Summary
Background
Preventive therapy for tuberculosis among HIV-infected patients is effective but has not been widely implemented in moderate/high-burden settings.
Objectives
To determine the impact of widespread use of isoniazid preventive therapy on rates of tuberculosis and death in HIV-infected individuals in Brazil.
Design
Stepped wedge, cluster randomized trial
Participants
Patients actively enrolled in 29 HIV clinics in Rio de Janeiro, Brazil
Control period
Standard of care
Intervention period
Staff training in tuberculosis screening, performance of tuberculin skin tests and use of isoniazid preventive therapy.
Randomization
Clinics were randomly allocated a date to begin the intervention period with two clinics beginning the intervention every 2 months starting September 1 2005.
Main outcome measures
Tuberculosis incidence alone or combined with death in the control versus intervention periods through August 31 2009.
Results
Among 17,413 patients in the THRio cohort, 12,816 were eligible for the intervention. Overall, there were 475 tuberculosis cases and 838 deaths. The intervention increased the rate of patients receiving skin tests from 19/100 person-years to 59/100 person-years, and from 36/100 person-years to 144/100 person-years for those eligible for isoniazid preventive therapy. In the control period, 221 tuberculosis cases were diagnosed (1·31/100 person-years) compared to 254 (1·10/100 person-years) in the intervention (unadjusted hazard ratio (HR)=0·87;95%CI:0·69–1·10). Rates of tuberculosis incidence or death were 3·64 and 3·04/100 person-years, respectively (HR=0·76; 95%CI:0·66–0·87). When adjusted for age, sex, entry CD4 count and use of antiretroviral therapy, the HR for tuberculosis was 0·73 (95%CI:0·54–0·99) and for tuberculosis or death was 0·69 (95%CI:0·57–0·83). Among 12,196 patients remaining in care (secondary analyses, 399 tuberculosis cases and 656 deaths), the adjusted HR of tuberculosis alone and combined with death were 0·42 (95%CI:0·29–0·60) and 0·45 (95%CI:0·35–0·56), respectively,
Conclusions
Operational training aimed at increasing tuberculosis screening, provision of tuberculin skin tests and use of isoniazid preventive therapy in Brazilian HIV clinics significantly reduced incident tuberculosis and death. Thus, scale-up of preventive therapy for HIV-infected patients in moderate tuberculosis incident settings is achievable and should be strongly considered in Brazil and elsewhere.
Trial registration
This trial is registered at clinicaltrials.gov (NCT00107887)
Funding
Bill & Melinda Gates Foundation; National Institutes of Health
INTRODUCTION
Background and objectives
Isoniazid preventive therapy has long been recognized as an effective intervention for reducing risk of tuberculosis at the individual and population level.1 Studies in people with HIV infection demonstrate that preventive therapy reduces tuberculosis rates, particularly in HIV-infected patients with positive tuberculin skin tests.2 Evidence from observational studies in Brazil and South Africa show substantial reductions in tuberculosis risk for HIV-infected patients who received both isoniazid preventive therapy and antiretroviral therapy3,4. Although use of isoniazid preventive therapy has been recommended by the World Health Organization since 1998,5 uptake of this intervention in countries with high burdens of HIV and tuberculosis has been extremely poor, and most patients eligible for isoniazid are not receiving it.6 Brazil has a moderate tuberculosis incidence rate (38/100,000 in 2011) 6, and in Rio de Janeiro, a city where 10% of tuberculosis cases are co-infected with HIV, the incidence was 95.3/100,000 in 2009 and tuberculosis continues to be a leading cause of illness and death in people with HIV infection.7 We sought to determine the impact of an intervention to increase use of isoniazid preventive therapy and the subsequent effect on rates of tuberculosis and death in HIV-infected individuals in Brazil, where antiretroviral therapy has been freely available through the public sector since 1996.
METHODS
Trial design
The design of the THRio study has been previously described.8 Briefly, THRio was a stepped wedge, cluster randomized trial in which 29 of the 51 clinics providing antiretroviral therapy in Rio de Janeiro, representing 57% of all HIV-infected patients, were randomly assigned to the date when a training intervention would be introduced. Clinics ranged in size from 121 patients to 1749 patients and were chosen because they were under the administrative control of the City Health Department and were geographically dispersed throughout the city. A stepped wedge design was chosen for operational simplicity and to ensure that all patients eventually received the intervention.
