Abstract
This study was conducted to determine: 1) If healthy subjects can be conditioned to tolerate clinically useful electrically induced muscle contraction; and 2) If there is a gender difference in response to such conditioning. Healthy volunteers (10 males, 11 females, mean age of 27.6 ± 5.8 yrs) were tested during each of 6 testing sessions. Maximal voluntary isometric contractions (MVIC) of the right quadriceps femoris (RQF) recorded by a computerized dynamometer. Electrical stimulation delivered through two surface electrodes and stimulation amplitude increased until the subject indicated to stop. After a 1 min rest the amplitude increased again to the same phase charge level, and the electrically induced contraction (EIC) was recorded by the dynamometer. Measurements of stimulation amplitude were repeated in each of 10 stimulation bouts per session. Measurements of EIC were repeated in session six. Statistical analyses included Multivariate ANOVAs, and Newman-Kuel’s post-hoc tests (p < 0.01). Mean values of phase charge increased from session 1 to 6 for all subjects. Males tolerated significantly higher phase charge. The mean %MVIC torque generated by female subjects was initially only 11.2 ± 21.6% but reached 42.9 ± 25.4% at the end of the 6th session. Males’ %MVIC torque values were significantly higher reaching 49.0 ± 41.6% and 73.5 ± 18.7% in the first and last trials respectively. Using the criterion that electrically induced contractions must be at least 25% of MVIC to be considered clinically useful, 36% of females were below this threshold at the end of the last session. In contrast, all males exceeded the 25% MVIC threshold at the end of the study. Most healthy subjects can be conditioned to electrical stimulation of the quadriceps, but depending on the criteria of therapeutic value and gender, some males and even more females may not reach the desired stimulation goal in 6 sessions. Females may require more conditioning sessions to reach contraction levels of therapeutic benefits. The reason(s) for the confounding factor of gender remains unknown.
Key Points.
Neuromuscular electrical stimulation (NMES) can strengthen skeletal muscles
Tolerance to NMES improves within 6 sessions
Conditioning is a key to eliciting stronger contraction and to increasing the number of subjects that can benefit from NMES
Healthy males can tolerate higher stimulusintensity and higher electrically induced quadriceps femoris contraction.
Key Words: Neuromuscular electrical stimulation, tolerance, conditioning, gender
Introduction
Numerous reports favour the use of neuromuscular electrical stimulators (NMES) to help regain muscle strength and enhance recovery of motor control (Alon et al., 2003; Arvidsson et al., 1986; Delitto et al., 1988; Eriksson et al., 1981; Fitzgerald et al., 2003; Gould et al., 1983; Lieber et al., 1996; Morrissey et al., 1985; Neder et al., 2002; Oldham and Stanley, 1989; Parker et al., 2003; Stevens et al., 2004; Wigerstad-Lossing et al., 1988). In particular, previous investigators have found stimulation paradigms statistically and clinically useful in retarding disuse atrophy, (Arvidsson et al., 1986; Eriksson et al., 1981; Gibson et al., 1988; Gould et al., 1983; Morrissey et al., 1985, Oldham and Stanley, 1989) and in improving joint range of motion (Alon et al., 1998; Faghri et al., 1994; Morrissey et al., 1985 Neder et al., 2002; Oldham and Stanley, 1989; Pandyan et al., 1997; Werner et al., 1993). Various studies compared the clinical effectiveness of volitional exercise alone with exercise and electrical stimulation combined, and found that the combination of treatment interventions promotes significantly faster recovery of muscle torque generation (Alon, 1987; Eriksson et al., 1981; Hainaut and Duchateau, 1992; Kahanovitz et al., 1987; Wigerstad-Lossing et al., 1988). The literature, however, lacks unanimity regarding the beneficial effects of NMES. Several well- designed and executed NMES studies have failed to demonstrate significant strength gains over exercises alone(Paternostro-Sluga et al., 1999; Sisk et al., 1987).
In addition to discrepancies in clinical outcomes, researchers vary considerably regarding the minimum level of induced contraction deemed essential to produce strength gains (Alon et al., 2003; Fitzgerald et al., 2003; Lieber et al., 1996; Neder et al., 2002; Oldham and Stanley, 1989; Pandyan et al., 1997, Quittan et al., 2001; Snyder-Mackler et al., 1995; Stevens et al., 2004; Talbot et al., 2003). Based on the cited studies, a therapeutic window between 25% and 50% MVIC may be required to achieve and maintain with NMES in order to realize clinically meaningful outcomes. However, it also appears from these studies that the efficacy of NMES may depend on the targeted muscle, the nature and characteristics of the pathology present, the stimulation parameters, and the patient’s tolerance of electrically induced contraction.
Patients’ inability to tolerate NMES has been postulated as a primary cause of failure to achieve strength gain in healthy subjects (Kramer, 1987). Tolerance, in this context, may be defined as the maximal level of stimulation acceptable to the subject while producing a robust muscle contraction. Theoretically, increased tolerance to NMES could lead to adequate levels of contraction needed to produce effective neuromuscular training. One way to improve tolerance is to condition the subject to electrically induced contractions.
Conditioning, in this framework, refers to the concept whereby what is initially considered the maximal tolerance level, will become a sub-maximal level as the subject gains familiarity with the perception of the stimulation (Balogun et al., 1993). Despite supportive evidence for conditioning in the use of NMES, the percentage of subjects who cannot be conditioned, and thus may not benefit from electrically induced contraction is unknown. Likewise, the minimal number of conditioning sessions before the NMES output level reaches the therapeutic window is unknown.
