Abstract
Pre-eclampsia (PE), new onset hypertension with proteinuria during pregnancy, is associated with chronic inflammation and placental oxidative stress (ROS). Chronic IL-17 increases blood pressure (MAP), autoantibodies (AT1-AA) and ROS during pregnancy. The objective of this study was to determine if TH17 suppression via IL-17RC (recombinant receptor C) decreases pathophysiology associated with placental ischemia (RUPP). On gestation day 14, mini-osmotic pumps infusing 100 pg/day of IL-17RC were implanted into pregnant rats undergoing RUPP (Reduced Uterine Perfusion Pressure), gestation day18 carotid catheters were inserted, day 19 MAP was recorded, TH17 cells, oxidative stress and AT1-AA were measured and analyzed via one-way ANOVA. MAP increased from 101 ±2 mmHg in normal pregnant, NP (n=19), to 120 ±1 mmHg in RUPP (n=17),but decreased to 110±2 mmHg in RUPP+IL-17RC rats (n=22). Pup weight decreased from 2.28 ± 0.2 g in NP to 1.96 ± 0.3 g in RUPP rats, but was significantly increased to 2.01 ± 0.1 in RUPP+IL-17RC rats. TH17 cells were 1.77% in RUPP but decreased to 0.65% in RUPP+IL-17RC rats. Urinary isoprostanes normalized in RUPP +IL-17RC rats (52 pg/μg) compared to 89 pg/μg in RUPP controls. Placental ROS was 652 RLU in RUPP, but decreased to 337 RLU in RUPP+IL-17RC rats. AT1-AA was 17.27 ± 0.7 bpm in RUPP but decreased to 5.00 ± 0.5 bpm in RUPP+IL-17RC rats. With this study, we show that infusion of IL-17RC blunts TH17s, oxidative stress, AT1-AA, and hypertension in the RUPP model of PE indicating that TH17 cells may play an important role in disease pathophysiology.
Keywords: hypertension, pregnancy, inflammation, oxidative stress
Introduction
Pre-eclampsia (PE) is a disease that affects 5-8% of pregnancies in the United States 1, 2. Hallmark characteristics of pre-eclampsia are new onset hypertension with proteinuria, immune activation, and imbalances between pre-angiogenic factors (vascular endothelial growth factor/placental growth factor [VEGF/PlGF]) and anti-angiogenic factor, soluble fms-like tyrosine kinase-1 (sFlt-1) and oxidative stress (ROS) during pregnancy. The exact pathophysiologic mechanisms that lead to the development of pre-eclampsia have yet to be determined but are believed to involve shallow trophoblasts invasion and early alteration of natural killer cells and T cells 2-4. This shallow trophoblasts invasion is thought to lead to decreased vasculogenesis in the growing uteroplacental unit 2. The lack of vascular remodeling leads to lower oxygen and nutrient delivery to the placenta thereby contributing to the development of placental ischemia.
Placental ischemia/hypoxia is implicated as an important mechanism in the development of pre-eclampsia. Placental ischemia induces oxidative stress, an imbalance in pro-angiogenic and anti-angiogenic factors, and immune activation and endothelial dysfunction in the maternal vasculature 2, 5. It has been proposed that the imbalance between two CD4+ T cell types, T regulatory (TReg) and T-helper 17 (TH17), is involved in the pathophysiology of pre-eclampsia 6. We have previously shown that placental ischemia stimulates this CD4+ T helper cell imbalance which contributes to excess pro-inflammatory cytokine production, a shift in angiogenic factors and leads to hypertension during pregnancy 7. T-helper 17 cells are a subclass of CD4+ T cells, characterized by their secretion of the cytokine IL-17, which has also been implicated in a number of autoimmune disorders, including psoriasis, multiple sclerosis, rheumatoid arthritis, and irritable bowel syndrome (IBS) 8, 9. Recent studies have shown these cells to be elevated in the circulation of pre-eclamptic patients compared to those with normal pregnancies 6, 10. Other autoimmune activities during pre-eclampsia, such as the production of autoantibodies to the angiotensin II type I receptor (AT1-AA) and increased circulating IL-17, indicate many similarities between pre-eclampsia and autoimmune diseases 5, 11. We have previously shown both TH17 cells and IL-17 to be significantly elevated in the RUPP rat model of preeclampsia [7]. Importantly, we recently demonstrated a role for the cytokine IL-17, which is predominately secreted by TH17 cells, in causing placental oxidative stress and hypertension during pregnancy. By infusing IL-17 into normal pregnant rats, we found TH17 cells to be elevated, along with urinary isoprostanes, placental oxidative stress, AT1-AA, and blood pressure compared to the normal pregnant control rat. Administering a superoxide dismutase mimetic, Tempol, attenuated the hypertension and placental oxidative stress, and significantly blunted the AT1-AA in response to IL-17 infusion. Therefore, these data suggested that generated reactive oxygen species seemed to behave as signaling molecules to stimulate the production of autoantibodies which have been shown by ours and other laboratories to play an important role in the pathogenesis of pre-eclampsia 12. In addition this led us to question a role for endogenous TH17 cells stimulated in the RUPP rat to mediate the pathophysiology of preeclampsia. There are no specifically defined inhibitors of TH 17 cells routinely used and we've shown that infusion of IL-17 stimulated circulating TH 17 cells in normal pregnant rats, therefore, we turned to inhibitors of the IL-17 pathway.