Intervention
The intervention tested was training HIV and tuberculosis clinic staff to screen and treat patients for tuberculosis, perform tuberculin skin tests and treat latent tuberculosis infection with isoniazid preventive therapy. Staff receiving training included physicians, nurses, social workers, nutritionists and psychologists. All clinics received the study intervention at a randomly assigned time during a 2.5 year implementation period. Approximately 1 month prior to the intervention start date at each clinic, staff received HIV/tuberculosis education and training for tuberculin skin testing, and administration of isoniazid preventive therapy Thereafter clinics were provided with updated lists of patients eligible for tuberculin skin testing and isoniazid preventive therapy at least once annually. Annual meetings were held to provide indicators of clinic performance for tuberculin skin tests and initiation of isoniazid preventive therapy.
Study procedures and eligibility
Patients who made at least one visit to any of the 29 clinics between September 1, 2003 and September 1, 2005 and patients making their first clinic visit between September 1 2005 and August 31 2009 were eligible for inclusion in our analysis. Brazil policy for isoniazid preventive therapy recommends that HIV-infected patients with no history of tuberculosis diagnosis, treatment, or prior tuberculosis preventive therapy should undergo tuberculin skin testing and, if positive, receive isoniazid preventive therapy9 Patients were eligible for the THRio intervention if they had not been diagnosed with tuberculosis or completed isoniazid preventive therapy prior to the study. Staff were instructed to screen all HIV-infected patients for tuberculosis symptoms and to perform annual tuberculin skin testing using PPD RT 23 (Statens Serum Institut, Denmark) on eligible individuals. Patients with tuberculin skin tests reactions ≥ 5mm were asked about tuberculosis symptoms and had a chest radiograph. Tuberculin-positive patients and those with documented prior positive tuberculin skin tests who had not received isoniazid preventive therapy were to be given isoniazid 300 mg with pyridoxine 25 mg daily for 6 months, refilled at 30 or 90 days depending on clinic as were patients with chest radiographic scars compatible with previous healed tuberculosis and no prior documented anti-tuberculosis therapy. Physicians recorded “completion” in medical records when patients reported taking 180 doses of isoniazid preventive therapy.
Randomization and Power
The unit of randomization was the HIV clinic. Clinics were randomly allocated a date to begin the intervention period of the study with two clinics beginning the intervention every 2 months starting September 1, 2005. Randomization was done using a highly restricted randomization design to achieve close balance with respect to clinic-level covariates including mean CD4 count, clinic size, average education, tuberculosis treatment levels, existence of a supervised tuberculosis therapy (DOTS) program and geography.10–12
With a Type I error of 0.05, and a coefficient of variation of 0.2 for TB incidence rates across clinics (based on a sample of 240 patients in 10 clinics10), we estimated the study would have 80% power to detect a 40% reduction in TB incidence.
Data collection
Medical record data for all patients were abstracted onto standardized forms at baseline and at approximately nine-month intervals by trained study personnel uninvolved in clinical care. Completed forms were mailed to the data management center for entry. Seven abstraction visits were conducted during the study and through nine months following study conclusion to ensure complete data capture. Information collected included HIV-related diagnoses, CD4 cell counts, HIV viral loads, antiretroviral therapy use and all diagnostic and treatment data for both active and latent tuberculosis. Supervisors re-abstracted 5% of records for quality control and an external study monitor consistently reported < 1% of randomly reviewed records had minor inconsistencies. To ensure all tuberculosis cases and deaths were captured, we cross-matched the THRio registry with the Rio de Janeiro City tuberculosis and mortality surveillance databases.
Statistical Analysis
The primary outcomes were crude and adjusted incidence of active tuberculosis and incidence of active tuberculosis or death before and following exposure to the intervention. 13 Tuberculosis was defined according to the Brazilian guidelines14 as 1) at least one positive culture for Mycobacterium tuberculosis; or 2) positive acid fast bacilli smear; or 3) clinical and radiographic presentation consistent with tuberculosis and response to treatment.
Rates of tuberculin skin testing and isoniazid preventive therapy initiation were compared between control and intervention periods across clinics using a Poisson regression accounting for within-clinic correlation via inclusion of a scale parameter for overdispersion. The denominator time for the tuberculin skin test rates started at entry into the cohort and for isoniazid preventive therapy rates on the date of a first positive tuberculin skin test. Incidence rates were calculated for both primary outcomes with total person-time in each period as the denominator and 1) all tuberculosis events, and 2) all tuberculosis events and deaths, as the numerator, standardized to 100 person-years of observation. The start of follow-up time was September 1, 2005, or the date of first clinic visit for patients who enrolled in care after the study began. Patients were followed through the first of death, tuberculosis diagnosis or August 31, 2009 (administrative censoring). Person-time was allocated to the control period from the time of entry into the study until the patient attended his or her clinic after the intervention was introduced. Patients who died or were diagnosed with tuberculosis within 60 days of their first clinic visit, whether that visit was during the clinic’s control or intervention period, were excluded from the analysis because they were considered to have entered the study with an incipient endpoint.