Evidence suggests that gender differences may be a confounding factor in such conditioning (Lautenbacher and Rollman, 1993). Despite a growing literature regarding these differences, we were unable to find any study that documented the percentage of subjects of either gender who were unable to tolerate NMES regardless of conditioning. Thus, there are specific gaps in our understanding of the interacting factors associated with successful application of NMES.
A clear understanding of such information will help the clinical decision making process by identifying subjects who may and those who may not be candidates for NMES treatment.
The purposes of the present study were to: 1) determine the percentage of healthy subjects in a sample of volunteers that can or cannot be conditioned to tolerate clinically useful contraction within 6 sessions over two weeks, and 2) determine if there is a gender difference in the ability to tolerate NMES.
Methods
Subjects
A convenient sample of 21 healthy subjects (10 males, 11 females) ranging in age between 18 and 50 were recruited to participate in this study. Their physical profile is summarized in Table 1. Each subject agreed to stimulate the right quadriceps femoris (RQF) and signed an informed consent approved by the University of Maryland, Baltimore, Institutional Review Board (IRB).
Table 1.
Subjects description and physical activity profile. Data are means (±SD).
| Gender | Age | Height | Weight | Physical Activity | Times per | |
|---|---|---|---|---|---|---|
| (years) | (m) | (kg) | (km*) | (hours**) | (week) | |
| Females (n =11) | 26.5 (8.3) | 1.64 (.05) | 59.9. (5.9) | 8.2 (5.6) | 1.1 (.6) | 2.7 (.9) |
| Males (n = 10) | 28.7 (3.3) | 1.80 (.09) | 79.5 (8:8) | 7.4 (3.7) | 1.4 (.8) | 3.1 (1.1) |
Note: Only 6 of 11 females (54%) and 9 of 10 males (90%) were engaged in physical training activity. The physical activity included running and various exercises routines. Means SD are calculated based on active subjects only.
* For distance related activities (Km per day)
** For time related activities (hours per week)
Testing Procedures
Testing was conducted in research laboratories of the Department of Physical Therapy & Rehabilitation Sciences, School of Medicine. The subject sat on a KinCom® AP125 table with the knee positioned at 60 deg, the hip at 110 deg flexion and the pelvis tightly secured to the table with a 10 cm wide strap. The right knee axis was aligned with the axis of the electric motor. The subject leg was strapped onto the dynamometer’s lever arm that contained a force transducer.
Each subject performed 3 sub-maximal and 1 maximal volitional knee extension contractions on a Kin-Com® AP125 isokinetic dynamometer. These 4 warm-up volitional contractions allowed the subject to detect any discomfort in the set-up, and permitted the researchers to confirm the function of the equipment. Recording maximal volitional isometric contraction (MVIC) of the RQF followed. The Kincom® was activated and the subject was asked to perform an MVIC of knee extension for a period of 3 seconds. The subject was given a 1 min rest, and then repeated the procedure two more times to produce a total of 3 MVICs.
Obtaining the Electrically Induced Contraction (EIC) of the RQF was done in the same sitting position as described for the MVIC. Two rectangular surface electrodes covered with wet saline sponges, each 7.7 x 12.7 cm were used. Electrode placement was determined in the following manner: An initial (central) mark bisecting the thigh longitudinally was made that represented half the distance between the inguinal crease and the base of the patella. A second mark was made 4 cm lateral to the central mark. A third mark was made 4 cm medial to the central mark. The more proximal electrode’s inferior border coincided with the lateral mark and extended upward. The more distal electrode superior border coincided with the medial mark and extended downward. Each electrode was secured with two elastic straps (Figure 1). The electrodes were place identically in each of the 6 sessions of the study.
Figure 1.
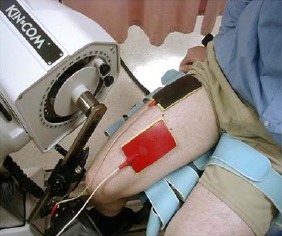
Electrodes position and method of securing them over the quadriceps.
The parameters setting for the constant voltage stimulator (VMS II, Chattanooga Corp. Chattanooga, TN) were symmetric biphasic waveform, 300 sec phase duration, a pulse rate of 50 pulses per second (pps) and 2 sec ramp up and down. Clinicians and researchers commonly use these parameters (Alon et al., 1992; 1999; Gibson et al., 1988; Kantor et al., 1994; Laufer et al., 2001; McMiken et al., 1983; Nordin et al., 1987). A hand switch connected to the stimulator was used to activate the stimulator and maintain a constant level of intensity between repeated bouts of contraction. The subject was instructed to remain inactive and not to add volitional contraction during stimulation. The procedural algorithm to obtain the EIC is summarized in Table 2. At the end of the session, each subject was asked to subjectively describe the sensation that prevented him/her from tolerating more stimulation. The sensation list was selected by the investigators based on clinical and previous research experience with NMES (Alon et al., 1992; 1999; Alon and Taylor, 1997) and included pins and needles, muscle cramping, other noxious sensations or any combination of these descriptors. Subjects returned for testing every other day for a total of 6 sessions. The 3 MVICs were recorded only on day 1 and day 6, while EIC were recorded for bout 1 and 10 during each of the six sessions.
Table 2.
