Abstract
Clostridium difficile infection (CDI) is a common problem encountered in solid organ transplant (SOT) recipients and the incidence is increasing. Generally, SOT recipients have an incidence of CDI that is similar to other post-operative patients, but this group has several unique risk factors that may contribute to more severe disease. Recent studies in non-transplant patients have indicated that treatment choices should be based on the severity of the illness. Although there continues to be a lack of well designed, randomized, controlled trials to support the management decisions that must be made for SOT recipients with CDI, the available evidence is reviewed and summarized for these treatment guidelines.
Keywords: Clostridium difficile, solid organ transplant, antibiotic-associated diarrhea, nosocomial infection, pseudomembranous colitis
I. Epidemiology and Risk Factors
Clostridium difficile is a spore-forming, anaerobic, Gram-positive bacillus. It causes 10–25% of cases of antibiotic-associated diarrhea, up to 75% of antibiotic-associated colitis, and over 90% of cases of antibiotic-associated pseudomembranous colitis (1). C. difficile causes inflammatory diarrhea and colonic mucosal injury through production of two exotoxins, toxin A and toxin B, which trigger a cytotoxic response, neutrophilic infiltrate, and cytokine release (1). The resulting inflammatory response results in the visible yellow plaques that form the characteristic pseudomembrane. This finding is less commonly seen in patients on immunosuppressive medications (2).
The overall incidence of C. difficile infection (CDI) in hospitalized patients is 1–2% and has been increasing worldwide (3). The increases in CDI incidence have been associated with the emergence of the North American pulsed field gel electrophoresis type 1 (NAP1) epidemic strain. The NAP1 strain is also associated with more severe CDI as well (3). CDI is a more frequently encountered problem in SOT recipients. The incidence of CDI is estimated to be 3–7% in liver recipients, 3.5–16% in kidney recipients, 1.5–7.8% in pancreas–kidney recipients, 9% in intestinal recipients, 15% in heart recipients, and 7–31% in lung recipients (4). Fulminant colitis develops in up to 8% of immunocompetent patients and 13% of SOT recipients with CDI (5). The incidence of CDI in SOT recipients is highest within the first 3 months after the procedure probably because of more frequent antimicrobial exposure, intense immunosuppression, and increased exposure to the healthcare setting (6). Late-onset CDI occurs months to years after the transplant and is usually associated with either antimicrobial exposure or intensified immunosuppression to treat graft rejection (6). It is not known how the NAP1 strain has impacted the incidence and severity of CDI in SOT recipients relative to the general hospital population.
Antimicrobial exposure is the most important risk factor for development of CDI (7). Any antimicrobial agent may predispose to CDI, but clindamycin, ampicillin, cephalosporins, and fluoroquinolones are most frequently implicated (7). The use of multiple antimicrobial agents and extended treatment courses have also been identified as risk factors (7). Antimicrobial agent administration has been associated with CDI in nearly all immunocompetent inpatients with CDI. However, only 80% of transplant recipients who develop CDI have recent antimicrobial exposures (7). The reduced relationship with antimicrobial exposure in SOT recipients may be secondary to alterations in the normal flora and impaired immunity due to immunosuppressive medications, severe pretransplant illness, and surgical intervention.
Immune system dysfunction may also be an important factor in the development of CDI in SOT recipients. The importance of the humoral immune response is demonstrated by a four-fold greater incidence of symptomatic disease in patients who are newly infected and lack preexisting immunity (8). A brisk humoral response to C. difficile toxins after infection reduces the likelihood of symptomatic disease (9). The hypogammaglobulinemia commonly associated with lung, heart, and liver transplants may result in a poor immune response and increase the incidence of CDI by five-fold in some patient subsets (10).