IL-17 receptor C (IL-17RC), functions as a receptor for IL-17 A and F, the bioactive heterodimer of IL-17 that is suspected to play a pathophysiological role in preeclampsia and autoimmune disease[13]. The stoichiometry is unclear and heterodimers and/or trimers of these molecules are thought to be essential for complete inhibition of the actions IL-17. It has been suggested that the soluble version of IL-17RC could be an effective inhibitor of IL-17 function and could be a potential treatment for autoimmune type diseases 13. In this current study, we investigated a role for endogenously stimulated IL-17 and TH17 cells in mediating hypertension in response to placental ischemia. We tested our hypothesis by administering IL-17 receptor C to block the IL-17 signaling cascade and thereby reduce circulating TH 17 cells, oxidative stress, AT1-AA, and hypertension associated with placental ischemia in the reduced uterine perfusion pressure (RUPP) rat model of pre-eclampsia.
Materials and Methods
Pregnant Sprague-Dawley rats purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN) were used in this study. Animals were housed in a temperature-controlled room (23 °C) with a 12:12-hour light/dark cycle. All experimental procedures executed in this study were in accordance with the National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
Administration of IL-17 RC to RUPP rats
On gestational day 14, under isoflurane anesthesia, normal pregnant (NP) rats underwent a reduction in uterine perfusion pressure (RUPP) with the application of a constrictive silver clip (0.203 mm) to the aorta superior to the iliac bifurcation performed while ovarian collateral circulation to the uterus was reduced with restrictive clips (0.100 mm) to the bilateral uterine arcades at the ovarian end. Recombinant mouse IL-17 receptor C (IL-17RC) (100 pg/day) (RnD Systems, Minneapolis, MN) was infused, intraperitoneally, from day 14 through day 19 of gestation via mini-osmotic pumps (model 2002, Alzet Scientific Corporation) in 22 RUPP rats. Murine IL-17RC has 87% homology and 86% identity to rat IL-17 RC indicating high biological similarity to the naturally occurring rat protein. The dose was determined based on binding ability of the soluble receptor to IL-17 A-F, as performed by the manufacturer. The groups of rats examined in this study were normal pregnant (NP, n=19) reduced uterine perfusion pressure (RUPP, n=17), and IL-17RC infused RUPP (RUPP+IL-17RC, n=22)
Measurement of mean arterial pressure into chronically instrumented conscious rats
Under isoflurane anesthesia, on day 18 of gestation, carotid arterial catheters were inserted for blood pressure measurements. The catheters inserted are V3 tubing (SCI) which is tunneled to the back of the neck and exteriorized. On day 19 of gestation, arterial blood pressure was analyzed after placing the rats in individual restraining cages. Arterial pressure was monitored with a pressure transducer (Cobe III Tranducer CDX Sema) and recorded continuously for one hour after a one hour stabilization period. Subsequently, a blood and urine sample was collected, kidneys, placentas, and spleens were harvested, and litter size and pup weights were recorded under anesthesia.
Placental UARI in response to IL-17 RC in RUPP rats
In a method described by Tam Tam [14] Power Doppler velocimetry measurements were performed on anesthetized pregnant dams on gestational day 18 at an imaging station with a Vevo 770 unit (Visual sonics) using a 30 Hz transducer and an insonating angle <30°. The peak systolic flow velocity (PSV) and end diastolic flow velocity (EDV) were recorded using the uterine artery Doppler waveform. The uterine artery resistive index (UARI) was calculated using the following formula: UARI= (PSV-EDV)/PSV. UARI was determined for the uterine artery bilaterally at three levels and the mean UARI was calculated for control RUPPs and compared to RUPPs + IL-17 RC.