In the primary intent-to-treat analysis, all patients eligible to receive either tuberculin skin test or isoniazid preventive therapy were included. We considered patients exposed to the intervention once they attended a clinic that had entered the intervention period of the trial, whether or not they received tuberculin skin testing or isoniazid preventive therapy. Patients who transferred clinics contributed follow up time to each clinic during the time they attended it. However, once a patient had observation time in a clinic in the intervention period, they were analyzed as having received the intervention. Tuberculosis incidence and mortality rates on each day were compared between patients who had been exposed to the intervention to those who had not yet been exposed to the intervention. Because this was a stepped wedge design, clinics provided different lengths of time to the control and intervention periods.
A secondary analysis of “stayers” was performed to assess the impact of the intervention on patients retained in care, as a number of patients eligible to receive tuberculosis testing and isoniazid preventive therapy never returned to the clinic after implementation of the intervention. The stayers analysis was a post-hoc analysis designed prior to initiation of data analysis and included those patients who had consistent contact with a clinic, defined as receiving at least one CD4 determination per year. In principle, patients should be seen at clinic, with routine CD4 testing, thrice yearly. Thus, patient time was censored one year after the last CD4 result in these analyses.
Cox proportional hazards modeling on a calendar time axis to account for secular trends with gamma-distributed random effects for clinic-level shared frailty was conducted to compare instantaneous risk of outcomes of those in intervention status to those in control status. Crude Cox models were conducted for the primary analysis and adjusted models were generated accounting for important covariates potentially associated with the intervention and the outcomes. These factors were sex, age at entry, antiretroviral therapy at baseline, and time-varying (last observation carried forward) CD4 count. 15 A time-varying binary covariate tracking intervention status was fit using definitions of times-at-risk in each period given above. These analyses were conducted with R version 2, and fit separately in Stata/MP 12.1 (College Station, TX) for verification. This trial is registered at clinicaltrials.gov (NCT00107887)
Ethics and Sponsor’s Role
Approval was granted by the Comite de Etica em Pesquisa of the Municipal Health Secretariat of Rio de Janeiro and the Johns Hopkins Medicine Institutional Review Board (Baltimore, US). The need for informed consent was waived because the intervention under study was training clinic staff to better perform practices that were already standard recommended practices in these clinics. Approval for the study was also obtained from the director of each clinic. An independent Data Safety and Monitoring Board reviewed the protocol and interim results annually. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
RESULTS
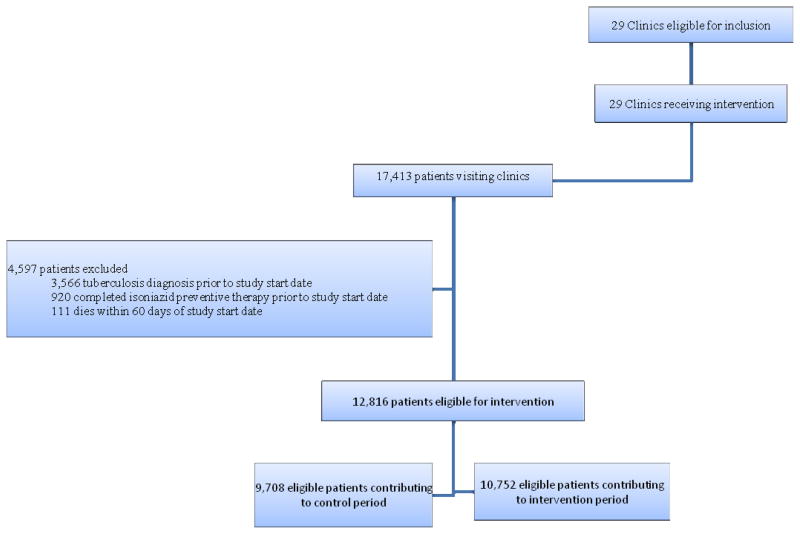
The initial THRio cohort consisted of 17,413 HIV-infected patients, 11,629 who had visited study clinics prior to trial start and 5,784 patients who entered clinical care between September 1, 2005 and August 31, 2009. According to a priori exclusion criteria, 4,597 (26%) patients were excluded from the analysis for the following reasons: diagnosed with tuberculosis prior to study start date plus 60 days (n=3,566); completed isoniazid preventive therapy prior to the study plus 60 days (n=920); or died within 60 days of study start (n=111). After these exclusions, 12,816 patients remained in our study sample.