The use of medications that suppress gastric acid, such as proton pump inhibitors and H2 receptor antagonists, is common in SOT recipients and may also serve as a significant risk factor for the development of CDI. The acidic environment of the stomach is usually fatal to vegetative forms of C. difficile and may prevent germination of the spore form of the organism. Proton-pump inhibitors (PPIs) may also cause disturbances in the gastrointestinal flora that can allow C. difficile to more easily colonize the bowel. As a result of these changes, hospitalized patients using PPIs are over twice as likely to develop CDI (11).
Other risk factors commonly cited in the literature include age greater than 65 years old, severe underlying disease, uremia, gastrointestinal surgery, presence of a nasogastric or endotracheal tube, and prolonged hospitalization (11). SOT recipients frequently have a combination of these risk factors; however the incidence of CDI in this group is not significantly greater than that of the general surgical population. The transplant procedure itself has not been linked to development of CDI. The use of trimethoprim-sulfamethoxazole in the prevention of opportunistic infections has also not been linked to the development of CDI in SOT recipients, although associations have been seen with trimethoprim-sulfamethoxazole and CDI in other immunocompromised states.
Of note, infants under the age of 1 are generally not thought to be at risk for CDI; however asymptomatic carriage of C. difficile in this population is common (12). Detection of C. difficile or its toxins should not be assumed to be the cause of diarrhea until alternate causes of diarrhea are ruled out.
Antimicrobial exposure, advanced age, immune system dysfunction or immunosuppression, and gastric acid suppression are important risk factors for CDI (II-2).
Although SOT recipients frequently have multiple risk factors for the development of CDI, the incidence in this population is generally not significantly higher than that of the general surgical population (II-2).
II. Diagnosis
CDI is diagnosed by confirming the presence of toxigenic C. difficile or one of its toxins in the stool of a symptomatic patient. Anaerobic culture of the organism is rarely used due to difficulty, cost, and inability to differentiate toxigenic from nontoxigenic strains (13). The laboratory gold standard for C. difficile toxin detection is the cytotoxicity cell assay; however its use is also limited due to the cost and the delay of at least 24h before interpretation (13). Therefore, most institutions in the United States currently use commercially available ELISAs for C. difficile toxin detection (13). These assays have a rapid turnaround and are relatively inexpensive. ELISAs are generally only 60–90% sensitive compared with cytotoxicity assays, but newer assays continue to improve detection rates (14). Even with the relatively low sensitivity, the negative predictive value of a negative toxin ELISA is greater than 95% and repeat testing increases the likelihood of a false positive result. Therefore additional diagnostic and treatment decisions after an initial negative toxin assay should be based on clinical suspicion of disease rather than automatically repeating the test (14). Several polymerase chain reaction (PCR)-based assays have recently become available for the diagnosis of CDI. The major advantage of these assays is enhanced sensitivity compared to toxin ELISAs (15). Currently, commercially available PCR-based assays are several times more expensive than toxin ELISAs and require specialized equipment.
In cases where the presentation is atypical or the presence of ileus results in a lack of diarrhea, clinicians will need to rely on supportive examination and laboratory findings in order to diagnose CDI. Fever, abdominal pain, and abdominal distension are typically present in severe colitis even in the absence of diarrhea (16). Striking bandemia and leukocytosis of more than 30,000 cells/mm2 are seen in nearly one-half of SOT recipients with CDI. CT scan findings suggestive of severe colitis include significant bowel wall edema and ascities. These exam and laboratory findings usually precede organ dysfunction. A high index of suspicion for CDI is necessary in SOT patients with these otherwise unexplained exam and laboratory findings.
Testing of stool for C. difficile and/or its toxins should only be performed in symptomatic patients (II-2).
If the initial test is negative, testing should be repeated only if there is a high index of suspicion for CDI and if test results will alter clinical management (II-2).
Otherwise unexplained fever, abdominal pain, and leukocytosis should prompt the clinician to consider CDI despite a lack of diarrhea (II-2).
