Abstract
Broad-spectrum antiviral drugs are urgently needed to treat individuals infected with new and re-emerging viruses, or with viruses that have developed resistance to antiviral therapies. Mammalian natural host defense peptides (mNHP) are short, usually cationic, peptides that have direct antimicrobial activity, and which in some instances activate cell-mediated antiviral immune responses. Although mNHP have potent activity in vitro, efficacy trials in vivo of exogenously provided mNHP have been largely disappointing, and no mNHP are currently licensed for human use. Mastoparan is an invertebrate host defense peptide that penetrates lipid bilayers, and we reasoned that a mastoparan analog might interact with the lipid component of virus membranes and thereby reduce infectivity of enveloped viruses. Our objective was to determine whether mastoparan-derived peptide MP7-NH2 could inactivate viruses of multiple types, and whether it could stimulate cell-mediated antiviral activity. We found that MP7-NH2 potently inactivated a range of enveloped viruses. Consistent with our proposed mechanism of action, MP7-NH2 was not efficacious against a non-enveloped virus. Pre-treatment of cells with MP7-NH2 did not reduce the amount of virus recovered after infection, which suggested that the primary mechanism of action in vitro was direct inactivation of virus by MP7-NH2. These results demonstrate for the first time that a mastoparan derivative has broad-spectrum antiviral activity in vitro and suggest that further investigation of the antiviral properties of mastoparan peptides in vivo is warranted.
Keywords: Mastoparan, Antiviral, Host defense peptide
1. Introduction
There is a pressing need to identify novel, broad-spectrum, antimicrobial agents that can be used as therapeutics to treat emerging multidrug resistant bacterial and viral pathogens. In the past decade, natural host defense peptides (NHP), which are produced by plants, invertebrates, and vertebrate animals, have emerged as a promising class of antimicrobials [45,56]. Host defense peptides comprise a broad array of endogenous peptide antibiotics that are produced either constitutively [29], or in response to wounding and mechanical stress [30], inflammation [37], or microbial infection [2,20]. A significant advantage of NHP over other types of antimicrobials is that although resistance to NHP can be induced in vitro [39] and exists naturally in rare instances [50], it occurs at rates that are orders of magnitude lower than that for conventional antimicrobials [58].
We undertook the present study to determine whether a putative host defense peptide derived from the wasp peptide mastoparan had antiviral properties. Although the antibacterial and antifungal properties of invertebrate NHP have been well studied, only a small number of reports have ascribed antiviral activity to an invertebrate peptide [1,31,33,52]. Mastoparan is a venom component, and may also contribute to acellular immune defense against pathogens in the same manner as the better characterized cecropins and attacins of other insects (reviewed in [9]). Consistent with that idea, mastoparan induces exocytosis in hemocytes [5] which are the primary mediators of immune defense in invertebrates. In addition, a mastoparan-B isolated from hornet Vespa basalis restricted growth of various Gram positive and negative bacteria in vitro [57]. Finally, several molecular features of mastoparan peptides suggest that they might be effective antimicrobial agents. Mastoparan is unstructured in aqueous medium, but forms an amphipathic α-helix when in contact with lipids [23]. Perpendicular insertion of the mastoparan α-helix into the lipid membrane of synthetic vesicles loaded with dye transiently opens “pores”, through which dye is released intermittently [4,55]. Mastoparan also triggers mast cell degranulation in vitro [34], and activates G-proteins [22]. Receptors for histamines, chemokines, and prostaglandins signal through heterotrimeric G proteins (reviewed in [12]) and mastoparans would therefore be expected to have diverse effects on immune responses in vivo.
For these reasons we hypothesized that a mastoparan-derived peptide analog (mastoparan-7, MP7-NH2) that has enhanced activity relative the parent peptide mastoparan [11,28] might be able to reduce infectivity of enveloped viruses directly, via disruption of the envelope structure, and that it might also promote cell-mediated antiviral responses. The studies described here will help guide future development of mastoparan-derived peptides as antiviral agents in vivo.