Determination of circulating T-helper TH17 lymphocytes
Circulating TH17 T-helper cell population was determined from peripheral blood leukocytes (PBL) collected at day 19 of gestation from NP, RUPP, and RUPP+IL- rats. We utilized flow cytometry analysis to detect specific CD4+ T cell populations; CD4+CD25−RORγ+ (retinoic acid receptor-related organ receptor gamma) isolated from NP, RUPP, and RUPP+IL-17RC rats. At the time of harvest, plasma was collected and PBLs were isolated from plasma by centrifugation on a cushion of Ficoll-Hypaque (Lymphoprep, Accurate Chemical & Scientific Corp., Westbury, NY) according to the manufacturer's instructions. For flow cytometric analysis, equal number of leukocytes (1 × 106) were incubated for 30 minutes at 4 °C with antibodies against rat CD4 and rat CD25 (BD Biosciences, San Jose, CA). After washing, cells were labeled with secondary Fluorescein isothiocyanate (FITC; Southern Biotech, Birmingham, AL) and phycoerythrin with cyanin-5 (PE-Cy5; Santa Cruz Biotechnology Inc., Santa Cruz, CA) antibody for 30 minutes at 4 °C. Cells were washed, permeabilized, and stained with rabbit polyclonal RORγ (Santa Cruz Biotechnology Inc., Santa Cruz, CA) for 30 minutes at 4 °C. After washing, cells were labeled with secondary phycoerythrin (PE; Southern Biotech, Birmingham, AL) for 30 minutes at 4 °C. As a negative control for each individual rat, cells were treated exactly as described above except they were incubated with anti-FITC, anti-PE-Cy4, and anti-PE secondary antibodies alone. Subsequently, cells were washed and resuspended in 500 μL of Rosswell Park Memorial Institute medium (RPMI) and analyzed for single and double staining on a Gallios flow cytometer (Beckman Coulter, Brea, CA). The percent of positive staining cells above the negative control was collected for each individual rat and mean values for each experimental group (NP (n=10), RUPP (n=12), RUPP + IL-17RC (n=17)) were calculated.
Determination of urinary Isoprostane
On day 19 of gestation, urine was collected from NP, RUPP, and RUPP + IL-17RC rats for determination of excreted isoprostanes measured via ELISA from Oxford Biomedical Research (Oxford, MI). The assay displayed a sensitivity of 0.05 ng/mL, inter-assay variability was 4.2% and intra-assay variability was 4.7%.
Determination of placental ROS
Superoxide production in the placenta was measured by using the lucigenin technique as we have previously described 14, 15. Rat placentas from NP, RUPP, and RUPP + IL-17RC rats were snap frozen in liquid nitrogen directly after collection and stored at −80 °C until further processing. Placentas were removed and homogenized in RIPA buffer (phosphate-buffered saline, 1%Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and a protease inhibitor cocktail; Santa Cruz, Santa Cruz, CA) as described previously. 14, 15 The samples were centrifuged at 16,000g for 30 min, the supernatant aspirated and the remaining cellular debris discarded. The supernatant was incubated with lucigenin at a final concentration of 5μmol/l. The samples were allowed to equilibrate for 15min in the dark, and luminescence was measured every second for 10 sec with a luminometer (Berthold, Oak Ridge, TN). Luminescence was recorded as relative light units (RLU) per min. An assay blank with no homogenate but containing lucigenin was subtracted from the reading before transformation of the data. Each sample was repeated 5 times and the average used for data transformation. The protein concentration was measured using a protein assay with BSA standards (Pierce, Rockford, IL). The data are expressed as RLU/min/mg protein.
Determination of Circulating AT1-AA
On day 18 of gestation blood was collected and immunoglobulin was isolated from 1 ml of serum by ammoniumsulfate precipitation. This IgG fraction was used in a bioassay. The AT1-AA activity was measured using spontaneously beating neonatal rat cardiomyocytes and characterized and antagonized specifically using AT1 receptor antagonists. The results express the difference between the basal beating rate of the cardiomyocytes and the beating rate measured after the addition of the AT1-AA (increase in number of beats/min or Δ beats/min 14, 16-20. AT1-AAs were assessed in NP, RUPP controls and RUPP + IL-17RC-infused rats.
Statistical Analysis
All of the data are expressed as mean ± SEM. Comparisons of control with experimental groups were analyzed by analysis of variance with Student's T test as post hoc analysis. A value of P < .05 was considered statistically significant.
Results
IL-17RC infusion significantly blunted hypertension in RUPP rats
Mean arterial pressure (MAP) was measured on day 19 of gestation in NP, RUPP, and RUPP + IL-17RC rats. The MAP increased significantly from 101 ± 2 mm Hg in NP rats, to 120 ± 1 mmHg in RUPP rats (p < 0.0001; Figure 1). This increase in MAP in RUPP rats was blunted significantly to 110 ± 2 mmHg in RUPP + IL-17RC compared to RUPP rats (p = 0.004; Figure 1).