III. Treatment
Severity of CDI can be divided into three categories: mild-to-moderate, severe, and severe with complications (17). Mild-to-moderate CDI is diarrhea and mild abdominal pain without systemic symptoms. Severe disease includes abdominal pain, leukocytosis, and fever or other systemic symptoms along with abundant diarrhea. Severe disease with complications includes the symptoms of severe disease accompanied by life-threatening conditions such as paralytic ileus, toxic megacolon, or multiorgan failure secondary to CDI. The disease severity may rapidly progress so clinicians should frequently reassess and adjust therapy if necessary.
The first intervention that should occur in any patient with CDI is cessation of the inciting antimicrobial agent whenever possible. Removing antimicrobial pressure on the normal flora was curative in roughly 15–25% of immunocompetent patients prior to the NAP1 epidemic (1). If antimicrobial agents must be continued in order to treat another ongoing bacterial infection, clinicians may consider changing to a more narrow-spectrum regimen or an alternate antimicrobial agent with less association with CDI.
Oral metronidazole and oral vancomycin are the accepted first line antimicrobial agents for treatment of CDI in both immunocompetent and SOT recipients. Only oral vancomycin is FDA approved treatment for C. difficile, but oral metronidazole has considerable historical use as first-line therapy for CDI. Metronidazole undergoes biliary excretion and crosses the inflamed colonic mucosa so it also reaches adequate levels in the feces when given intravenously. This route of administration has not been rigorously studied, but is supported by several case series (18). In contrast to metronidazole, vancomycin does not reach adequate levels in the feces when given intravenously and should never be administered intravenously to treat CDI.
Two recent studies support basing the initial antibiotic choice on the severity of CDI (17, 19) (Figure 1). Oral metronidazole is usually the drug of choice for mild-to-moderate disease due to comparable efficacy in both the general population and SOT recipients. Metronidazole has a significant cost advantage over oral vancomycin. There has also been a long-held concern that the use of oral vancomycin will increase the incidence of vancomycin-resistant enterococci, but recent studies have not substantiated this effect. A major disadvantage of metronidazole use in SOT recipients is an interaction with medications such as tacrolimus, so that levels of tacrolimus should be monitored during treatment.
Figure 1.
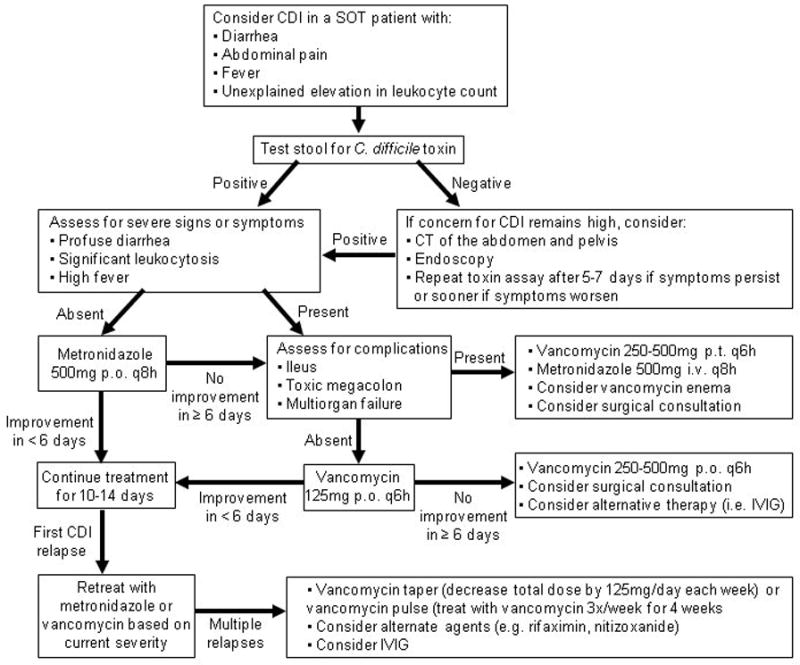
Recommended Approach to the Diagnosis and Treatment of CDI in Adult SOT Recipients
Oral vancomycin is the preferred therapy for severe CDI. Several recent studies demonstrated improved response rates with vancomycin compared to metronidazole in severe disease. Two randomized studies found that 85–97% of patients with severe CDI were cured with vancomycin therapy, but only 65–76% of patients were cured with oral metronidazole (17, 19). These same studies continue to show no significant difference between the two antimicrobial agents in mild-to-moderate disease (17, 19). Vancomycin typically is administered at 125 mg four times daily in adults because higher doses have increased cost without improved efficacy. This regimen achieves stool vancomycin concentrations that are hundreds of times greater than the minimum inhibitory concentration (MIC) of C. difficile (20). The usual dose of oral vancomycin for children is 40mg/kg daily given in 3 or 4 divided doses.
