TGF-β1 and MAGE3/6 observed in Western blots suggests that at least a fraction of these exosomes originates from tumor cells. Further, total exosomal protein levels and levels of these tumor-associated proteins in exosomes appear be able to distinguish exosomes obtained from plasma collected at diagnosis from those obtained following chemotherapy. These results suggest that exosomal protein levels as well as heir molecular profiling could serve as markers of the disease presence and progression.
In this small study, we followed several patients looking for changes in exosomal protein levels during/after chemotherapy. Levels of exosomal proteins variably changed, in some cases increasing and others decreasing, following chemotherapy. Correlations between exosomal protein levels and clinical data suggested these measurements could be useful in evaluating patients’ responses to therapy. In patients who had failed treatment with taxol and carboplatin or topotecan, exosome protein levels remained unchanged from the pre-therapy levels. In contrast, patients who responded to therapy exhibited either decreased or increased exosomal protein levels relative to pre-therapy levels. It could be surmised that patients with higher levels of exosomal proteins after therapy also failed treatment and became chemotherapy resistant. Their ovarian cancer cells were perhaps able to expel drugs via an intense release of exosomes. A study by Luciani et al. suggested that tumor-derived exosomes could be responsible for removing cytotoxic drugs from tumor cells thus reducing the anti-tumor potential of chemotherapy [24]. Also, Safaei et al. showed that cisplatin-resistant OvCa patients secreted higher quantities of microvesicles which carried the cisplatin export transporters, MRP2, ATP7A and ATP7B [22]. Moreover, enhanced microvesicle production and resistance to cisplatin were associated with higher expression of genes whose products are known to be responsible for membrane fusion, vesicle trafficking and export of drugs by microvesicles [22]. On the other hand, patients whose exosomal protein levels decreased after therapy were clinical responders, as exemplified by patients #8 and #10, who had no evidence of the disease and whose CA125 and HE4 level dramatically decreased after TK chemotherapy. In these patients, a dramatic drop in exosomal protein levels could reflect tumor cell elimination and decreased tumor burden. Our data, while preliminary and performed with only a few patients, suggest that exosomal protein quantification in plasma should be further evaluated as a potential biomarker of response to therapy. Also, it is likely that a selective molecular profile of tumor-derived exosomes, which have been reported to be enriched in selected tumor-associated molecules, might be more informative in predicting responses and prognosticating than total protein exosomal protein levels.
Acknowledgments
This study was supported in part by the Foundation for Polish Science (Parent Bridge Program/2011/186) grants to MS; PO-1CA109688 to TLW and the Browning Memorial Fund to TLW.
REFERENCES
- 1.Poole EM, Merritt MA, Jordan SJ, Yang HP, Hankinson SE, et al. Hormonal and reproductive risk factors for epithelial ovarian cancer by tumor aggressiveness. Cancer Epidemiol Biomarkers Prev. 2013;22:429–437. doi: 10.1158/1055-9965.EPI-12-1183-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 3.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czystowska M, Han J, Szczepanski MJ, Szajnik M, Quadrini K, et al. IRX-2, a novel immunotherapeutic, protects human T cells from tumor-induced cell death. Cell Death Differ. 2009;16:708–718. doi: 10.1038/cdd.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes) Biochem Soc Trans. 2013;41:245–251. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 9.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, et al. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98–110. [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33:441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 11.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96:1302–1309. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS One. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu S, Liu C, Su K, Wang J, Liu Y, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 14.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, et al. Melanoma exosomes educate bone marrow progenitor cells toward a prometastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Ba Y, Ma L, Cai X, Yin Y, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 18.Khan S, Jutzy JM, Valenzuela MM, Turay D, Aspe JR, et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One. 2012;7:e46737. doi: 10.1371/journal.pone.0046737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lässer C, Eldh M, Lötvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012:e3037. doi: 10.3791/3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 23.Shao H, Chung J, Balaj L, Charest A, Bigner DD, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18:1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702–1713. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]