Among these 12,816, 61% were male, the median age was 37 years (interquartile range (IQR) 30–45 years), the median CD4 was 408 cells/mm3 (IQR 248–598), and 60% were receiving combination antiretroviral therapy at baseline (Table 1). At first visit in the intervention period, the median CD4 was 426 cells/ mm3 (IQR 268–619).
Table 1.
Patient characteristics
| Total number of patients in analysis | 12,816 |
|---|---|
| Men (%) | 7,789 (61%) |
| Median age at entry, years (interquartile range) | 37 (30–45) |
| Median CD4 at entry, per mm3 (interquartile range)* | 408 (248–598) |
| Years since HIV diagnosis at entry (interquartile range) | 2.4 (0.4–5.8) |
| Receiving antiretroviral therapy at entry (%) | 7,657 (60%) |
2319 (18%) patients had no CD4 result available within 180 days of entry
2813 (22%) patients had no CD4 result available within 180 days of intervention
Table 2 describes the tuberculin skin test and isoniazid preventive therapy experience among the 12,816 eligible patients. Among these patients, 472 had a positive tuberculin skin test prior to THRio start date and were eligible for isoniazid preventive therapy. Among the 12,344 eligible for a tuberculin skin test, 7,361 (60%) had at least one tuberculin skin test placed and read. Among 6,224 eligible for a second tuberculin skin test, 2,919 (47%) received at least one more test. In total, 1,455 (20%) of the 7,361 patients receiving at least one test had a positive result. Among all tuberculin-positives, 1,186 (82%) started isoniazid preventive therapy and 1,003 (85%) completed therapy. Rates of tuberculin skin tests were significantly greater in the intervention period (59/100 person-years) compared to the control period (19/100 person-years, p<0.001), as were rates for initiation of isoniazid preventive therapy amongst those with a positive tuberculin skin test (144/100 vs. 36/100 person-years, p<0.001). An additional 286 patients initiated isoniazid preventive therapy for reasons other than a new positive tuberculin skin test; 211 had a previous positive tuberculin skin test, 70 had a previous positive chest radiograph, and 5 had both a prior positive tuberculin skin test and chest x-radiograph. Treatment completion was 83% in these patients. Among all patients initiating preventive therapy, 1·5% (N=22) reported adverse events leading to therapy interruption, mostly pruritis (n=7) and gastrointestinal disturbances (n=5), with only 3 patients experiencing liver toxicity.
Table 2.
Rates of tuberculin skin testing and initiation of isoniazid preventive therapy among tuberculin skin test positives
| Control Period (per 100 person-years) | Intervention Period (per 100 person-years) | p-value | |
|---|---|---|---|
| Rate of tuberculin skin testing | 19 | 59 | <0.001 |
| Rate of initiating isoniazid preventive therapy | 36 | 144 | <0.001 |
During the THRio study period, 725 patients were diagnosed with tuberculosis, of whom 250 (34%) were excluded because tuberculosis was diagnosed within 60 days of clinic entry and was classified as prevalent. Among the 475 tuberculosis cases included in the analysis, 404 (85%) were identified from medical records and 71 (15%) from the tuberculosis registry. Among 838 deaths, 294 (35%) were found in medical records and 544 (65%) in the mortality registry. In the control period, there were 221 patients diagnosed with tuberculosis and 391 deaths during 16,830 person-years of follow up, for a tuberculosis incidence rate of 1·31/100 person-years and a tuberculosis or death incidence rate of 3·64/100 person-years. The estimated coefficient of variation of the TB rates, calculated as the square root of the variance of the gamma-distributed rate multipliers, was 0·22.16 In the intervention period, there were 254 patients diagnosed with tuberculosis and 447 deaths during 23,093 person-years of follow up, for a tuberculosis incidence rate of 1·10/100 person-years and a tuberculosis or death incidence rate of 3·04/100 person-years (Table 3). The unadjusted Cox proportional hazard ratios were 0·87 (95% CI: 0·69–1·10; p=0·24) for tuberculosis and 0·76 (0·66–0·87; p<0·001) for tuberculosis or death. After adjustment for age, sex, baseline highly active antiretroviral therapy and time-varying CD4 cell count, the adjusted hazard ratio for tuberculosis was 0·73 (95% CI 0·54–0·99; p=0·04) and was 0·69 (95% CI 0·57–0·83; p<0·001) for tuberculosis or death. The full adjusted model is provided in the online supplement, along with the unadjusted hazard ratio for the patients included in the full model.
Table 3.