Procedure to obtain maximal tolerance of electrically induced contraction (EIC)
|
Data reduction and analyses
An in house, custom written software program was used to reduce the 100 Hz torque-time raw data and to calculate each trial’s peak torque as the average of 10 data samples (each represents 0.1 sec interval) during the highest 1 sec on the torque-time curve (Figure 2). We then determined the MVIC and EIC peak torques as well as percent EIC (EIC/MVIC x 100). From these data, we calculated the peak MVIC (Session 1), the EIC for bout 1 and 10 (in sessions 1 and 6). We also collected phase charge data directly from the stimulator digital output display as representing stimulation intensity for bout 1 and 10 in each sessions (Alon et al., 1999; Kantor et al., 1994). Primary outcome data were organized into a 2 x 2 x 2 factorial design (Bout; Session; Gender) and subjected to repeated measures (Bout and Session) ANOVA and Newman-Keul’s post-hoc tests. Reported differences in test means achieved at least p < 0.01.
Figure 2.
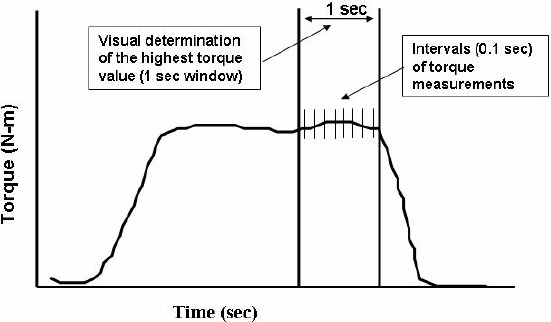
Illustration of MVIC and EIC determination. An interval of the highest magnitude on the torque-time curve was visually determined. The MVIC peak torque was calculated as the average of 10 values collected at 0.1 sec intervals within the 1 sec interval. EIC was likewise calculated and further presented as percent MVIC using the formula EIC/MVICx100.
Results
Electrically induced quadriceps contraction (EIC) as percent of MVIC was significantly greater among males compared to females throughout the study. Furthermore, the EIC was significantly greater at the last bout of each session compared to the first bout, and in the last session compared to the first session. These data are illustrated in Figure 3.
Figure 3.
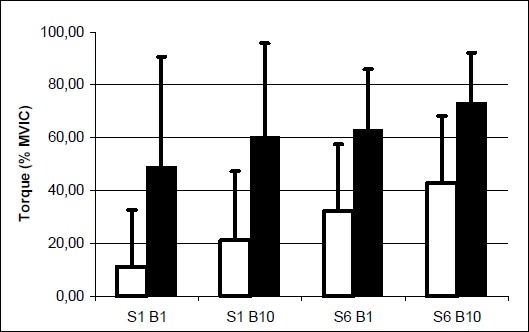
Means and standard deviations of the electrically induced contraction (EIC) as percent of MVIC at the beginning and end of sessions 1 and 6. Empty bars = females, Filled bars = males, S = Session, B = Bout.
Similarly, the results revealed that males tolerated significantly higher phase charge than the female subjects. The significant increase in phase charge was also evident from the first to sixth session and between the first and tenth stimulation bout within each session. No significant interactions occurred among these three main factors. Figure 4 shows the mean phase charge of both genders. Females’ data yielded phase charge ranges from a minimum of 8.6 ± 4.5 µC (bout 1, session 1) to a maximum of 16.9 ± 8.0 µC (bout 10, session 6). The men had a minimum of 17.7 ± 9.5 µC (bout 1, session 1) to a maximum of 31.5 ± 8.7 µC (bout 10, session 6). The percent-changes of both electrically induced contraction and phase charge from the end of session 1 to the end of session 6 are summarized in Table 3. Males increased the MVIC by 22% while females more than doubled their percent MVIC. Increase tolerance to stimulation, as indicated by the phase charge, followed a zig-zag pattern for both males and females. As seen in Figure 5, the tolerance at the end of each session was attenuated, but not completely lost at the beginning of the next session.
Figure 4.
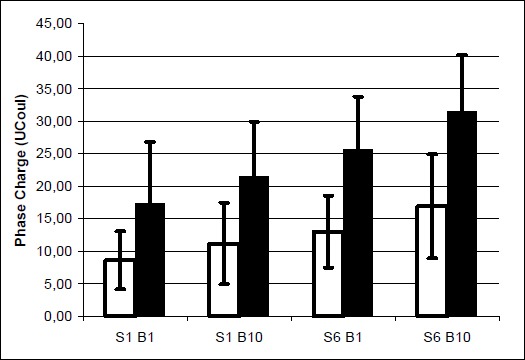
Means and standard deviations of the amount of phase charge at the beginning and end of sessions 1 and 6. Empty bars = females, Filled bars = males, S = Session, B = Bout.
Table 3.
Summary of the mean changes in electrically induced contraction expressed as percent MVIC and corresponding mean changes of phase charge measured in microcoulombs (µC) recorded at the end of session one and six. Data are means (±SD)
| Session 1 | Session 6 | % Change | ||
|---|---|---|---|---|
| Females | %MVIC | 21.3 (24.8) | 44.6 (26.6) | 109.4 |
| Charge(µC) | 11.2 (6.2) | 16.9 (8.0) | 50.8 | |
| Males | %MVIC | 60.7 (31.9) | 74.1 (18.2) | 22.0 |
| Charge(µC) | 21.4 (8.5) | 31.5 (8.6) | 47.2 |
As seen, inter-subject variability was considerable in the studied sample. Note: The reported values would probably be different particularly in session 6 if the stimulator was more powerful (i.e., had higher maximal phase charge)
Figure 5.
