Abstract
Hypercholesterolemia is a risk factor for estrogen receptor (ER) positive breast cancers and is associated with a decreased response of tumors to endocrine therapies. Here we show that 27-Hydroxycholesterol (27HC), a primary metabolite of cholesterol and an ER and Liver X receptor (LXR) ligand, increases ER-dependent growth and LXR-dependent metastasis in mouse models of breast cancer. The effects of cholesterol on tumor pathology required its conversion to 27HC by the cytochrome P450 oxidase CYP27A1, and were attenuated by treatment with CYP27A1 inhibitors. In human breast cancer specimens, CYP27A1 expression levels correlated with tumor grade. In high-grade tumors, both tumor cells and tumor-associated macrophages exhibited high expression levels of the enzyme. Thus, lowering circulating cholesterol levels or interfering with its conversion to 27HC may be a useful strategy to prevent and/or treat breast cancer.
Obesity and the metabolic syndrome are risk factors for estrogen receptor (ER)-positive breast cancer in postmenopausal women (1, 2). This has been attributed to increases in circulating insulin and insulin-like growth factors, local production of estrogens in adipose tissue, and the influence of adipokines and inflammatory cytokines on tumors and their microenvironment (3). Recently, hypercholesterolemia, an established comorbidity of obesity, has been identified as an independent risk factor for breast cancer in postmenopausal women (4-6). Whereas studies of the impact of HMGCoA reductase inhibitors (statins) on breast cancer risk have yielded equivocal results (7), there is strong evidence that disease-free survival is improved in breast cancer survivors who are taking statins prior to diagnosis (8, 9).
It has been proposed that the beneficial effects of statins in breast cancer result from their ability to directly inhibit cell proliferation. This hypothesis is difficult to reconcile with the observation that statin concentrations of 1-200μM are required to inhibit cancer cell proliferation in vitro whereas the extrahepatic levels of statins do not normally exceed 10-200nM in humans (10-12). An alternative explanation is that tumor cell growth is negatively impacted by reducing the levels of circulating cholesterol. Of significance in this regard are the recent observations that the oxysterol, 27-hydroxycholesterol (27HC), an abundant primary metabolite of cholesterol, is a Selective Estrogen Receptor Modulator (SERM) and liver X receptor (LXR) agonist that exerts a spectrum of activities in bone and in the cardiovascular system in mice (13-16). Furthermore, we performed a comprehensive analysis of the molecular pharmacology of 27HC in cellular models of breast cancer, revealing that it exhibited significant ER and LXR partial agonist activity at concentrations that are expected to be found in humans (figs. S1-3) (17). These findings prompted us to evaluate the extent to which 27HC impacts tumor pathophysiology in animal models of breast cancer.
The first objective of our studies was to determine whether or not the estrogenic activity of 27HC was sufficient to promote the growth of MCF7 cell-derived breast xenografts when propagated in ovariectomized mice. The estrogen dependency of this model was demonstrated by showing that 17β-estradiol (E2), but not vehicle treatment, promoted tumor growth (Fig. 1A, fig. S4). 27HC also promoted the growth of these tumors, and this activity was inhibited by cotreatment with the pure antiestrogen, ICI 182,780, or upon cessation of 27HC supplementation. Gene expression studies revealed a potential association between 27HC exposure and the development of tamoxifen resistance (fig. S1A), prompting an evaluation of the pharmacology of 27HC in a mouse model of tamoxifen resistance (TamR) (18). In this model, as in the tumors of patients with tamoxifen resistant disease, tamoxifen exhibits robust agonist activity. It was significant, therefore, that 27HC promoted tumor growth as well as, or better than, tamoxifen or E2 in this model (Fig. 1B).