Incidence rates during control and intervention periods, and Cox proportional hazards model results for primary outcomes (tuberculosis and tuberculosis or death), intent-to-treat analysis
| Outcome | Control Period (n=9708; person-years=16830) | Intervention period (n=10752; person-years=23093) | Unadjusted Hazard Ratio (95% CI) | p-value | Adjusted Hazard Ratio* (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Tuberculosis | 221 | 254 | --- | --- | --- | --- |
| Incidence Rate (per 100 person-years) | 1.31 | 1.10 | --- | --- | ||
| Intervention | 0.87 (0.69–1.10) | 0.24 | 0.73 (0.54–0.99) | 0.04 | ||
| Tuberculosis or Death | 612 | 701 | --- | --- | --- | --- |
| Incidence Rate (per 100 person-years) | 3.64 | 3.04 | ||||
| Intervention | 0.76 (0.66–0.87) | <0.001 | 0.69 (0.57–0.83) | <0.001 |
Adjusted model excludes 1,982 patients because they did not have a CD4 count; the adjusted model includes 345 tuberculosis cases and 891 tuberculosis cases and deaths for the two models, respectively
Model is adjusted for age, sex, highly active antiretroviral therapy at baseline, time-varying CD4
In the stayers analysis, 621 patients were excluded and 2083 had their person-time reduced during the control period, and 1009 patients were excluded and 2969 patients had their person-time reduced from the intervention period. A total of 5523 and 5085 person-years were lost in the control and intervention periods, respectively, accounting for 33% and 22% of follow-up time in the periods. In the control period, 200 patients were diagnosed with tuberculosis and 302 deaths were reported during 11,307 person-years of follow up for a tuberculosis incidence rate of 1·77/100 person-years and a tuberculosis or death incidence rate of 4·44/100 person-years (Table 4). In the intervention period, 199 tuberculosis cases and 354 deaths were reported among 18,008 person-years for rates of 1·11/100 person-years for tuberculosis incidence and 3·07/100 person-years for tuberculosis or death. Crude hazard ratios for tuberculosis and tuberculosis or death were 0·42 (95%CI: 0·31–0·58; p<0.001) and 0·50 (0·41–0·60; p<0.001), respectively. Adjusted models revealed similar hazard ratios. The full adjusted stayers model is provided in the online supplement, along with the unadjusted hazard ratio for the patients included in the full model. In the stayers analysis sample, rates of tuberculin skin tests were significantly greater in the intervention period (74/100 person-years) compared to the control period (29/100 person-years, p<0.001), as were rates for initiating IPT (172/100 vs. 52/100 person-years, p<0.001).
Table 4.
Incidence rates during control and intervention periods, and Cox proportional hazards model results for primary outcomes (tuberculosis and tuberculosis or death), secondary “stayers” analysis
| Outcome | Control Period (n=9087; person-time=11307) | Intervention period (n=9743; person-time=18008) | Unadjusted Hazard Ratio (95% CI) | p-value | Adjusted Hazard Ratio* (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Tuberculosis | 200 | 199 | --- | --- | --- | --- |
| Incidence Rate (per 100 person-years) | 1.77 | 1.11 | --- | --- | ||
| Intervention† | 0.42 (0.31–0.58) | <0.001 | 0.42 (0.29–0.60) | <0.001 | ||
| Tuberculosis or Death | 502 | 553 | --- | --- | --- | --- |
| Incidence Rate (per 100 person-years) | 4.44 | 3.07 | ||||
| Intervention† | 0.50 (0.41–0.60) | <0.001 | 0.45 (0.35–0.56) | <0.001 |
Adjusted model includes 293 tuberculosis cases and 763 tuberculosis cases and deaths for the two models, respectively
Model is adjusted for age, sex, highly active antiretroviral therapy at baseline, time-varying CD4
DISCUSSION
The THRio intervention of training clinicians to screen for tuberculosis, perform tuberculin skin testing and offer isoniazid preventive therapy to HIV-infected patients with access to antiretroviral therapy had a modest impact on tuberculosis incidence (13% reduction) and a larger and statistically significant impact on tuberculosis or death (24% reduction). Upon adjustment for important covariates such as age, sex, CD4 count and receipt of antiretroviral therapy, the intervention had greater effect, with 27% and 31% reductions in tuberculosis and tuberculosis or death, respectively. Our stayers analysis examined the impact of the intervention on those patients who were retained in care, and here the impact was even greater (58% and 55% reductions in tuberculosis or tuberculosis and death, respectively).