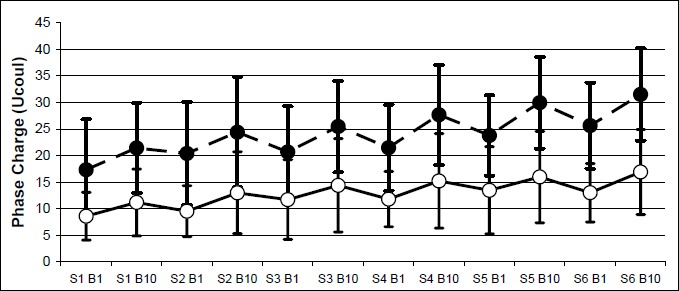
The fluctuating yet upward increase tolerance to stimulus intensity within and between sessions. Filled circle = males, Empty circle = females, S = Session, B = Bout.
Using criterion that clinically useful electrically induced contractions of the quadriceps must reach a threshold of at least 25% of MVIC (Lieber et al., 1996; Quittan et al., 2001), only 1 female (9%), and 6 males (60%) achieved that level in the first bout of the first session. The numbers increased to 4 (36%) and 7 (70%) at the end of the first session for females and males, respectively.
At the start of the 6th session 5 females (45%) and 8 males (80%) were able to achieve 25% MVIC and at the 10th bout of the 6th session 7 females (64%) and all males (100%) did 25% or better.
If the threshold leading to successful clinical outcome is assumed to be 50% (Snyder-Mackler et al., 1994; 1995) only one female (9%), and 4 males (40%) achieved that level in the first bout of the first session. At the end of the first session 3 females (27%) and 7 males (70%) met or exceeded that level. At the start of the 6th session 4 females (36%) and 8 males (80%) exceeded the threshold of 50% MVIC and these numbers improved to 5 females (45%) and 9 males (90%) at the 10th bout of the 6th session. Stated conversely, 55% of females and 10% of males could not be conditioned to tolerate electrically induced contraction at 50% MVIC after six sessions of stimulation. These numbers improved if the threshold was lowered to 25% MVIC were only 36% of females could not be conditioned and all males were conditioned.
Two females (18.2%) and 7 males (70%) were able to tolerate more phase charge (stimulus intensity) than the stimulator was capable of producing. Table 4 documents the session when individual subjects were able to tolerate the stimulator’s maximal phase charge output. The mechanism underlying the variability of the maximum tolerated phase charge values is inherent in the design of the stimulator as a constant voltage device (Stecker, 2004).
Table 4.
The session number at which each of 9 individual subjects exceeded the maximal stimulator output.
| Session | Phase Charge (Ucoul) |
|
|---|---|---|
| Female | 6 | 29.8 |
| Female | 3 | 31.8 |
| Male | 5 | 27.9 |
| Male | 5 | 40.0 |
| Male | 1 | 37.1 |
| Male | 2 | 35.5 |
| Male | 4 | 40.1 |
| Male | 6 | 40.3 |
| Male | 3 | 32.3 |
Painful muscle cramps sensation was the leading cause for subjects’ request to stop the stimulation (50% of subjects), while intense pins and needles (18%), combination of cramps-pins/needle (9%) and other noxious sensations (deep pressure, sharp pain, and nausea; 18%) provided the remaining reasons to become intolerant of stimulation intensity. Five percent of the subjects had no specific complaints of discomfort, but noted that the stimulation felt “weird”.
Discussion
This study provides several important findings that should help to guide clinicians in the proper application of NMES. First, the tolerance of women and men to electrical stimulation is likely to improve within and between sessions, thereby improving the likelihood of therapeutic benefit from NMES. But the degree of conditioning achieved is likely to vary considerably among both men and women. Inspection of the raw data suggests a general observation whereby subjects that exhibited strong electrically elicited contractions initially, were more likely to reach the highest percentage of MVIC during the sixth session.
A second important finding is that males were able to tolerate considerably more electrical stimulation than females. Our reported data are consistent with a previous study by Alon et. al. (1999) in which males had a higher tolerance of phase charge and recorded higher EIC during a single session of plantar flexor stimulation. Laufer et al reported similar results when the stimulation was applied over the quadriceps femoris muscle in responsiveness to painful stimuli. Why females are less tolerant of electrical stimulation overall is not immediately clear. In a recent review, Rollman, (2003) acknowledged this phenomenon and suggested that the musculo-skeletal system of females responds to pain differently than that of man. Can it also be that the fitness level affects tolerance to electrical stimulation? In the present study, 9 of 10 males were involved in some form of physical training while only 6 of 11 females had similar training experiences. However of the remaining four, two sedentary females tolerated the maximum stimulator output within the third to sixth sessions. In contrast, two of the more physically active females were among the last subjects to increase their tolerance to the stimulation. Thus, it appears that fitness levels may not correlate with tolerance or to susceptibility to conditioning.
Most subjects reported they were unable to tolerate further increases in stimulation secondary to discomfort. The leading cause of discomfort was muscle cramps with 50% of subjects indicating intolerance of further cramping was their principal reason to stop the stimulation. Delitto et al. (1992) suggested that high electrically induced contractile force is likely to result in substantial discomfort associated with muscle contraction but did not elaborate if there were differences between cramping and other expressions of discomfort. The second leading cause of discomfort in the present study was a “pins and needles ”parasthesia. The origin of this noxious perception may be stimulation of free nerve endings (C fibers) in dermal and sub-dermal connective and adipose tissues, (Burke and Applegate, 1989; Sakakibara et al., 1995) while the cramps are likely to originate from within the peripheral motor system (Baldissera et al., 1994). Whereas distinct differentiation of response is clearly demonstrated during interscalene brachial plexus block,(Bollini et al., 2003; Urmey and Stanton, 2002) documentation that differentiates neural pathways under the present test conditions is not available. Nor is it clear whether the conditioning noted in the present study is related to the phenomenon of habituation to sensory stimuli(Chang et al., 2002).