Figure 1.
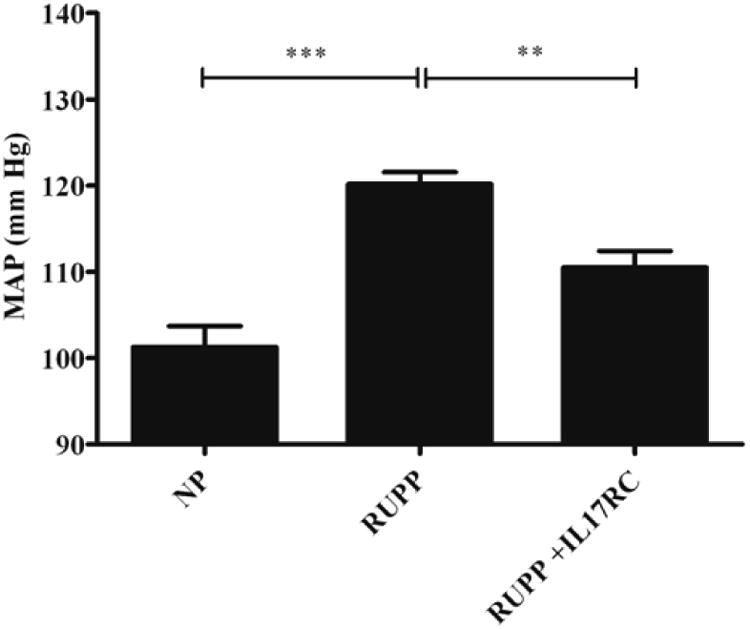
Blood pressure is increased in response to RUPP in pregnant rats (P<0.01). IL-17 receptor C significantly decreased blood pressure in RUPP rats (P<0.01).
IL-17RC infusion significantly increased pup and placenta weight and improved uterine artery resistance in RUPP rats
In Figure 2, pup weight of litters from RUPP rats (1.96 ± 0.3 g) was significantly lower than pup weight from NP rats (2.28 ± 0.2 g, p = 0.028). However, IL-17RC infusion into RUPP rats offset the decreased pup weight. Average pup weight in RUPP + IL-17RC rats was significantly increased compared to that in RUPP rats (2.01 ± 0.1 g; p = 0.05) but did not reach that of a normal pregnant rat offspring. Moreover, average placenta weight significantly decreased from 0.5 ±0.02 g in NP to 0.47±0.02 g in RUPP (p= 008), while infusion of IL-17RC into RUPP rats normalized placenta weight (0.52±0.2 g, p=0.04). Importantly, maternal body weight was not significantly increased by infusion of IL-17 RC into RUPP rats indicating the effect of IL-17 RC to increase pup weight may specific to the placental fetal unit (data not shown). Furthermore, uterine artery resistance index was improved in RUPP rats treated with IL-17 RC compared to control RUPP rat. We have shown that UARI increases with placental ischemia from 0.60+/−0.03 (n=4) in NP rats to 0.71+/−0.04 (n=7) in RUPP rats. In this study administration of IL-17RC improves UARI in RUPP rats to 0.64+/−0.063 in RUPP+IL-17 RC(n=5). However, this did not reach statistical significance but does indicate a potential improvement in blood and nutrient supply to the growing utero placental unit.
Figure 2.
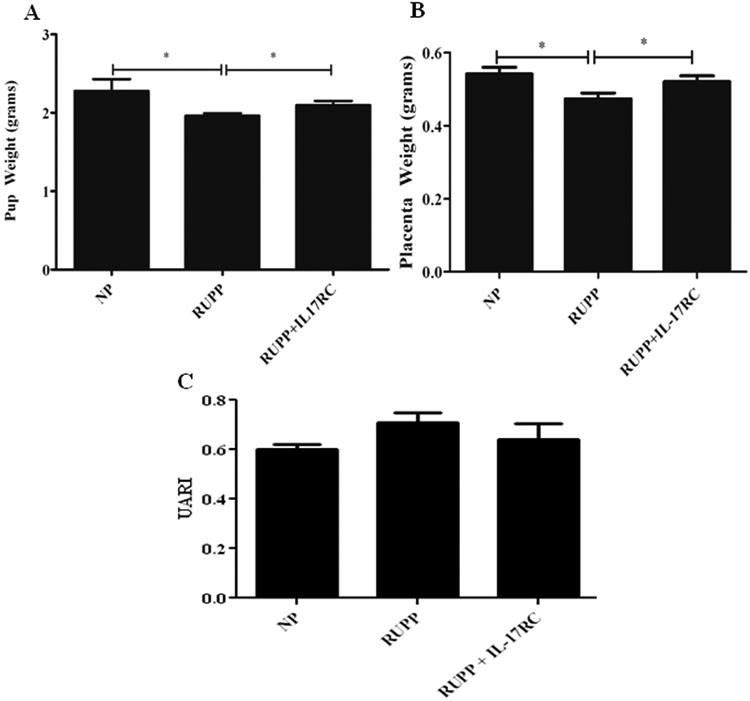
Pup and placental weights are decreased in response to placental ischemia in RUPP rats however, infusion of IL-17 RC significantly improved weights of offspring in RUPP rats (*P<0.05). Uterine resistance index is increased in RUPP rats compared to NP rats, but was lowered with administration of IL-17 RC.