2. Materials and methods
Ethics statement. All animal studies were reviewed and approved by the Duke University Institutional Animal Care and Use Committee.
2.1. Inoculation of mice
Eight to ten-week-old female C57BL/6 mice were obtained from Charles River Laboratories and housed for at least 1 week before experiments were initiated. Mice were housed in microisolator cages in a biosafety level 2-equipped animal facility. Viral stocks were diluted to appropriate titers in serum-free DMEM. For intranasal (i.n.) inoculation, mice were lightly anesthetized with isoflurane using a vaporizer and administered 106 PFU of VSV (in the presence or absence of 166 μM MP7-NH2, as indicated) in 30 μl total volume. After infection animals were monitored twice daily for the development of symptoms. Mice were weighed daily and were humanely euthanized if they lost 15% or more of their pre-infection body weight. The Institutional Animal Care and Use Committee of Duke University approved all animal experiments.
2.2. Cell culture and viruses
BHK-21 cells (baby hamster kidney, ATCC CCL-10) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal calf serum and 1% penicillin-streptomycin (DMEM 5) unless otherwise indicated. BHK cells were used to propagate and titer vesicular stomatitis virus (VSV; Indiana strain). Vero cells (African Green Monkey kidney cells, ATCC CCL-81) were cultured in DMEM supplemented with 10% fetal calf serum and 1% penicillin-streptomycin (DMEM 10) and were used to propagate and titer Herpes simplex virus 1 (HSV-1) West Nile virus strain NY99 (WNV NY99), yellow fever virus strain 17D (YFV-17D), and the chimeric virus WNV-DENV4 30. BSC-1 cells (African Green Monkey kidney cells, ATCC CCL-26) were cultured in DMEM 10 and were used to propagate and titer vaccinia virus Western Reserve (vvWR), and cowpox Brighton Red (CPXV-BR). HEp-2 cells (ATCC CCL-23) were cultured in MEME supplemented with 10% fetal calf serum, 1% penicillin-streptomycin, 1% non-essential amino acids, and 1% sodium pyruvate (MEME 10) and were used to propagate and titer human respiratory syncytial virus (RSV). A549 cells (human lung epithelial cells, ATCC CCL-185) were cultured in DMEM 10 and were used to propagate and titer adenovirus (AdV). RAW 264.7 cells (Abelson murine leukemia virus transformed macrophage cells, ATCC TIB-71) were cultured in MEME 10. MDCK cells (Madin-Darby canine kidney, ATCC CCL-34) were cultured in MEME 10 and used in influenza TCID50 assays.
2.3. Peptide
MP7-NH2 peptide (INLKALAALAKALL-NH2) was synthesized by CPC Scientific, Sunnydale, CA.
2.4. Direct inactivation of virus infectivity by peptide
Viruses were incubated in a total volume of 500 μl serum-free DMEM with the indicated concentration of MP7-NH2 peptide (5–50 μM). Incubations were at room temperature unless otherwise indicated, and were allowed to proceed for 30 min with vortexing every 10 min unless otherwise noted. At the end of the incubation period 4.5 ml serum-free DMEM was added to each sample, which lowered the peptide concentration to ≤5 μM. Samples were then serially diluted, and applied to monolayers of the appropriate cell type for determination of virus by plaque assay as described [7,8,17]. Influenza titers were determined by TCID50 assay as follows. MDCK cells in 96-well microtiter plates were infected with serial dilutions of virus-containing supernatants, and TCID50 was determined 4 days later by the method of Reed and Muench. Infected wells were identified by the presence or absence of agglutination after Turkey red blood cells were added to the wells. The percent reduction in infectivity was determined for each virus as follows: % reduction in titer = 100 – [(titer of peptide treated sample in PFU/ml)/(titer of untreated sample in PFU/ml) × 100].