These effects are noteworthy, as the THRio study included patients with free access to antiretroviral drugs, which we and others have demonstrated reduce the risk of tuberculosis substantially16. The larger effect on tuberculosis and death in the unadjusted analysis could reflect deaths from tuberculosis in the control period that were not diagnosed pre-mortem.17 Thus, THRio shows that isoniazid preventive therapy to tuberculin skin test-positive patients with access to antiretroviral therapy has additional benefits for reducing the risk of tuberculosis and death. These benefits are substantial and important, as the risk of tuberculosis remains elevated in HIV-infected individuals receiving antiretroviral therapy compared to HIV-uninfected people from the same population. Baseline data from this study found individual level effects of isoniazid preventive therapy and antiretroviral therapy, with a multiplicative impact of the two,3 though the baseline study revealed many limitations of the current implementation of tuberculin skin testing and isoniazid preventive therapy in HIV-infected patients in Rio de Janeiro. Preliminary results from a recent randomized clinical trial among HIV-infected patients receiving antiretroviral therapy in South Africa reported a 37% reduced risk for tuberculosis among patients receiving 12 months of isoniazid preventive therapy versus placebo (panel).18
The THRio study evaluated the impact of a short course (6 months) of isoniazid preventive therapy in an HIV-infected population in a setting with a moderate incidence of tuberculosis. Several recent individual clinical trials in very high incidence settings in southern Africa have shown improved efficacy of extended durations of isoniazid preventive therapy in HIV-infected patients, and protection waned once patients stopped treatment.19,20 Brazil’s tuberculosis epidemic is considerably less severe than southern Africa’s, thus the durability of a short course of isoniazid preventive therapy for HIV-infected patients with positive tuberculin skin tests appears to be robust, as reinfection is less likely. Whether the combination of antiretroviral therapy and a short course of isoniazid are sufficient for providing durable protection against tuberculosis in high burden settings is uncertain. It is also possible that treatment of latent tuberculosis with a more sterilizing regimen, such as a rifamycin-based regimen, might be more efficacious than treatment with isoniazid alone.21
Our intent was to conduct a study under normal clinic conditions in an effort to represent a sustainable model, without the stringent conditions of a clinical trial. Thus, a large number of patients either never attended or had limited attendance at the clinic during the intervention and were therefore not exposed to the intervention. One marker of attendance is the reporting of CD4 counts, which Brazilian guidelines recommend be done three times annually. However, 1,982 (15.5%) patients included in the primary analysis never had a reported CD4 count, and were subsequently excluded from the adjusted analysis, as CD4 count was a potential explanatory variable included in the model. Tuberculosis rates were considerably higher in patients with no CD4 reported, and these patients tended to be newly diagnosed HIV-infected patients during the intervention period who had little to no contact with the clinic following their HIV diagnosis and prior to their TB diagnosis. Thus, adjusted analyses reported marginally better protection. When we restricted our analysis to HIV patients who had at least yearly encounters with a study clinic and were therefore more likely to be exposed to the intervention, we observed an even larger and highly significant reduction in the incidence of tuberculosis (58%) and tuberculosis or death (55%). The estimates of the effectiveness of our intervention range from the intent-to-treat results and the results seen in the subset of patients who have regular contact with the clinic: i.e. from the 13% and 24% reductions (and their confidence intervals) observed in the intent-to-treat analysis and the >50% reductions observed in the stayers analysis. The stayers analysis results emphasize the need for strategies to maintain attendance in clinic to improve HIV outcomes.
The overall impact of our intervention was diminished by patients, newly diagnosed with HIV, presenting with tuberculosis before preventive measures could be offered. Twenty-one percent of all tuberculosis diagnoses during the THRio study period were among patients newly diagnosed with HIV. These patients were excluded from our analyses a priori because they did not have an opportunity to benefit from the study intervention. This finding emphasizes the need for strategies to diagnose HIV earlier and start preventive therapy and antiretroviral therapy as soon as possible after diagnosis to reduce tuberculosis risk.
Because tuberculosis deaths are likely underreported among HIV-infected patients in Rio de Janeiro, we combined the outcome of TB and death to capture effects related to prevention of tuberculosis deaths by the intervention.22 Our finding that the intervention reduce the risk of tuberculosis and death more substantially than tuberculosis alone suggests that there was underascertainment of tuberculosis deaths, consistent with reports from South Africa.23 The THRio intervention was a health services intervention, based on training clinic staff, including physicians, nurses, social workers, psychologists and nutritionists, to better recognize and treat tuberculosis among HIV-infected patients. Though the provision of tuberculin skin tests and isoniazid preventive therapy was the primary focus, an increased awareness by all those receiving the training may have provided additional inestimable benefits, such as earlier tuberculosis diagnosis or earlier initiation of antiretroviral therapy, thus decreasing mortality24–26. Though our study horizon was only four years, patient care may have improved over this time period (60% receiving antiretroviral therapy at study start compared to 73% at study end), thus providing more benefit to patients in the intervention period. Although only 9% of the population actually received isoniazid preventive therapy, these patients, those screened for latent and active tuberculosis, may have stayed more connected to the clinic and benefited accordingly. The stepped wedge design was chosen for operational simplicity, though this study design did present several challenges. Though the analysis accounted for secular trends, it is still possible there were interactions between secular trends and some covariates. Finally, increased tuberculosis screening and use of isoniazid preventive therapy could have had secondary benefits, decreasing transmission of Mycobacterium tuberculosis in health facilities and thereby decreasing incidence more broadly.