Whereas both genders demonstrated an ability to become conditioned to NMES, the mechanism involved in the conditioning process is not specifically described in the literature and may include peripheral and central neural adaptation as well as peripheral alteration in tissue conductance. Alon et al. (1987) reported previously that tissue impedance estimated from the voltage/current ratio decreased over 12 sessions of stimulation. The authors interpreted this finding to indicate an increase in tissue conductivity and hypothesized that the changes may have been due to an increase of blood flow and interstitial fluid volume, or a decrease in subcutaneous fat content or both. The observation that soft tissue opposition to current flow is likely to diminish with stimulation has been reproduced, (Alon et al., 1992) but the source(s) of that reduction remain unknown.
A central question in this study was whether or not men and women can be conditioned to tolerate electrical stimulation to a degree that will make it useful for strengthening of weak muscles or promote improvement in motor control. The answer seems to depend on the target muscle, the methodology, and the quantification criteria used by investigators. It may also be associated with the percentage of MVIC that electrical stimulation must induce. Scott et al. (1990) demonstrated that low-level EIC that was well accepted by children with Duchenne Muscular Dystrophy produced significant strength gains. We estimate that the data reported by Scott et al. generated about 5-10% of MVIC that resulted in improved strength and endurance of the dorsiflexors. Alon and Taylor (1997) who subsequently stimulated the abdominal muscles at minimal visible contraction for 4 weeks reported similar results. In contrast, Snyder-Mackler at el. (1994; 1995) compared what they termed “clinical high intensity ”to “portable low intensity ”stimulators in the production of quadriceps muscle force after ACL injury. They argued that 50% MVIC or better must be achieved to produce an adequate training effect. Lieber et. al. (1996) did not support the findings of Snyder-Mackler et. al. as they used between 15% and 45% MVIC and reported significant strength gains after ACL repair. Similarly, Quinttan et al. (2001) reported that stimulation of the quadriceps and hamstrings of patients with chronic congestive heart failure at 25-30 % MVIC resulted in significant strength gains. Other investigators did not report percentages, (Alon et al., 1998; Alon and Taylor, 1997; Caggiano et al., 1994; Fitzgerald et al., 2003; Gibson et al., 1988; Oldham and Stanley, 1989, Petterson et al., 1994) but were successful in regaining muscle strength and better motor control following stimulation. If based on the current evidence, we assume a minimum therapeutic effect at 25% MVIC, then 60% of the males achieved this criterion in the first session compared to only 9% of females. Using a threshold criterion for therapeutic effect at 50% MVIC and 6 stimulation sessions, 90% of males and 45% of females may benefit from NMES training while 10% of males, and 55 % of females would not benefit from NMES induced strengthening of the quadriceps.
Lastly, our finding of gradual conditioning to electrical stimulation further refines the current knowledge regarding tolerance by recognizing the phenomenon of partial reversal between sessions. This partial, not complete end of previous session-start of next session decline of tolerance, and yet the overall session-to-session increase tolerance to stimulation implies a combination of accommodation and habituation to electrical stimulation. Accommodation refers to the transient but reversible increase threshold of nerve excitation. Habituation implies a long-term non-reversal adaptation to stimulation that may involve morphological and histochemical alteration (Gauthier et al., 1992; Gibson et al., 1988; Ogino et al., 2002; Pekindil et al., 2001, Quittan et al., 2001). In the present investigation we only tested these phenomena over a two-week period. Whether either or both accommodation and habituation are completely reversed a few weeks after cessation of stimulation requires further inquiry.
Two weaknesses should be recognized as limiting factors in the following discussion. First, 43% of subjects could tolerate higher stimulation intensity than the stimulator was capable of generating. Conceivably, a stronger stimulator would have produced different outcomes. However, the stimulator’s parameters used in the present study are widely used in clinical settings and the stimulator’s maximal phase charge is equal or better when compared to other commercially available stimulators. Moreover, we believe that the clinical utility of the present findings would not be altered even if the stimulator were more powerful due to the fact that our data is comparable to previously published studies. (Snyder-Mackler et al., 1995, Snyder-Mackler et al., 1994, Lieber et al., 1996, Laufer et al., 2001, Stevens et al., 2004, Fitzgerald et al., 2003, Parker et al., 2003, Neder et al., 2002) The second limitation is that the data were obtained from a single healthy non-impaired muscle. The possibility that neuromuscular impairment may alter patients’ responses to electrical stimulation necessitates that a caution is added to any conclusions derived from healthy muscles.