2.5. Electron microscopy
VSV stock (100 μl) was concentrated by Airfuge (Beckman, Fullerton, CA) centrifugation at 30,000 psi for 30 min. All but 10 μl of the supernatant was removed and replaced with 300 mOsmol HEPES buffered saline (HBS). Centrifugation and buffer exchange was repeated and virus was resuspended in 30 μl of HBS. Multiple tubes were combined and mixed. Resuspended virus (29 μl) was treated with MP7-NH2 at final concentrations of 0 μM, 10 μM, or 50 μM MP7-NH2 in 50 μl final volume. Incubations were carried out for 30 min at RT. After the incubation, virus was immobilized on a carbon-coated formvar 300 mesh grid for 30 s, removed through blotting, washed with water, and blotted again. The grid was stained with 2% uranyl acetate for 10 s and removed by blotting. Virus was imaged using a Philips/FEI CM 12 transmission electron microscope. Microscopy was performed at the Duke Electron Microscopy Service.
2.6. Cell viability assay
BHK cells (20,000 cells/well) were plated in 96 well flat bottom tissue culture plates and allowed to attach overnight. Cells were treated with serum-free media containing 0–50 μM concentrations of MP7-NH2. After 30 min or 24 h, peptide-containing media was removed and wells were washed with phenol red-free media prior to addition of MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) reagent. Cell viability was assessed using the CellTiter 96 AQueous Assay (Promega, Madison, WI) and following the manufacturer's protocol. The absorbance was read at 490 nm using a Multi-Detection Microplate Reader (BioTek, Highland Park, USA).
2.7. Assay for cell-mediated antiviral activity
Semi-confluent monolayers of three different cell types; BHK-21, A549, and RAW 264.7 (1 × 105 cells/well) in a 12 well plate were washed in serum-free DMEM (SF-DMEM), then incubated with 5 μM of MP7-NH2 peptide in a total volume of 1 ml SF-DMEM. After 1 h the peptide was removed and cells were washed 3× in SF-DMEM. Cells were then infected with 1 × 105 PFU per well VSV (MOI = 1) in a total volume of 100 μl. After a 30 min adsorption the inoculum was removed, cells were washed 3× in SF-DMEM and 1 ml complete medium containing 5% fetal bovine serum was added to each well. Supernatants were collected at 6 h after infection, and were titered via plaque assay on BHK cells as described [8].
2.8. ELISA for binding antibody
EasyWash high binding ELISA plates (96 well, polystyrene) (Corning) were coated with purified CPXV B5 (200 ng per well) in bicarbonate buffer (0.1 M NaHCO3) overnight at 4 °C. Wells were washed, blocked with Superblock (15% Normal Goat Serum, 0.04% Whey in PBS-Tween) and incubated with diluted sera. Antibody was detected with alkaline-phosphatase conjugated goat anti-mouse Ig and antibody reactivity was detected by adding pNPP substrate (4-nitrophenyl phosphate Sigma N2640) at 1 mg/ml as directed by the manufacturer incubating for 45 min and reading Abs at 405 nm. Serum Ig endpoint titers are reported as the reciprocal of the highest dilution that gave absorbance greater than 2× over background, where background is the absorbance for pre-immune serum at the same dilution. All animals were assayed individually, and compared to individual pre-immune titers.