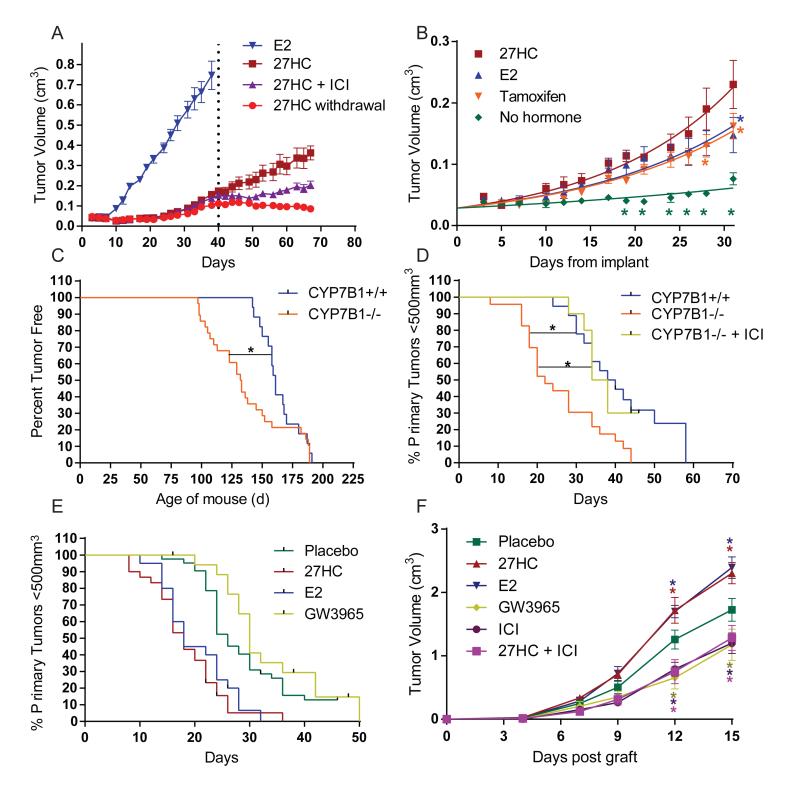
Fig. 1. The oxysterol, 27-hydroxycholesterol, increases tumor growth in several animal models of estrogen receptor positive breast cancer.
(A) The estrogenic activity of 27-hydroxycholesterol (27HC) is sufficient to support the growth of human MCF7 cell xenografts when propagated in ovariectomized mice. MCF7 cells were injected into the axial mammary pad of ovariectomized, immunocompromized mice and administered 27HC by daily injection or were given an E2 pellet as indicated. At day 40, the 27HC treated mice were randomized into three groups: continued 27HC, 27HC + the antiestrogen ICI 182,780 (ICI), or vehicle treatment (27HC withdrawal) (mean +/− SEM, n = 9-10). (B) 27HC supports the growth of tamoxifen resistant, MCF7 cell derived, breast tumors. Tamoxifen resistant MCF7 cells (TamR) were injected into ovariectomized, immunocompromized mice and treated for 30 days with E2 (pellet), tamoxifen (pellet), 27HC (injection) or without supplementation. Colored stars (*) indicate significant differences from 27HC treated tumors (mean +/− SEM, p<0.05, n = 5-9). The latency (C) and growth (D) of tumors in the MMTV-PyMT mouse model of breast cancer was evaluated in mice in which catabolism of 27HC is attenuated (CYP7B1−/− background). Significance between curves is indicated by a connecting black line and * (p<0.05, n = 10-28). (E) Tumor growth in MMTV-PyMT mice is increased by 27HC and attenuated by LXR agonists. MMTV-PyMT mice were injected daily with either 27HC, E2, GW3965 or vehicle as indicated. The growth of tumors in the 27HC and E2 treated mice were significantly different from those grown in Placebo and GW3965 treated animals (p<0.05, n = 110 total). (F) The growth of the ER-positive E0771 murine cell-derived xenografts was stimulated by 27HC when grown syngenically. Treatments were by injection as indicated. Colored stars indicate significant difference from placebo at the selected time point (mean +/− SEM, p<0.05, n = 7).