In cases of severe CDI with complications, decreased gastrointestinal motility may limit the efficacy of oral vancomycin by preventing the drug from reaching the site of infection. In these patients, higher doses of oral vancomycin may be warranted in an attempt to increase the probability that adequate levels of vancomycin will be achieved in the colon as quickly as possible. Several case reports also support the use of vancomycin administered by enema in cases of ileus (21). Bloodstream infections from colonic flora have been reported following administration of vancomycin enemas so clinicians should exercise caution when considering this approach (21). Intravenous metronidazole may also be administered with oral vancomycin and vancomycin enemas in an attempt to ensure sufficient drug gets to the site of infection.
Antimicrobial therapy alone may be insufficient treatment in patients with severe CDI and surgical intervention may be a necessary addition. Less than 3% of immuncompetent patients with CDI develop fulminant pseudomembranous colitis that requires colectomy; however, colectomy is performed in up to 13% of SOT recipients with CDI (5). This increase in surgical intervention in SOT recipients may represent a true increase in disease severity within this population, but may also be confounded by a bias to more rapidly return these patients to the operating room. Surgical intervention within the first 48 hours of a failure to respond to medical therapy, bowel perforation, or multiorgan failure may reduce mortality in patients with severe disease (5). Patients at higher risk for postoperative mortality include those admitted for a diagnosis other than CDI, suffering mental status changes, and requiring vasopressor support prior to colectomy (22).
Intravenous immunoglobulin (IVIG) has been attempted with variable success in the treatment of CDI. IVIG is known to contain C. difficile antitoxin antibodies; but its use is supported only by case studies and series. A retrospective analysis of 18 pair-matched patients with severe CDI did not show any benefit to combining IVIG with standard antimicrobial therapy; however, this study did not control for the time from onset of symptoms to IVIG administration (23). In a retrospective review of heart transplant recipients with hypogammaglobulinemia, a lower incidence of CDI was noted in the patients treated with IVIG (10); however, these results were not statistically significant. At this time, IVIG remains a treatment option that is worth further study, but cannot be broadly recommended.
More than one-third of patients with CDI will suffer at least one recurrence (24). Treatment of the first relapse should again be guided by the disease severity since relapse is not related to the development of antimicrobial resistance to the first course of treatment (24). Management of patients with multiple recurrences has not been thoroughly studied, but there are reports of success with either a prolonged tapering or pulse dosing schedule of oral vancomycin.
There has been great interest in the use of adjunctive therapies with conventional antibiotics in order to reduce the frequency of CDI relapses. Several retrospective studies and case series in patients suffering from recurrent disease have revealed a modest benefit after treatment with IVIG or probiotics (25, 26). Clear benefits have not been reported in placebo-controlled trials for either treatment. Probiotic use also carries the risk of infection from the organisms in probiotic formulas, but this complication appears rare. Fecal flora restoration appears beneficial at preventing relapses in immunocompetent hosts (27). However, similar to recommendations supporting avoidance of probiotics in immunocompromised hosts because of risk of infection, it also appears prudent to avoid fecal flora restoration in SOT recipients as well. Cholestyramine and colestipol have also been investigated as adjunctive therapy in case studies and series since they bind the C. difficile toxins in vitro, but have demonstrated inconsistent clinical results. Caution should be used when the binding resins are administered in conjunction with vancomycin since cholestyramine has been shown to complex with it in vitro and may result in subtherapeutic fecal concentrations. A small case series indicates rifaximin may be of benefit to prevent relapses, however there are concerns for the rapid development and dissemination of resistance (28, 29).