The THRio intervention was successful in increasing the use of tuberculin skin tests and isoniazid preventive therapy, as recommended by the Brazilian Ministry of Health. Median times to skin testing and to isoniazid for skin test-positive patients were markedly reduced following the intervention compared to before.21 Nevertheless, 36% of tuberculosis cases diagnosed after the intervention were among patients who had not yet received a tuberculin skin test. Strategies for delivering tuberculosis preventive therapy more expeditiously are urgently required. Use of interferon-gamma release assays linked to routine CD4 cell count measurements could potentially simplify the process of diagnosing latent tuberculosis infection and speed the time to initiation of preventive therapy. Alternatively, providing tuberculosis preventive therapy to all HIV-infected patients, as recommended by the World Health Organization5, could ensure broader uptake. While earlier trials have shown either very modest or no benefit of isoniazid preventive therapy for tuberculin-negative individuals2, 20, recent results from Rangaka and colleagues demonstrate that isoniazid is highly protective in tuberculin- and interferon-gamma-negative individuals receiving antiretroviral therapy, though these results may not be applicable in Brazil where TB exposure and transmission are both markedly less than in South Africa.27
Despite limitations inherent to a study conducted in a real-world setting, we showed that tuberculin skin test screening and provision of isoniazid preventive therapy to HIV-infected patients with access to highly active antiretroviral therapy significantly reduced the risk of tuberculosis and death and should be widely implemented. In settings similar to those of Brazil, where rates of latent tuberculosis infection are reasonably high but repeat reinfection with tuberculosis is uncommon, provision of one 6 month regimen of isoniazid preventive therapy is likely to have lasting protection from tuberculosis. Providing preventive therapy on a large scale through routine HIV clinic care is an effective way to significantly reduce tuberculosis incidence and death among HIV-infected patients.
Supplementary Material
Figure 1.
Trial Profile (CONSORT Diagram modified for stepped wedge cluster-randomized trial)
Panel: Research in context.
Systematic Review
We searched PubMed for articles published in the HIV era with the search terms “isoniazid preventive therapy” or “tuberculosis preventive therapy” or “tuberculin skin test” and “HIV” and “tuberculosis.” Only English language titles were included. We also included results reported at recent scientific meetings. These results were combined with a published systematic review.2
Interpretation
Isoniazid preventive therapy reduces the risk of tuberculosis in HIV-infected individuals2, especially those with a positive tuberculin skin test.2, 20 Despite being recommended for more than 15 years by the World Health Organization, relatively few people with HIV received isoniazid preventive therapy until recently. We trained HIV clinicians to screen for latent and active tuberculosis and to provide isoniazid preventive therapy to patients with a positive tuberculin skin test. We have shown that significant reductions of tuberculosis and mortality risk can be achieved in the population of HIV-infected patients enrolled in care through this intervention in a setting with a moderate tuberculosis epidemic.
Acknowledgments
Funding was provided by the Bill and Melinda Gates Foundation, grant 19790.01 to the Consortium to Response Effectively to the AIDS-Tuberculosis Epidemic (CREATE). Additional support was received from the National Institutes of Health Fogarty International Center grant U2RTW006885 and National Institutes of Health grants AI066994 and AI001637. We thank Diane Pope, PhD, Richard Moore, MD, Jeanne Keruly, MSN, David Bishai, MD, and David Dowdy, MD, for advice and Grace Barnes, MPH, for study monitoring. Peter Small, MD, and Michael Kimerling, MD, of the Bill and Melinda Gates Foundation provided support and assistance. We are grateful to the members of the Data Safety Monitoring Board who closely monitored the study and provided critically useful guidance: Philip C. Hopewell, MD (Chair), Andrew Nunn, MSc, Francisco Pinkusfeld Bastos, MD, and Karin Weyer, MD.
Footnotes
Contributors
REC, LHM, JEG, BD and SCC designed the study. BD, VS, SCC, BK, SC, AE, REC, JEG participated in the implementation of the trial. BD, VS, SCC, and AE participated in administration and AE, BK, SC in regulatory support. LHM, JEG, VS, BK, SC and AE participated in data management. VS, BK, and SC participated in data collection. REC, LHM, JEG, VS, AGP, BK and SC participated in data analysis. JEG wrote the initial draft of the report and all authors revised and approved the final report.