The impact of 27HC on tumor pathology was next evaluated in an immune competent Mouse Mammary Tumor Virus-Polyoma Middle T-Antigen (MMTV-PyMT) mouse model. These mice, which express the MMTV-PyMT transgene, develop spontaneous ERα-positive mammary adenocarcinomas that metastasize to the lung (19, 20). For these studies, the MMTV-PyMT mice were crossed onto a CYP7B1+/+ or −/− background. The cytochrome p450 monooxygenase CYP7B1 is responsible for the catabolism of 27HC. In CYP7B1−/−/MMTV-PyMT mice it was determined that plasma and intra-tumoral 27HC concentrations were ~3 times higher than in CYP7B1+/+/MMTV-PyMT control mice. Tumor latency was dramatically reduced in the CYP7B1−/− mice (Fig. 1C). Once palpable tumors formed, they grew at a significantly increased rate in CYP7B1−/− than those in CYP7B1+/+ mice (Fig. 1D). Treatment of CYP7B1−/− mice with ICI 182,780 resulted in tumor growth rates similar to those in a CYP7B1+/+ background, confirming the role of ER in this process. In a separate study, at the time of tumor detection, CYP7B1+/+/MMTV-PyMT mice were treated daily with placebo, 27HC, E2 or the synthetic LXR agonist GW3965. As shown in Fig. 1E, treatment with E2 or 27HC significantly increased the growth of tumors compared to vehicle, while GW3965 slightly retarded tumor growth when compared to placebo, a result that mirrors the responses observed in vitro (fig. S2 B,C). The tumor promoting effects of 27HC were also confirmed in a second murine model of ER-positive breast tumors. For this study, E0771 cells, derived from a spontaneous mammary tumor in C57BL/6 mice, were propagated syngenically in ovariectomized mice. In this model, tumor growth was increased by either E2 or 27HC supplementation and the activity of 27HC could be inhibited by cotreatment with ICI 182,780. The LXR agonist GW3965 decreased tumor growth (Fig. 1F). Assessment of gene expression in the MMTV-PyMT model indicated that markers for proliferation, macrophage infiltration, angiogenesis and invasion were increased in 27HC treated mice (fig. S5). Elevated expression of both ER and LXR target genes was also observed. These studies confirm the ER and LXR agonist activity of 27HC and implicate ER as the mediator of the effects of this oxysterol on primary tumor growth.
Given the role of CYP7B1 in 27HC catabolism, we considered that differences in its expression might track with outcomes in ER-positive breast cancers. To address this question, we evaluated CYP7B1 mRNA expression in several different human breast cancer datasets and determined that its elevated expression is associated with better survival outcome in luminal A types (P=0.0469) (fig. S6a). This is an important finding as, in general, luminal A-breast cancers generally express ER and are most likely to respond to ER antagonists or aromatase inhibitors and would be expected to be influenced by the estrogenic activity of 27HC.
Expression of CYP27A1 (cytochrome p450 oxidase required for the conversion of cholesterol to 27HC) mRNA in breast tumors did not correlate with outcome in this disease, prompting a closer examination of CYP27A1 protein expression in tumors (fig. S6b). Previously, it has been determined that CYP27A1 is highly expressed in macrophages (21). Our studies revealed that, regardless of where macrophages reside in human breast tissue (benign vs. malignant or intraductal vs. stromal), they consistently stain strongly for CYP27A1 protein (fig. S7). The well-established correlation between macrophage infiltration and breast cancer outcome raises the possibility that macrophage-produced 27HC may be able to support the growth of ER-positive breast tumors (22). To test this hypothesis, we evaluated whether conditioned media from bone-derived macrophages could support the growth of ER-positive breast cancer cells and whether media quality was influenced by CYP27A1 activity. We found that (a) macrophage conditioned media stimulated MCF7 cell proliferation, and that this activity was inhibited by ICI 182,780, and (b) the basal effect of conditioned media from CYP27A1−/− macrophages on MCF7 cell proliferation was increased by 27HC supplementation (fig. S8). Importantly, the ability of conditioned media to support the growth of MCF7 cells was compromised when macrophages were treated with two different CYP27A1 inhibitors (GI268267X and GW273297X (23)), and the effects of these inhibitors could be reversed by adding 27HC. These data suggest that local production of 27HC by tumor-associated macrophages is likely to have a significant impact on tumor pathology.
In addition to macrophages, we also found that CYP27A1 protein was expressed, to varying degrees, in cancer cells themselves (fig. S9). More specifically, we determined that increased expression of this enzyme was observed in higher grade tumors (estimated odds ratio of 6.7, CI 1.7-27, p=0.0007; Table 1, fig. S9). Thus, in addition to paracrine production from macrophages, autocrine production of 27HC by cancer cells is likely to influence tumor pathology.
Table 1.