For mild to moderate CDI, oral metronidazole remains the drug of choice (I). The accepted dose of metronidazole is 250mg QID or 500mg TID for adults and 30–50mg/kg/day divided TID for pediatric patients (not to exceed adult dosing).
For severe CDI, oral vancomycin is the treatment of choice (I). The accepted dose of vancomycin is 125mg QID for adults and 40–50mg/kg/day divided QID for pediatric patients (not to exceed adult dosing).
In cases of severe CDI with complications, the dose of oral vancomycin may be increased up to 500mg orally QID (III), vancomycin may be administered by enema (II-2), and intravenous metronidazole may be added (II-3).
Surgical intervention should be considered in cases of complicated CDI (II-3).
Patients suffering from multiple recurrences of CDI may respond to prolonged courses of oral vancomycin, either in a tapering or pulse dose schedule (II-2).
There is insufficient evidence to recommend routine use of IVIG (II-2), probiotics (I), or toxin-binding resins (I) in the treatment of initial or recurrent CDI. Probiotics and toxin-binding resins may be potentially harmful due to the risk of bacteremia or reducing the effectiveness of antimicrobial therapy, respectively.
IV. Prevention/Prophylaxis
In addition to infection control measures (discussed below), prevention of CDI must focus on reducing the risk factors for developing the disease in patients that acquire C. difficile. The most significant modifiable risk factor for CDI remains antimicrobial exposure, especially to broad spectrum antimicrobial agents. Many institutions have succeeded in limiting the use of broad-spectrum antimicrobial agents through use of formulary restrictions and antimicrobial stewardship programs. This strategy was effective in reducing the incidence of CDI by 60% when a stewardship program was implemented during the nosocomial outbreak in Quebec (30). Programs that reduced broad spectrum antimicrobial agent use without altering overall antimicrobial use also resulted in significant reductions in the incidence of CDI (30). Other interventions that specifically limit only high-risk antimicrobial agents such as cephalosprins and clindamycin also meet with statistically significant reductions in CDI at many other centers (31).
There is no known effective prophylaxis against C. difficile. CDI can be caused by any antimicrobial therapy, including metronidazole and vancomycin, so it is recommended that no antimicrobial agent be given with the intention of preventing the disease. Pre-existing colonization with C. difficile also appears to be protective against development of CDI after a patient is hospitalized, so the presence of the organism or its toxin in an asymptomatic patient would not be cause for pre-emptive therapy (32). The use of probiotics as a preventative measure has also had inconsistent success in several small studies, and there are currently no adequate studies that specifically support the use of probiotics as effective prophylaxis against CDI. Vaccines may be beneficial in the future; however vaccine development has not progressed beyond animal and phase I studies at this time.
Limiting antimicrobial use through formulary restrictions and/or antimicrobial stewardship programs reduces the incidence of CDI (II-3)
Other modifiable risk factors for the development of CDI, such as gastric acid suppression or prolonged hospitalization, should be reduced if possible (III).
V. Infection Control Issues
Both strict hand hygiene and appropriate contact precautions are essential in order to limit the spread of C. difficile within institutions. There has been a shift from using soap toward the use of alcohol-based sanitizers for hand hygiene. These hand sanitizers do not kill C. difficile spores, but several studies have not demonstrated an increase in the incidence of CDI when these are used in place of soap (33–35). Several studies have shown that the incidence of new C. difficile disease is significantly decreased by the use of a gown and gloves when entering the room of a patient with CDI (36). Studies that evaluate the combination of improved hand hygiene with contact precautions have shown a reduction in horizontal spread of C. difficile by 60–80% (36).