IL17RC infusion blunts T-helper 17 cells, oxidative stress, and AT1-AA in RUPP rats
Circulating TH17 T-helper cells were 0.13± 0.09% of gated cells in NP rats, which increased to 6.29± 2.64% in RUPP rats. IL-17RC infusion into RUPP rats decreased the TH17 population to 0.69± 0.95% (Figure 3).
Figure 3.
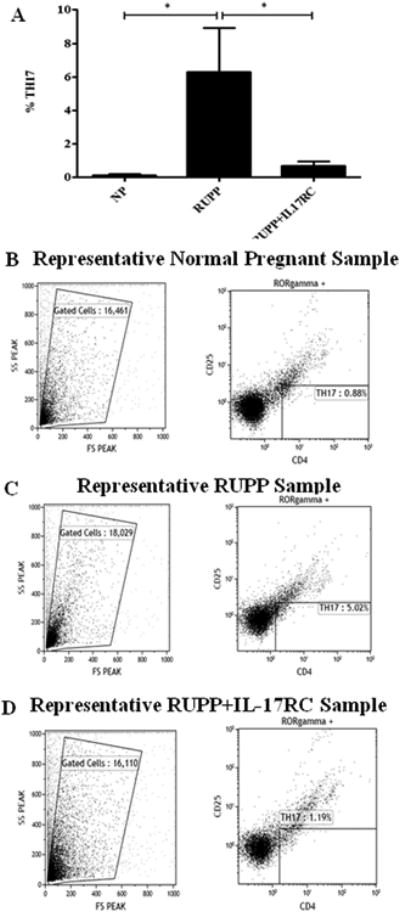
Circulating TH17 increases in response to placental ischemia in RUPP rats. Administration of IL-17 RC into RUPP rats blunted this increase. Circulating peripheral blood leukocytes (PBLs) isolated from normal pregnant (NP, n=9), placental ischemic (RUPP, n=10), and IL-17RC infused (RUPP + IL-17RC, n=12) rats were collected and analyzed by flow cytometry. Top panel (A) shows the graphed results indicating that TH17 cells (CD4+ RORγ+CD25−) are increased in RUPP compared to NP rats and are decreased in RUPP rats infused with IL-17RC (*P<0.05). The percent of CD4+RORγ+CD25− staining cells above the negative control was collected for each individual rat respectively and mean values for each experimental group (NP, RUPP, and RUPP + IL17-RC) was calculated. Panels (B–D) illustrate representative scatter plots and staining profiles of PBL triple stained with anti-CD4, anti-CD25, and anti-RORγ.
Urinary isoprostane levels were determined via ELISA. Isoprostane levels in RUPP rats increased to 89.03 pg/μg protein compared to NP rats at 56.41 pg/μg (Figure 4a). However, this was ablated by IL-17RC infusion into RUPP rats. Urinary isoprostane was normalized to 52.23 pg/μg protein in RUPP+IL-17 RC (Figure 4a).
Figure 4.