Conflict of Interest Statement
We declare that we have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Comstock GW, Baum C, Snider DE., Jr Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am Rev Respir Dis. 1979;119(5):827–30. doi: 10.1164/arrd.1979.119.5.827. [DOI] [PubMed] [Google Scholar]
- 2.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;(1):CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golub JE, Pronyk P, Mohapi L, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23:631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO guidelines 2011. Department of HIV/AIDS. Stop TB Department. World Health Organization; Geneva, Switzerland: Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. [Google Scholar]
- 6.Global tuberculosis report. World Health Organization; Geneva, Switzerland: 2012. WHO/HTM/TB/2012.6. [Google Scholar]
- 7.Brasil. [Accessed [2013 Mar 01]];SMS - Secretaria Municipal de Saúde. Available at: http://www.rio.rj.gov.br/web/smsdc/exibeconteudo?article-id=126082.
- 8.Moulton LH, Golub JE, Durovni B, et al. Statistical design of THRio: a phased implementation clinic-randomized study of a tuberculosis preventive therapy intervention. Clin Trials. 2007;4:190–199. doi: 10.1177/1740774507076937. [DOI] [PubMed] [Google Scholar]
- 9.Conde MB, Melo FA, Marques AM, et al. BTA Guidelines on Tuberculosis Work Group. III Brazilian Thoracic Association Guidelines on tuberculosis. J Bras Pneumol. 2009 Oct;35(10):1018–48. doi: 10.1590/s1806-37132009001000011. [DOI] [PubMed] [Google Scholar]
- 10.Raab GM, Butcher I. Balance in cluster randomized trials. Stat Med. 2001;20:351–65. doi: 10.1002/1097-0258(20010215)20:3<351::aid-sim797>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials. 2004;1:297–305. doi: 10.1191/1740774504cn024oa. [DOI] [PubMed] [Google Scholar]
- 12.Hayes RJ, Moulton LH. Cluster Randomized Trials. Boca Raton: CRC Press; 2009. [Google Scholar]
- 13.SINAN: Brasil. [Accessed 27 Nov 2012];Ministério da Saúde.Sistema de informação de agravos de notificação. Available at: http://dtr2004.saude.gov.br/sinanweb.
- 14.SIM: Brasil. [Accessed 27 Nov 2012];Ministério da Saúde.Sisitema de informações de mortalidade. Available at: < http://www2.datasus.gov.br/DATASUS/index.php?area=040701.
- 15.Gupta A, Wood R, Kaplan R, et al. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7(3):e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suthar AM, Lawn SK, del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Medicine. 2012;9:1–15. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selig L, Guedes R, Kritski A, et al. Uses of tuberculosis mortality surveillance to identify programme errors and improve database reporting. Int J Tuberc Lung Dis. 2009;13(8):982–8. [PubMed] [Google Scholar]
- 18.Rangaka MX, Boulle A, Wilkinson RJ, et al. Randomized controlled trial of isoniazid preventive therapy in HIV-infected persons on antiretroviral therapy. Presented at IAS; 2012; Washington, DC, US. [Google Scholar]
- 19.Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365(1):11–20. doi: 10.1056/NEJMoa1005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive therapy for tuberculosis in adults with HIV infection in Botswana: a randomized, double-blind, placebo-controlled trial. Lancet. 2011;377(9777):1588–98. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 21.Durovni B, Cavalcante SC, Saraceni V, et al. The implementation of isoniazid preventive therapy in HIV clinics: the experience from the TB/HIV in Rio (THRio) Study. AIDS. 2010;24(suppl 5):S49–56. doi: 10.1097/01.aids.0000391022.95412.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saraceni V, Nicolai CCA, Caridade MC, et al. Tuberculosis and multiple causes of death in Rio de Janeiro, Brazil: underestimation of TB burden. 41st Union World Conference on Lung Health; Berlin, Germany. 2010. [Google Scholar]
- 23.Charalambous S, Grant AD, Innes C, et al. Association of isoniazid preventive therapy with lower early mortality in individuals on antiretroviral therapy in a workplace programme. AIDS. 2010;(Suppl 5):S5–13. doi: 10.1097/01.aids.0000391010.02774.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangaka M, Wilkinson R, Wilkinson K, et al. Effect of tuberculin skin testing or interferon-release on the benefit of concurrent isoniazid preventive therapy with ART: subgroup analysis of a randomized controlled trial. Presented at CROI; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.