C. difficile spores are known to contaminate the environment, are resistant to standard disinfectants, and are capable of surviving for months on dry surfaces within a hospital room. It is not yet clear if routine environmental decontamination with sporicidal agents is necessary, although it is reasonable to consider during disease outbreaks. There are currently no U.S. Environmental Protection Agency (EPA) approved hospital disinfectants that have sporicidal activity, but 10% bleach solutions may be used and have been shown to be sporicidal within 10 minutes of exposure.
The combination of strict hand hygiene and contact precautions significantly reduces the incidence of CDI through limiting patient acquisition of C. difficile (II-3).
10% bleach solutions are sporicidal and may be used for environmental decontamination during outbreaks (II-3).
Acknowledgments
Funding Sources: Dr. Riddle: National Institutes of Health under Ruth L. Kirschstein National Research Service Award 5 T32 HD007507 from the NICHD. No commercial associations that might pose a conflict of interest. Dr. Dubberke: 1K12RR02324901.
Footnotes
Potential conflicts of interest: Consultant for Merck, Meridian, Becton-Dickinson.
VI. Key References
- 1.Aslam S, Musher DM. An update on diagnosis, treatment, and prevention of Clostridium difficile-associated disease. Gastroenterol Clin North Am. 2006;35(2):315–335. doi: 10.1016/j.gtc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Nomura K, Fujimoto Y, Yamashita M, Morimoto Y, Ohshiro M, Sato K, et al. Absence of pseudomembranes in Clostridium difficile-associated diarrhea in patients using immunosuppression agents. Scand J Gastroenterol. 2008:1–5. doi: 10.1080/00365520802321238. [DOI] [PubMed] [Google Scholar]
- 3.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 4.Riddle DJ, Dubberke ER. Clostridium difficile infection in solid organ transplant recipients. Curr Opin Organ Transplant. 2008;13(6):592–600. doi: 10.1097/MOT.0b013e3283186b51. [DOI] [PubMed] [Google Scholar]
- 5.Dallal RM, Harbrecht BG, Boujoukas AJ, Sirio CA, Farkas LM, Lee KK, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363–372. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albright JB, Bonatti H, Mendez J, Kramer D, Stauffer J, Hinder R, et al. Early and late onset Clostridium difficile-associated colitis following liver transplantation. Transpl Int. 2007;20(10):856–866. doi: 10.1111/j.1432-2277.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 7.Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40(1):1–15. doi: 10.1016/s0195-6701(98)90019-6. [DOI] [PubMed] [Google Scholar]
- 8.Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998;351(9103):633–636. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 9.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342(6):390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 10.Munoz P, Giannella M, Alcala L, Sarmiento E, Fernandez Yanez J, Palomo J, et al. Clostridium difficile-associated diarrhea in heart transplant recipients: is hypogammaglobulinemia the answer? J Heart Lung Transplant. 2007;26(9):907–914. doi: 10.1016/j.healun.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile--associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45(12):1543–1549. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 12.Dubberke ER, Gerding DN, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29 (Suppl 1):S81–92. doi: 10.1086/591065. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins TD, Lyerly DM. Clostridium difficile testing: after 20 years, still challenging. J Clin Microbiol. 2003;41(2):531–534. doi: 10.1128/JCM.41.2.531-534.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohan SS, McDermott BP, Parchuri S, Cunha BA. Lack of value of repeat stool testing for Clostridium difficile toxin. Am J Med. 2006;119(4):356, e357–358. doi: 10.1016/j.amjmed.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Stamper PD, Alcabasa R, Aird D, Babiker W, Wehrlin J, Ikpeama I, et al. Comparison of a commercial real-time PCR assay for tcdB detection to a cell culture cytotoxicity assay and toxigenic culture for direct detection of toxin-producing Clostridium difficile in clinical samples. J Clin Microbiol. 2009;47(2):373–378. doi: 10.1128/JCM.01613-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunenshine RH, McDonald LC. Clostridium difficile-associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73(2):187–197. doi: 10.3949/ccjm.73.2.187. [DOI] [PubMed] [Google Scholar]