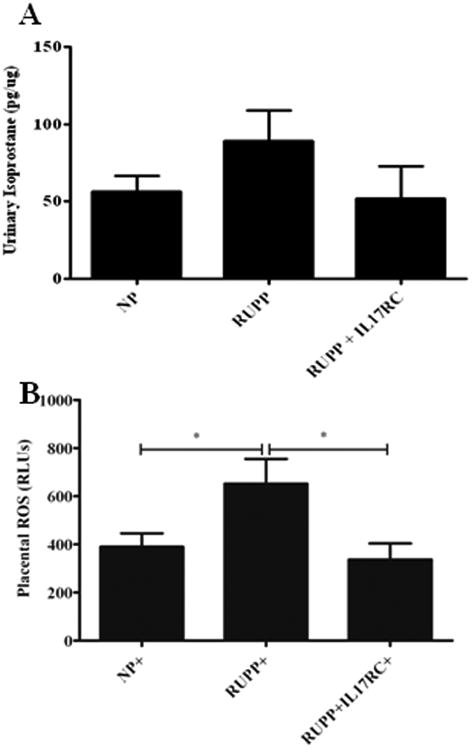
Oxidative stress is a hallmark of preeclampsia. In response to RUPP in pregnant rats urinary isoprostanes and placental ROS are significantly elevated. However, both are decreased with IL-17 RC infusion
Figure 4b shows that placental reactive oxygen species (ROS) was significantly increased in RUPP rats compared to NP rats (652 RLUs vs. 390 RLUs; p = 0.021). Infusion of IL-17RC into RUPP rats significantly reduced and normalized placental oxidative stress. Placental ROS was 337 RLUs in RUPP+IL-17RC rats (p = 0.013).
AT1-AA levels in NP rats were 0.40 ± 0.27 bpm. Autoantibody levels increased significantly in RUPP rats (Fig. 5, p < 0.0001). However, infusion of IL-17RC into RUPP rats decreased production of the AT1-AA to 5.99 ± 0.5 bpm from 17.27 ± 0.7 bpm in RUPP rats (Fig. 5, p < 0.001).
Figure 5.
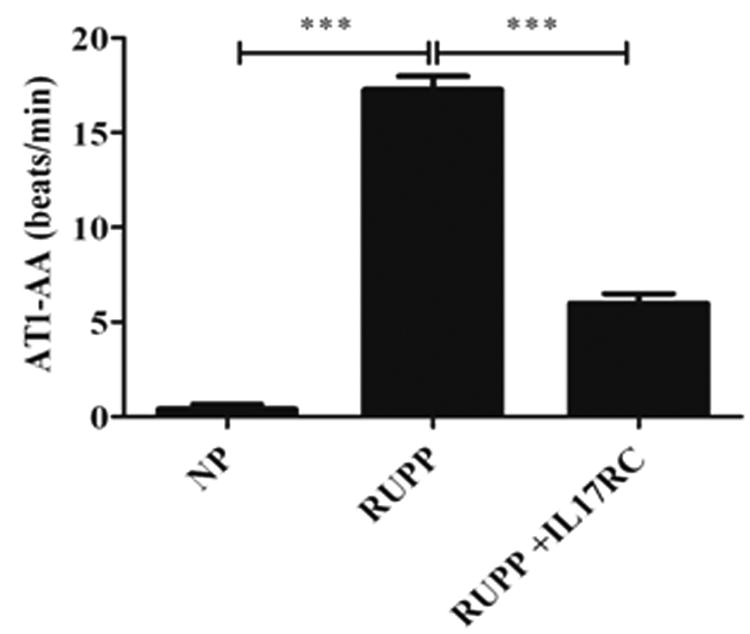
AT1-AA are significantly increased in RUPP rats and preeclamptic women. Administration of IL-17 RC significantly decreased circulating AT1-AA in RUPP rats.
Discussion
It has been established that IL-17 producing TH17 cells are mediators of several autoimmune diseases, to include psoriasis, rheumatoid arthritis, multiple sclerosis, and IBS 9. Recent research also proposes roles for IL-17 and TH17 cells as mediators of pre-eclamptic pathophysiology 6. We have recently published a study showing that chronic IL-17 infusion into pregnant rats increased blood pressure, circulating TH17 cells, placental ROS and AT1-AA. Therefore, in this study we sought to determine if infusion of soluble IL-17 receptor C would decrease circulating TH 17 cells, oxidative stress and hypertension in RUPP rat of model of pre-eclampsia. IL-17 soluble receptor C binds the most active forms of IL-17 superfamily, IL-17A and IL-17F. The stochiometry of this receptor is unclear as it may form a heterodimer in its active form, which would be important to biological activity since IL-17 cytokine is now considered a heterodimer between IL-17A:IL-17F.
Reduced uterine perfusion pressure (RUPP) in rats has been shown to increase blood pressure, while decreasing maternal and pup litter weight 21. We have recently showed IL-17 and TH17 cells to be increased in RUPP rats compared to NP rat controls. In this study, we demonstrate that IL-17RC infusion into pregnant RUPP rats significantly decreased blood pressure, AT1-AA production, and ROS in the placenta, while increasing pup and placenta weight and improving UARI (Figures 1-5). These data further support a role for IL-17 and TH17 cells in the pathophysiology of pre-eclampsia. Given that infusion of IL-17RC was initiated at the time of placental insult (gestational day 14), it could be that early suppression of TH17 could normalize the placental inflammation to some degree thereby correcting fetal demise. Though we have administered various anti-inflammatories to RUPP rats and have demonstrated an important role for suppression of inflammation to decreased blood pressure response in this model, this is the first of our studies to demonstrate a fetal protective mechanism to occur by inhibiting a specific immune pathway in RUPP rats. Therefore these data are very important to demonstrate the importance of immune suppression to improve both maternal and fetal health in response to placental ischemia.