The implication to practitioners is the need to recognize that while both males and females are likely to benefit from NMES to the quadriceps muscle, most females may need conditioning over a longer time period. Restricting the number of treatments to only 6 sessions may deprive women from potential benefits that NMES could offer. Second, all of our subjects reached at least 5% of MVIC by the end of 6 sessions. Whether this percentage is enough to produce significant strength gain remains a tentative hypothesis. Non-vigorous contraction NMES could be indicated for patients where intense contraction is unwarranted or intolerable (Fitzgerald et al., 2003). However, if a low contraction level is chosen it may require more repetitions. Alon and Taylor, (1997) Alon et al. (2003) and Scott et al. (1990) took the approach of using low-level contraction but extended the sessions to 3 hours each day over 4 to 12 weeks of stimulation. These studies provided NMES to the abdominal muscles, the wrist extensors/flexors and the dorsiflexors respectively and were able to produce significant strength gain at the end of the NMES training.
Third, setting stimulation intensities to the same level as was set in a previous visit without regard to patient discomfort may cause some patients to refuse further stimulation because of unduly discomforting stimulation. Increasing stimulus intensity each treatment with patient consent, rather then rigid dial setting, would seem a more appropriate procedure particularly when maximal stimulation is the treatment target (Snyder-Mackler et al., 1994; 1995). Borrowing from Delitto’s data (Delitto et al., 1992) that individuals may have different coping styles, the clinician should tailor their treatment protocols for a particular patient’s coping style to minimize patient discomfort.
Conclusions
Within the limits of this study, we concluded that most healthy subjects could be conditioned to tolerate electrical stimulation at a clinically meaningful electrically induced contraction of the quadriceps femoris. If the selected minimum criterion is as low as 5% MVIC, up to six sessions of conditioning are adequate to condition all subjects. If the minimum criterion is 25% all men, but only 64% of women would be conditioned; and if the threshold is at 50 % MVIC, 10 percent of males and 55 percent of females may not be candidates for neuromuscular strengthening program of the quadriceps muscle after six sessions of conditioning. Because females tolerate electrically induced contraction less than males, they may require more sessions of conditioning in order to benefit from the stimulation program.
Biographies

Gad ALON
Employment
Univ. of Maryland, School of Medicine, Department of Physical Therapy & Rehab. Science.
Degree
PhD,PT
Research interests
Electrical stimulation,pathological movements,neurorehabilitation.
E-mail: galon@som.umaryland.edu

V. Smith GERALD
Employment
Univ. of Central Florida, College of Health and Public Affairs, Department of Health Professions.
Degree
PhD,PT
Research interests
Neuroscience,neurorehabilitation.
E-mail: gesmith@mail.ucf.edu
References
- Alon G., Dar A., Katz-Behiri D. (1998) Efficacy of a hybrid upper limb neuromuscular electrical stimulation system in lessenning selected impairments and dysfunctions consequent to cerebral damage. Journal of Neurological Rehabiitation 12, 73-80 [Google Scholar]
- Alon G., Frederickson R., Gallager L., Rehwoldt C.T., Guillen M., Pement M.L., Barnhart J.B. (1992) Electrical stimulation of the abdominals: The effects of three versus five weekly treatments. Journal Clinical Electrophysiology 4, 5-11 [Google Scholar]
- Alon G., Kantor G, Smith G.V.(1999) Peripheral nerve excitation and plantar flexion force elicited by electrical stimulation in males and females. Journal of Orthopedic and Sports Physical Therapy 29, 208-214;discussion 215-217. [DOI] [PubMed] [Google Scholar]
- Alon G., Sunnerhagen K.S., Geurts A.C., Ohry A. (2003) A home-based, self-administered stimulation program to improve selected hand functions of chronic stroke. NeuroRehabilitation 18, 215-225 [PubMed] [Google Scholar]
- Alon G., Taylor D.J. (1997) Electrically elicited minimal visible tetanic contraction and its effect on abdominal muscles strength and endurance. European Journal of Physical Medicine and Rehabilitation 7, 2-6 [Google Scholar]
- Alon G.M., McCombe S.A., Koutsantonis S., Stumphauzer L.J., Burgwin K.C., Parent M.M., Bosworth R.A. (1987) Comparison of the effects of electrical stimulation and exercise on abdominal musculature. Journal of Orthopedic and Sports Physical Therapy 8, 567-573 [DOI] [PubMed] [Google Scholar]
- Arvidsson I., Arvidsson H., Eriksson E., Jansson E. (1986) Prevention of quadriceps wasting after immobilization: an evaluation of the effect of electrical stimulation. Orthopedics 9, 1519-1528 [DOI] [PubMed] [Google Scholar]
- Baldissera F., Cavallari P., Dworzak F.(1994) Motor neuron ‘bistability’. A pathogenetic mechanism for cramps and myokymia. Brain 117 (Pt 5)929-939 [DOI] [PubMed] [Google Scholar]
- Balogun J.A., Onilari O.O., Akeju O.A., Marzouk D.K. (1993) High voltage electrical stimulation in the augmentation of muscle strength: effects of pulse frequency. Archives Physical Medicine and Rehabilitation 74, 910-916 [PubMed] [Google Scholar]