3. Results
3.1. Viruses treated with MP7-NH2 have reduced infectivity
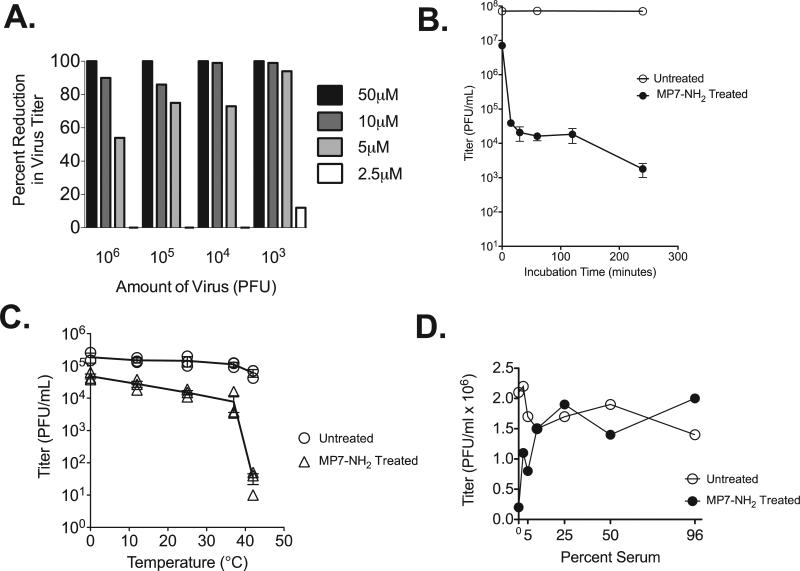
To determine whether mastoparan analog peptide MP7-NH2 had antiviral properties, we incubated increasing concentrations of MP7-NH2 (0–50 μM) with 103–106 PFU of vesicular stomatitis virus Indiana (VSV), then assayed residual infectivity of the virus via plaque assay on BHK cells. Incubations were performed at room temperature, in a total volume of 500 μl serum free medium, and samples were vortexed every 10 min to promote mixing of the virus and peptide. After the 30 min incubation, the reaction was “stopped” via addition of 4.5 ml serum free medium. Serial dilutions were made from the 5 ml culture, and 200 μl of the 1:10–1:1000 dilutions were applied to BHK cells for detection of residual infectious virus by plaque assay. As shown in Fig. 1A, MP7-NH2 peptide concentrations at and above 5 μM reduced or eliminated infectivity of VSV. The degree of inactivation was more complete at relatively higher peptide concentrations and when fewer input virions were used. Inactivation was also more efficient when the incubation time was increased (Fig. 1B). Those results suggested that in this instance virus inactivation was dependent upon direct contact between virus and peptide, and could occur in a cell free system.
Fig. 1.
MP7-NH2 inactivates VSV. (Panel A) Increasing amounts of purified VSV were incubated with increasing concentrations of MP7-NH2 as indicated. Reactions were performed at room temperature in a total volume of 500 μl with serum free DMEM as the diluent. After a 30 min incubation cultures were serially diluted, and applied to monolayers of BHK cells to detect residual infectious virus. Plaques were counted 48 h later. Bars on graph represent the percent reduction in virus titer when the untreated and treated samples were compared. (Panel B) 107 PFU of VSV was incubated with 50 μM MP7-NH2 at room temperature for the indicated amounts of time. Graph represents the average ±SEM of three experiments performed independently. (Panel C) 105 PFU of VSV was incubated with 10 μM MP7-NH2 at the indicated temperatures for 30 min. Graph shows results of three experiments performed independently. (Panel D) 106 PFU of VSV was incubated with 10 μM MP7-NH2 at room temperature for 30 min in the presence of the indicated concentration of non-heat inactivated fetal calf serum. Panel D shows one representative graph from four studies performed independently.
3.2. Factors that modulate direct antiviral activity of MP7-NH2 in vitro
Other studies have reported that the antimicrobial properties of cationic peptides are affected by changes in temperature or by the presence of serum or serum proteins [10,14,32]. To determine whether these factors affect the direct antiviral activity of MP7-NH2 we performed inactivation studies at different temperatures. As shown in Fig. 1C, the ability of MP7-NH2 to inactivate VSV was increased at relatively warmer temperatures (37 °C and 42 °C), and was decreased at cooler temperatures (0 °C and 10 °C). That observation agreed with other reports that showed increased antiviral activity for peptide antibiotics at higher temperatures [14,42] and raised the possibility that direct virus inactivation by MP7-NH2 might be facilitated by the increased fluidity of virus envelope lipids at higher incubation temperatures.