IL-17 is produced by a number of cells types, including γ δ T cells, natural killer T cell (NKT), neutrophils, eosinophils, and monocytes 22, 23. Furthermore, expression of cellular receptors are found almost ubiquitously in all tissues. IL-17 R is found on endothelial cells, fibroblast cells and many immune cells. Infusion of soluble IL-17 RC could therefore interfere with various cellular-immune reactions that are stimulated in response to placental ischemia of pregnancy. Importantly, we show that IL-17 RC attenuates the rise in circulating TH17 cells observed in response to placental ischemia [7].
The most well noted physiological role of IL-17 and IL-17 -producing TH17 cells is to recruit host defense cells, such as neutrophils, to sites of infection. IL-17 stimulates the production of other cytokines by neutrophils for cell to cell communication, and more importantly, it stimulates these cells to release antimicrobial substances. Reactive oxygen species production is a defense mechanism used by neutrophils and macrophages against bacterial infection. However, production of ROS by activated neutrophils can also damage normal host tissue. Here, we demonstrate that blockade of IL-17 decreases urinary isoprostane, and significantly decreases placental ROS that results from placental ischemia in RUPP rats (Figure 4). Therefore, the IL-17RC may be inhibiting the recruitment of neutrophils to the placental unit and therefore neutrophilic ROS production. Further, we demonstrated a significant reduction in the percent of circulating TH17 in pregnant RUPP rats infused with IL-17RC compared to control RUPP rats (Figure 3). This demonstrates a role for IL-17 in the CD4+ T cell imbalance that is seen in response to placental ischemia. However it remains to be determined if the CD4+ T cell imbalance is due to increased IL-17 or causes increased IL-17 in pre-eclampsia. This, along with our previous data, suggests an important role for elevated IL-17 to stimulate this imbalance among T cells. Elevated IL-17 level could begin early on with an imbalance in natural killer cells in the uterus. These cells have been shown to play a very important role in the success of pregnancies and since they secrete IL-17, they could therefore influence the T cell imbalance that occurs during pre-eclampsia. Therefore, IL-17 blockade by the soluble receptor could normalize the T cell balance by inhibiting NK-produced IL-17. However, uterine NK cells nor neutrophils were measure in this model nor in response to IL-17 RC. Therefore future studies are important to determine the potential effect IL-17 RC may have on inhibiting placental neutrophils or uterine natural killer cells and improvement of pregnancy outcomes in response to placental ischemia.
AT1-AAs have been shown to play an important role in hypertension during pregnancy. We recently showed that IL-17 induced hypertension was associated with AT1-AA and oxidative stress. Administration of the superoxide dismutase mimetic, Tempol, decreased blood pressure, placental ROS and surprisingly AT1-AA in response to IL-17 infusion during pregnancy. These data indicated the importance of ROS as signaling molecules in the pathophysiology hypertension during pregnancy. In the current study we show decreasing TH17 cells in RUPP rats decreased oxidative stress, autoantibodies and blood pressure in response to placental ischemia (Figure 5). Importantly, pup weight was significantly improved in RUPP rats with lower placental oxidative stress and AT1-AA, indicating the importance of not only ROS and AT1-AA in fetal demise but supports our hypothesis that TH17 cells play an important role in the hypertension and intrauterine growth restriction during pre-eclampsia. It is important to continue future research investigating the role of immune alterations to mediate the pathophysiology of pre-eclampsia in order to develop novel and innovative treatment strategies that can improve both maternal and fetal health in the face of this devastating disease.
Perspective
This study demonstrates an important role for IL-17 to mediate the pathophysiology of hypertension and intrauterine growth restriction in the preeclamptic RUPP rat model. The data presented suggest that IL-17 may be a therapeutic target in preeclamptic women. Inhibition of IL-17 may help to regulation the angiotensin system and significantly reduce blood pressure and placental oxidative stress, as well as lead to an increase in fetal weight. These perceived benefits could result in a longer, healthier pregnancy, therefore, leading to a decrease maternal and infant morbidity that is associated with pre-eclampsia.
Novelty and Significance.
What is new?