Overexpression of CYP27A1 increases likelihood of having a higher tumor grade. Immunohistochemical analysis of CYP27A1 expression in human breast cancer tissue microarrays. CYP27A1 expression was determined to be low or high and correlated with tumor grade, estrogen receptor (ER), progesterone receptor (PR) or Human Epidermal Growth Factor Receptor 2 (HER2) status. A Fisher’s exact test was used to determine P-values for the likelihood of association. Ordinal logistic regression was used to estimate the odds ratio. N/A: not applicable due to too small of sample number.
| Grade | ER | PR | HER2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | 1 | 2 | 3 | + | − | + | − | + | − | |
| CYP27A1 (low) | 48 | 19% | 65% | 17% | 81% | 19% | 73% | 27% | 8% | 92% |
| CYP27A1 (high) | 11 | 0% | 45% | 55% | 71% | 29% | 68% | 32% | 0% | 1% |
| P value | 0.02 | 0.02 | 0.03 | 0.19 | ||||||
| Odds Ratio | 6.7 | 0.19 | 0.21 | N/A | ||||||
Recently, it was reported that a diet high in both fat and cholesterol decreases latency and increases tumor growth and metastasis in MMTV-PyMT mice (24). It has also been shown that tumor xenografts grew faster when propagated in the hyperlipidemic Apoe−/− mouse model (25). Because a high-fat, high-cholesterol diet was used in both of these studies, the specific contribution of cholesterol (or its metabolites) on tumor biology could not be assessed. To directly address this question, we evaluated tumor pathology in the MMTV-PyMT model as a function of a high cholesterol diet (HCD). In this study, CYP27A1+/+/MMTV-PyMT mice fed a HCD from weaning developed palpable tumors earlier than mice on a control diet (Fig. 2A). Furthermore, once tumors were detected, they grew at a faster rate in mice fed a HCD compared to mice on a control diet (Fig. 2B). We confirmed that the intratumoral concentrations of 27HC in HCD-fed mice were elevated and reflect the levels observed in human patients without any known genetic predisposition to elevated cholesterol (15, 26) (fig. S10). However, in CYP27A1−/−/MMTV-PyMT mice, in which 27HC is undetectable, it was observed that tumor latency was increased and tumor growth decreased when mice were fed a control diet, and the effect of HCD on tumor pathology was negated in the CYP27A1 null background (Fig. 2A, B). Importantly, the impaired growth of mammary tumors in CYP27A1−/− mice could be restored to that observed in CYP27A1 intact mice by daily injection of 27HC from the point of detection of a palpable tumor. These data indicate that 27HC, and not cholesterol per se, is pathologic in breast tumors.
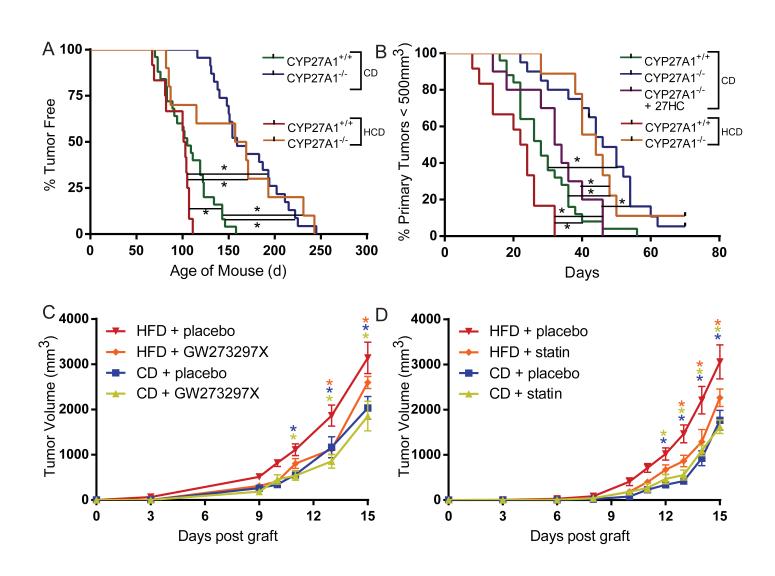
Fig. 2. Genetic or pharmacological inhibition of 27-hydroxycholesterol production attenuates hypercholesterolemia-promoted tumor growth in mice.