- Bollini C.A., Urmey W.F., Vascello L., Cacheiro F. (2003) Relationship between evoked motor response and sensory paresthesia in interscalene brachial plexus block. Regional Anaesthesiology Pain Medicine 28, 384-388 [DOI] [PubMed] [Google Scholar]
- Burke D., Applegate C..(1989) Paraesthesiae and hypaesthesia following prolonged high-frequency stimulation of cutaneous afferents. Brain 112(Pt 4), 913-929 [DOI] [PubMed] [Google Scholar]
- Caggiano E., Emrey T., Shirley S., Craik R.L. (1994) Effects of electrical stimulation or voluntary contraction for strengthening the quadriceps femoris muscles in an aged male population. Journal of Orthopaedic and Sports Physical Therapy 20, 22-28 [DOI] [PubMed] [Google Scholar]
- Chang Q.Y., Lin J.G., Hsieh C.L. (2002) Effect of electroacupuncture and transcutaneous electrical nerve stimulation at Hegu (LI.4) acupuncture point on the cutaneous reflex. Acupuncture Electrotherapy Research 27, 191-202 [DOI] [PubMed] [Google Scholar]
- Delitto A., Rose S.J., McKowen J.M., Lehman R.C., Thomas J.A., Shively R.A. (1988) Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate ligament surgery. Physical Therapy 68, 660-663 [DOI] [PubMed] [Google Scholar]
- Delitto A. Strube M.J. Shulman A.D, and Minor S.D.(1992) A study of discomfort with electrical stimulation. Physical Therapy 72, 410-421; discussion on 421-424. [DOI] [PubMed] [Google Scholar]
- Eriksson E., Haggmark T., Kiessling K.H., Karlsson J. (1981) Effect of electrical stimulation on human skeletal muscle. International Journal Sports Medicine 2, 18-22 [DOI] [PubMed] [Google Scholar]
- Faghri P.D., Rodgers M.M., Glaser R.M., Bors J.G., Ho C., Akuthota P. (1994) The effects of functional electrical stimulation on shoulder subluxation, arm function recovery, and shoulder pain in hemiplegic stroke patients. Archives Physical Medicine Rehabilitation 75, 73-79 [PubMed] [Google Scholar]
- Fitzgerald G.K., Piva S.R., Irrgang J.J. (2003) A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. Journal of Orthopaedic and Sports Physical Therapy 33, 492-501 [DOI] [PubMed] [Google Scholar]
- Gauthier J.M., Theriault R., Theriault G., Gelinas Y., Simoneau J.A. (1992) Electrical stimulation-induced changes in skeletal muscle enzymes of men and women. Medicine Science Sports Exercise 24, 1252-1256 [PubMed] [Google Scholar]
- Gibson J.N., Smith K., Rennie M.J. (1988) Prevention of disuse muscle atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet 2, 767-770 [DOI] [PubMed] [Google Scholar]
- Gould N., Donnermeyer D., Gammon G.G., Pope M., Ashikaga T. (1983) Transcutaneous muscle stimulation to retard disuse atrophy after open meniscectomy. Clinical Orthopedics 190-197 [PubMed] [Google Scholar]
- Hainaut K., Duchateau J. (1992) Neuromuscular electrical stimulation and voluntary exercise. Sports Medicine 14, 100-113 [DOI] [PubMed] [Google Scholar]
- Kahanovitz N., Nordin M., Verderame R., Yabut S., Parnianpour M., Viola K., Mulvihill M. (1987) Normal trunk muscle strength and endurance in women and the effect of exercises and electrical stimulation. Part 2: Comparative analysis of electrical stimulation and exercises to increase trunk muscle strength and endurance. Spine 12, 112-118 [DOI] [PubMed] [Google Scholar]
- Kantor G., Alon G., Ho H.S. (1994) The effects of selected stimulus waveforms on pulse and phase characteristics at sensory and motor thresholds. Physical Therapy 74, 951-962 [DOI] [PubMed] [Google Scholar]
- Kramer J.F. (1987) Effect of electrical stimulation current frequencies on isometric knee extension torque. Physical Therapy 67, 31-38 [DOI] [PubMed] [Google Scholar]
- Laufer Y., Ries J.D., Leininger P.M., Alon G. (2001) Quadriceps femoris muscle torques and fatigue generated by neuromuscular electrical stimulation with three different waveforms. Physical Therapy 81, 1307-1316 [PubMed] [Google Scholar]
- Lautenbacher S., Rollman G.B. (1993) Sex differences in responsiveness to painful and non-painful stimuli are dependent upon the stimulation method. Pain 53, 255-264 [DOI] [PubMed] [Google Scholar]
- Lieber R.L., Silva P.D., Daniel D.M. (1996) Equal effectiveness of electrical and volitional strength training for quadriceps femoris muscles after anterior cruciate ligament surgery. Journal Orthopedic Research 14, 131-138 [DOI] [PubMed] [Google Scholar]
- McMiken D.F., Todd-Smith M., Thompson C. (1983) Strengthening of human quadriceps muscles by cutaneous electrical stimulation. Scandinavian Journal Rehabilitation Medicine 15, 25-28 [PubMed] [Google Scholar]
- Morrissey M.C., Brewster C.E., Shields C.L., Jr., Brown M. (1985) The effects of electrical stimulation on the quadriceps during postoperative knee immobilization. American Journal Sports Medicine 13, 40-45 [DOI] [PubMed] [Google Scholar]
- Neder J.A., Sword D., Ward S.A., Mackay E., Cochrane L.M., Clark C.J. (2002) Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD). Thorax 57, 333-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M., Kahanovitz N., Verderame R., Parnianpour M., Yabut S., Viola K., Greenidge N., Mulvihill M. (1987) Normal trunk muscle strength and endurance in women and the effect of exercises and electrical stimulation. Part 1: Normal endurance and trunk muscle strength in 101 women. Spine 12, 105-111 [DOI] [PubMed] [Google Scholar]