- 17.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 18.Johnson S, Peterson LR, Gerding DN. Intravenous metronidazole and Clostridium difficile-associated diarrhea or colitis. J Infect Dis. 1989;160(6):1087–1088. doi: 10.1093/infdis/160.6.1087. [DOI] [PubMed] [Google Scholar]
- 19.Louie T, Gerson M, Grimard D, Johnson S, Poirier A, Weiss K, et al. Results of a phase III trial comparing tolevamar, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhea (CDAD). 47th Annual ICAAC; 2007. Chicago. Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007. [Google Scholar]
- 20.Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother. 2007;51(8):2716–2719. doi: 10.1128/AAC.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis. 2002;35(6):690–696. doi: 10.1086/342334. [DOI] [PubMed] [Google Scholar]
- 22.Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008;143(2):150–154. doi: 10.1001/archsurg.2007.46. discussion 155. [DOI] [PubMed] [Google Scholar]
- 23.Juang P, Skledar SJ, Zgheib NK, Paterson DL, Vergis EN, Shannon WD, et al. Clinical outcomes of intravenous immune globulin in severe Clostridium difficile-associated diarrhea. Am J Infect Control. 2007;35(2):131–137. doi: 10.1016/j.ajic.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38(6):2386–2388. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surawicz CM. Role of probiotics in antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, and recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2008;42 (Suppl 2):S64–70. doi: 10.1097/MCG.0b013e3181646d09. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox MH. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother. 2004;53(5):882–884. doi: 10.1093/jac/dkh176. [DOI] [PubMed] [Google Scholar]
- 27.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 28.Curry SR, Marsh JW, Shutt KA, Muto CA, O’Leary MM, Saul MI, et al. High frequency of rifampin resistance identified in an epidemic Clostridium difficile clone from a large teaching hospital. Clin Infect Dis. 2009;48(4):425–429. doi: 10.1086/596315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44(6):846–848. doi: 10.1086/511870. [DOI] [PubMed] [Google Scholar]
- 30.Valiquette L, Cossette B, Garant MP, Diab H, Pepin J. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin Infect Dis. 2007;45 (Suppl 2):S112–121. doi: 10.1086/519258. [DOI] [PubMed] [Google Scholar]
- 31.Davey P, Brown E, Fenelon L, Finch R, Gould I, Hartman G, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2005;(4):CD003543. doi: 10.1002/14651858.CD003543.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Gerding DN, Muto CA, Owens RC., Jr Measures to control and prevent Clostridium difficile infection. Clin Infect Dis. 2008;46 (Suppl 1):S43–49. doi: 10.1086/521861. [DOI] [PubMed] [Google Scholar]
- 33.Boyce JM, Ligi C, Kohan C, Dumigan D, Havill NL. Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs. Infect Control Hosp Epidemiol. 2006;27(5):479–483. doi: 10.1086/504362. [DOI] [PubMed] [Google Scholar]
- 34.Rupp ME, Fitzgerald T, Puumala S, Anderson JR, Craig R, Iwen PC, et al. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol. 2008;29(1):8–15. doi: 10.1086/524333. [DOI] [PubMed] [Google Scholar]
- 35.Vernaz N, Sax H, Pittet D, Bonnabry P, Schrenzel J, Harbarth S. Temporal effects of antibiotic use and hand rub consumption on the incidence of MRSA and Clostridium difficile. J Antimicrob Chemother. 2008;62(3):601–607. doi: 10.1093/jac/dkn199. [DOI] [PubMed] [Google Scholar]
- 36.Muto CA, Blank MK, Marsh JW, Vergis EN, O’Leary MM, Shutt KA, et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin Infect Dis. 2007;45(10):1266–1273. doi: 10.1086/522654. [DOI] [PubMed] [Google Scholar]