The latency and growth of tumors in the MMTV-PyMT mouse model of breast cancer were evaluated in mice in which the conversion of cholesterol into 27HC was inhibited by disruption of the CYP27A1 gene (CYP27A1−/−). For this study, MMTV-PyMT mice were bred onto a CYP27A1+/+ or a −/− background and (A) tumor latency and (B) tumor growth were measured in mice on a control diet (CD) or on a high cholesterol diet (HCD) from weaning. Note that in the tumor growth studies daily injection of 27HC overcame the inhibitory effect of CYP27A1 deletion. Significance between curves is indicated by a connecting black line and * (p<0.05, n = 9-25). The growth of the ER-positive E0771 murine cell-derived grafts were evaluated when grown in syngeneic APOE3 mice fed a control diet or a HFD in following coadministration of (C) the CYP27A1 inhibitor GW273297X (or vehicle) or (D) the statin atorvastatin (or vehicle) as indicated. Stars (*) indicate statistically significant differences with the HFD + placebo group (mean +/− SEM, p<0.05, n = 6-12).
Unlike humans, mice do not normally become hypercholesterolemic when fed a high fat diet (HFD). Therefore, we made use of an APOE3 targeted replacement mouse model, in which the mouse Apoe gene has been replaced with the human APOE3 allele (27). A HFD significantly increases circulating levels of both total cholesterol and 27HC in these mice. Importantly, the elevated 27HC can be decreased by treatment with a CYP27A1 inhibitor (GW273297X) (fig. S11). Using the ER-positive E0771 model, we demonstrated that tumors grew faster in HFD APOE3 mice compared to mice on a control diet, and that tumor growth was attenuated by treatment with GW273297X administered by daily injection (Fig. 2C). As plasma 27HC concentrations are correlated with circulating cholesterol (26), we tested whether inhibition of de novo cholesterol synthesis would impact tumor growth. Indeed, we found that oral administration of the statin atorvastatin, reduced the level of circulating cholesterol and attenuated the enhanced tumor growth associated with a HFD (Fig. 2D, S12). Importantly, MMTV-PyMT mice on a HFD do not develop hypercholesterolemia and tumor growth in these animals was indistinguishable from those on a control diet (fig. S13). Thus, in this APOE3 mouse model, a HFD enhances tumor growth, an effect that can be partially reversed by treatment with agents that inhibit the biosynthesis of cholesterol or 27HC.
We next addressed whether 27HC impacted metastasis in the MMTV-PyMT model. As shown in Fig. 3A, a greater number of metastatic foci were observed in lungs from mice treated with daily 27HC injections compared to mice injected with vehicle alone. Metastasis was evaluated when total tumor burden had reached 2cm3. The effect of 27HC on metastasis in this model was confirmed by measuring lung PyMT mRNA expression. By this measure, decreased metastasis was observed in the CYP27A1−/− mice, with increased metastasis being evident in the CYP7B1−/− mice. A similar relationship between elevated 27HC and metastasis (as assessed by PyMT mRNA expression) was observed in mice receiving daily injections of 27HC (Fig. 3B). In contrast to the effects on the growth of primary tumors (above), the actions of 27HC on metastasis do not appear to involve ER, as treatment of animals with E2 was without effect in this model. Conversely, whereas LXR activation attenuates E2-dependent breast cancer cell proliferation in vitro and in the tumor models described above, the LXR agonist GW3965 increased lung metastasis, albeit less efficiently than 27HC. We conclude from these studies that LXR activation by 27HC increases tumor metastasis and that these activities occur independently of ER.
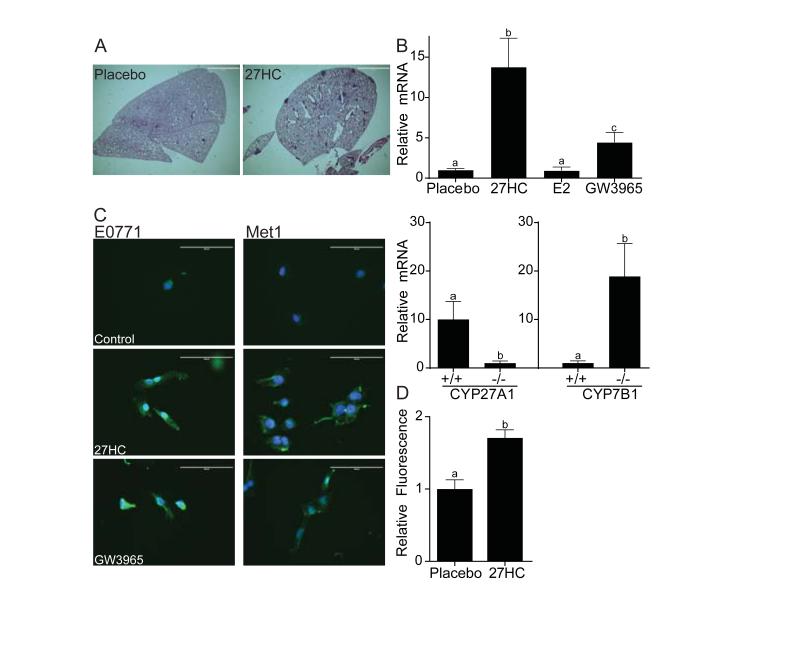
Fig. 3. Increased metastasis of breast cancer cells to lung is observed in mice in which circulating 27-hydroxycholesterol is elevated.