- Ogino M., Shiba N., Maeda T., Iwasa K., Tagawa Y., Matsuo S., Nishimura H., Yamamoto T., Nagata K., Basford J.R. (2002) MRI quantification of muscle activity after volitional exercise and neuromuscular electrical stimulation. American Journal Physical Medicine Rehabilitation 81, 446-451 [DOI] [PubMed] [Google Scholar]
- Oldham J.A., Stanley J.K. (1989) Rehabilitation of atrophied muscle in the rheumatoid arthritic hand: a comparison of two methods of electrical stimulation. Journal Hand Surgery [Br] 14, 294-297 [DOI] [PubMed] [Google Scholar]
- Pandyan A.D., Granat M.H., Stott D.J. (1997) Effects of electrical stimulation on flexion contractures in the hemiplegic wrist. Clinical Rehabilitation 11, 123-130 [DOI] [PubMed] [Google Scholar]
- Parker M.G., Bennett M.J., Hieb M.A., Hollar A.C., Roe A.A. (2003) Strength response in human femoris muscle during 2 neuromuscular electrical stimulation programs. Journal of Orthopedic and Sports Physical Therapy, 33, 719-726 [DOI] [PubMed] [Google Scholar]
- Paternostro-Sluga T., Fialka C., Alacamliogliu Y., Saradeth T., Fialka-Moser V. (1999) Neuromuscular electrical stimulation after anterior cruciate ligament surgery. Clinical Orthopedic Related Research 368, 166-175 [PubMed] [Google Scholar]
- Pekindil Y., Sarikaya A., Birtane M., Pekindil G., Salan A. (2001) 99mTc-sestamibi muscle scintigraphy to assess the response to neuromuscular electrical stimulation of normal quadriceps femoris muscle. Annals Nuclear Medicine 15, 397-401 [DOI] [PubMed] [Google Scholar]
- Petterson T., Smith G.P., Oldham J.A., Howe T.E., Tallis R.C. (1994) The use of patterned neuromuscular stimulation to improve hand function following surgery for ulnar neuropathy. Journal Hand Surgery [Br] 19, 430-433 [DOI] [PubMed] [Google Scholar]
- Quittan M., Wiesinger G.F., Sturm B., Puig S., Mayr W., Sochor A., Paternostro T., Resch K.L., Pacher R., Fialka-Moser V. (2001) Improvement of thigh muscles by neuromuscular electrical stimulation in patients with refractory heart failure: a single-blind, randomized, controlled trial. American Journal Physical Medicine Rehabilitation 80, 206-214; quiz 215-216, 224. [DOI] [PubMed] [Google Scholar]
- Rollman G.B. (2003) Introduction: Sex makes a difference: experimental and clinical pain responses. Clinical Journal of Pain 19, 204-207 [DOI] [PubMed] [Google Scholar]
- Sakakibara H., Hirata M., Hashiguchi T., Toibana N., Koshiyama H., Zhu S.K., Yamada S. (1995) Digital nerve conduction velocity for evaluation of peripheral nerve impairments in vibration syndrome. Central European Journal Public Health 3 Suppl, 52-53 [PubMed] [Google Scholar]
- Scott O.M., Hyde S.A., Vrbova G., Dubowitz V. (1990) Therapeutic possibilities of chronic low frequency electrical stimulation in children with Duchenne muscular dystrophy. Journal of Neurological Science 95, 171-182 [DOI] [PubMed] [Google Scholar]
- Sisk T.D., Stralka S.W., Deering M.B., Griffin J.W. (1987) Effect of electrical stimulation on quadriceps strength after reconstructive surgery of the anterior cruciate ligament. American Journal Sports Medicine 15, 215-220 [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler L., Delitto A., Bailey S.L., Stralka S.W. (1995) Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. Journal Bone Joint Surgery [Am] 77, 1166-1173 [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler L., Delitto A., Stralka S.W., Bailey S.L. (1994) Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Physical Therapy, 74, 901-907 [DOI] [PubMed] [Google Scholar]
- Stecker M.M. (2004) Nerve stimulation with an electrode of finite size: differences between constant current and constant voltage stimulation. Computers Biology and Medicine, 34, 51-94 [DOI] [PubMed] [Google Scholar]
- Stevens J.E., Mizner R.L., Snyder-Mackler L. (2004) Neuromuscular electrical stimulation for quadriceps muscle strengthening after bilateral total knee arthroplasty: a case series. Journal of Orthopedic and Sports Physical Therapy 34, 21-29 [DOI] [PubMed] [Google Scholar]
- Talbot L.A., Gaines J.M., Ling S.M., Metter E.J. (2003) A home-based protocol of electrical muscle stimulation for quadriceps muscle strength in older adults with osteoarthritis of the knee. Journal Rheumatology 30, 1571-1578 [PubMed] [Google Scholar]
- Urmey W.F., Stanton J. (2002) Inability to consistently elicit a motor response following sensory paresthesia during interscalene block administration. Anesthesiology 96, 552-554 [DOI] [PubMed] [Google Scholar]
- Werner S., Arvidsson H., Arvidsson I., Eriksson E. (1993) Electrical stimulation of vastus medialis and stretching of lateral thigh muscles in patients with patello-femoral symptoms. Knee Surgery Sports Traumatology Arthroscopy 1, 85-92 [DOI] [PubMed] [Google Scholar]
- Wigerstad-Lossing I., Grimby G., Jonsson T., Morelli B., Peterson L., Renstrom P. (1988) Effects of electrical muscle stimulation combined with voluntary contractions after knee ligament surgery. Medicine Science Sports Exercise 20, 93-98 [DOI] [PubMed] [Google Scholar]