Administration of IL-17 inhibitor decrease immune cells and signaling molecules in a rat model of hypertension during pregnancy
This decrease of immune cells and molecules resulted in lower blood pressures and improved pup weights.
What is relevant?
Blockade of this inflammatory cascade could be used to improve pregnancy outcomes preeclamptic women
Summary
This study highlights the importance of immune mechanisms to cause the increase in blood pressure and decrease in fetal weight that is associated with preeclampsia, both of which can be corrected by administration of specific anti-inflammatories during the later gestational stages.
Acknowledgments
Sources of Funding: This work was supported by NIH grants HL78147 and HL51971 and HD067541. RD is supported by the German Research Foundation (DFG 631/7-1).
Footnotes
Disclosures: None
References
- 1.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nature clinical practice Nephrology. 2005;1:98–114. doi: 10.1038/ncpneph0035. quiz 120. [DOI] [PubMed] [Google Scholar]
- 2.George EM, Granger JP. Recent insights into the pathophysiology of preeclampsia. Expert review of obstetrics & gynecology. 2010;5:557–566. doi: 10.1586/eog.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 4.Lindheimer MD, Romero R. Emerging roles of antiangiogenic and angiogenic proteins in pathogenesis and prediction of preeclampsia. Hypertension. 2007;50:35–36. doi: 10.1161/HYPERTENSIONAHA.107.089045. [DOI] [PubMed] [Google Scholar]
- 5.Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva ginecologica. 2010;62:105–120. [PMC free article] [PubMed] [Google Scholar]
- 6.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, Nanan R. Systemic increase in the ratio between foxp3+ and il-17-producing cd4+ t cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 7.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN, Jr, LaMarca B. Cd4+ t-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57:949–955. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mai J, Wang H, Yang XF. Th 17 cells interplay with foxp3+ tregs in regulation of inflammation and autoimmunity. Frontiers in bioscience : a journal and virtual library. 2010;15:986–1006. doi: 10.2741/3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of th17 cells in human disease. Trends in immunology. 2011;32:603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toldi G, Rigo J, Jr, Stenczer B, Vasarhelyi B, Molvarec A. Increased prevalence of il-17-producing peripheral blood lymphocytes in pre-eclampsia. Am J Reprod Immunol. 2011;66:223–229. doi: 10.1111/j.1600-0897.2011.00987.x. [DOI] [PubMed] [Google Scholar]
- 11.Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. American journal of hypertension. 2001;14:178S–185S. doi: 10.1016/s0895-7061(01)02086-6. [DOI] [PubMed] [Google Scholar]
- 12.Dhillion P, Wallace K, Herse F, Scott J, Wallukat G, Heath J, Mosely J, Martin JN, Jr, Dechend R, LaMarca B. Il-17-mediated oxidative stress is an important stimulator of at1-aa and hypertension during pregnancy. American journal of physiology Regulatory, integrative and comparative physiology. 2012;303:R353–358. doi: 10.1152/ajpregu.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the il-17 receptor related molecule il-17rc as the receptor for il-17f. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN, Jr, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: Role of b lymphocytes. Hypertension. 2011;57:865–871. doi: 10.1161/HYPERTENSIONAHA.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrish MR, Wallace K, Tam Tam KB, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, Martin JN, Dechend R, LaMarca B. Hypertension in response to at1-aa: Role of reactive oxygen species in pregnancy-induced hypertension. American journal of hypertension. 2011;24:835–840. doi: 10.1038/ajh.2011.62. [DOI] [PubMed] [Google Scholar]
- 16.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. At(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 17.Dechend R, Homuth V, Wallukat G, Muller DN, Krause M, Dudenhausen J, Haller H, Luft FC. Agonistic antibodies directed at the angiotensin ii, at1 receptor in preeclampsia. Journal of the Society for Gynecologic Investigation. 2006;13:79–86. doi: 10.1016/j.jsgi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 18.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin ii type i receptor (at1-aa) in pregnant rats: Role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin ii type i receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. American journal of hypertension. 2010;23:911–916. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parrish MR, Ryan MJ, Glover P, Brewer J, Ray L, Dechend R, Martin JN, Jr, Lamarca BB. Angiotensin ii type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gender medicine. 2011;8:184–188. doi: 10.1016/j.genm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert J, Dukes M, LaMarca B, Cockrell K, Babcock S, Granger J. Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in pregnant rats. American journal of hypertension. 2007;20:686–691. doi: 10.1016/j.amjhyper.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Lockhart E, Green AM, Flynn JL. Il-17 production is dominated by gammadelta t cells rather than cd4 t cells during mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. Il-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: Il-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]