(A) Representative lung sections from MMTV-PyMT mice reveals an increased number of metastatic lesions in mice following injection of 27HC or placebo. Metastasis was evaluated when total tumor burden had reached 2cm3. (B) Quantification of PyMT mRNA, a surrogate for mammary tumor cell metastasis, in MMTV-PyMT-mice in which circulating 27-hydroxycholesterol production is inhibited (CYP27A1−/− background) or increased (CYP7B1−/−). Different letters denote statistical significance (mean +/− SEM, p<0.05, n= 4-13). (C) Both 27HC and the LXR agonist GW3965 induce the expression of vimentin and induce EMT-like morphological changes in E0771 breast cancer cells in vitro. Overlaid images of green (vimentin) and blue (dapi nuclear stain). All images were adjusted to 90% brightness and 100% contrast. Scale bar = 100μm (D) Cell intrinsic effects of 27HC on metastatic potential of E0771 breast cancer cells. E0771 cells, tagged by expressing infrared florescence protein (IRFP), were treated in vitro for 72 hours with 27HC or vehicle and injected into the tail veins of syngeneic mice. Twenty-eight days later, lung colonization was evaluated by assessing fluorescence in harvested lungs (mean +/− SEM, p<0.05, n= 5).
Gene expression analysis revealed that 27HC and LXR agonists induce the expression of several genes involved in epithelial-mesenchymal-transition (EMT) (fig. S14). In addition, breast cancer cell lines treated in vitro with 27HC or with a synthetic LXR agonist adopt a spindle-like morphology, which mirrors the increased expression of vimentin, Snail1 and FAPα, established markers of EMT (Fig. 3C, fig. S14). To examine the in vivo relevance of these observations, we assessed the impact of 27HC treatment on the metastatic potential of ER-negative, LXR-positive Met1 cells. It was determined that when these cells were pretreated in vitro with 27HC and injected i.v. into animals, they readily metastasized to the lung (Fig. 3D). These results are consistent with the hypothesis that 27HC, acting through LXR, increases lung metastasis secondary to effects on EMT.
In summary, we have shown the pathologic actions of cholesterol on ER-positive breast cancer require its conversion to 27HC. Further, it was demonstrated that the actions of 27HC on primary tumor growth are dependent on ER, whereas its actions in metastasis require LXR. These results may have implications for both the treatment and prevention of breast cancer. The SERMs tamoxifen and raloxifene were recently approved in the US as breast cancer chemopreventive agents in post-menopausal women at high risk for the disease. These agents can have adverse side effects and may not be well suited for women at average risk of breast cancer. However, our data suggest that a reduction in breast cancer risk may also be achieved by lowering total cholesterol and highlight the need for additional studies to evaluate the efficacy of this approach. Studies to evaluate the impact of lowering cholesterol on response to endocrine therapy in breast cancer are also warranted. Finally, the observation that LXR, but not ER, is required for metastasis in mice with elevated 27HC highlights the potential relevance of our findings to other cancers in which LXR expression is apparent.
Supplementary Material
Acknowledgements
Funded by the National Institutes of Health NIH: K99CA172357 (ERN), NIH R37DK048807 (DPM) and Department of Defense: DOD BC085585 (ERN), DOD BC094960 (DPM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Department of Defense. Expression profiling data on 27HC in MCF7 cells were uploaded to GEO (accession number GSE46924) and was part of a larger study (GSE35428).
References
- 1.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 2.Capasso I, et al. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol Ther. 2011;10:1240. doi: 10.4161/cbt.10.12.13473. [DOI] [PubMed] [Google Scholar]
- 3.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, McGuire V. Evidence of association between plasma high-density lipoprotein cholesterol and risk factors for breast cancer. J Natl Cancer Inst. 1990;82:460. doi: 10.1093/jnci/82.6.460. [DOI] [PubMed] [Google Scholar]
- 5.Ferraroni M, et al. HDL-cholesterol and breast cancer: a joint study in northern Italy and southern France. Int J Epidemiol. 1993;22:772. doi: 10.1093/ije/22.5.772. [DOI] [PubMed] [Google Scholar]
- 6.Kitahara CM, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat. 2012;135:261. doi: 10.1007/s10549-012-2154-x. [DOI] [PubMed] [Google Scholar]
- 8.Ahern TP, et al. Statin Prescriptions and Breast Cancer Recurrence Risk: A Danish Nationwide Prospective Cohort Study. J Natl Cancer Inst. 2011 doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 10.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10. [PubMed] [Google Scholar]
- 11.Gerber JG, et al. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of AIDS Clinical Trials Group 5108 Study. J Acquir Immune Defic Syndr. 2005;39:307. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- 12.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 13.DuSell CD, et al. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology. 2010;151:3675. doi: 10.1210/en.2010-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson ER, et al. The oxysterol, 27-hydroxycholesterol, links cholesterol metabolism to bone homeostasis through its actions on the estrogen and liver x receptors. Endocrinology. 2011;152:4691. doi: 10.1210/en.2011-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umetani M, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 17. Materials and methods are available as supplementary material on Science Online.
- 18.Connor CE, et al. Circumventing tamoxifen resistance in breast cancers using antiestrogens that induce unique conformational changes in the estrogen receptor. Cancer Res. 2001;61:2917. [PubMed] [Google Scholar]
- 19.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin EY, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson M, Ellis E, Hunt MC, Schmitz G, Babiker A. Marked induction of sterol 27-hydroxylase activity and mRNA levels during differentiation of human cultured monocytes into macrophages. Biochim Biophys Acta. 2003;1593:283. doi: 10.1016/s0167-4889(02)00398-1. [DOI] [PubMed] [Google Scholar]
- 22.Sica A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Lyons MA, Brown AJ. Metabolism of an oxysterol, 7-ketocholesterol, by sterol 27-hydroxylase in HepG2 cells. Lipids. 2001;36:701. doi: 10.1007/s11745-001-0775-8. [DOI] [PubMed] [Google Scholar]
- 24.Llaverias G, et al. Role of cholesterol in the development and progression of breast cancer. Am J Pathol. 2011;178:402. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alikhani N, et al. Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model. Oncogene. 2013;32:961. doi: 10.1038/onc.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karuna R, et al. Plasma levels of 27-hydroxycholesterol in humans and mice with monogenic disturbances of high density lipoprotein metabolism. Atherosclerosis. 2011;214:448. doi: 10.1016/j.atherosclerosis.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan PM, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 28.Wardell SE, Kazmin D, McDonnell DP. Research resource: Transcriptional profiling in a cellular model of breast cancer reveals functional and mechanistic differences between clinically relevant SERM and between SERM/estrogen complexes. Mol Endocrinol. 2012;26:1235. doi: 10.1210/me.2012-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haibe-Kains B, Schroeder M, Bontempi G, Sotiriou C, Quackenbush J. genefu: Relevant Functions for Gene Expression Analysis, Especially in Breast Cancer. R package version 1.8.0. 2012 [Google Scholar]
- 30.Parker JS, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heller G, et al. Downregulation of TSLC1 and DAL-1 expression occurs frequently in breast cancer. Breast Cancer Res Treat. 2007;103:283. doi: 10.1007/s10549-006-9377-7. [DOI] [PubMed] [Google Scholar]
- 32.RCoreTeam . R: A language and environment for statistical computing. R Foundation for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria; 2012. [Google Scholar]
- 33.Szeles L, et al. Research resource: transcriptome profiling of genes regulated by RXR and its permissive and nonpermissive partners in differentiating monocyte-derived dendritic cells. Mol Endocrinol. 2010;24:2218. doi: 10.1210/me.2010-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.