To determine whether the presence of bovine serum affected direct antiviral activity of MP7-NH2 we performed direct inactivation of VSV in media containing different amounts of non-heat inactivated fetal calf serum. The experiment was performed four times, and a representative result is shown in Fig. 1D. These studies consistently showed that serum concentrations at and above 2.5% decreased the ability of MP7-NH2 to inactivate VSV, although some activity was retained in media containing 2.5% and 5% serum. This compared favorably to reports of other NHP that were completely inactive at serum concentrations at or above 0.1% [14], and suggested that MP7-NH2 might retain antiviral activity in vivo.
3.3. VSV treated with MP7-NH2 has minimal residual infectivity in vivo
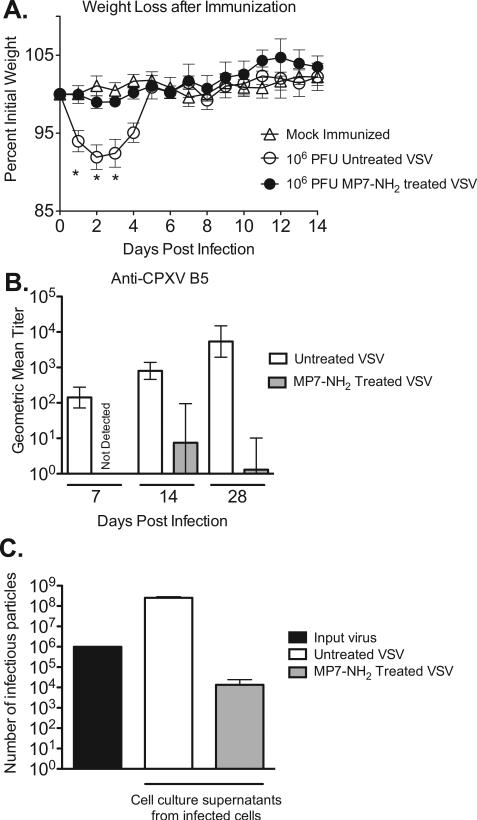
The preceding studies demonstrated that MP7-NH2 treatment could reduce infectivity of VSV in vitro in a dose and time dependent manner. To determine whether VSV that had been inactivated by MP7-NH2 was pathogenic in vivo, we challenged groups of mice (n = 4–5 per group) intranasally with 106 PFU of a recombinant VSV expressing a model vaccine antigen (cowpox protein B5, CPXV B5) which either had or had not been pre-incubated for 30 min with MP7-NH2. Mice infected intranasally with live VSV display clinical signs of acute illness and can lose up to 20% of their pre-infection body weight in the first four days after challenge [40,41]. As shown in Fig. 2A, mice infected with untreated VSV lost approximately 10% of their pre-infection body weight by the second day after challenge (open circles, Fig. 2A) and did not fully regain their pre-infection weight until five days after challenge. In contrast, mice infected with VSV that had been pre-incubated with MP7-NH2 (filled circles, Fig. 2A), did not lose significantly more weight than mock-challenged control mice (P > 0.05, two-tailed Student T test) at any time during the study, and did not exhibit other clinical signs of illness. The difference in weight loss between the two VSV infected groups was significant on days 1–3 after infection (P < 0.05, two-tailed Student T test), with mice inoculated with MP7-NH2 treated VSV losing significantly less weight than those inoculated with untreated virus. Those results supported the idea that VSV pre-incubated with MP7-NH2 was non-infectious and could not replicate in vivo. As a second measure to detect residual infectivity in vivo, we used a binding ELISA to detect serum antibody reactive against the vaccine antigen (CPXV-B5) encoded by the VSV. CPXV-B5 was encoded in the viral RNA and is only produced as a result of virus replication; it was not present on the outer envelope of the VSV particles in the inoculum. Thus, if mice developed detectable immune responses to CPXV-B5, it could be inferred that some amount of infectious virus was present in the inoculum. As shown in Fig. 2B, mice infected with the MP7-NH2 treated virus had significantly reduced antibody responses relative those receiving the untreated virus (P < 0.05 via unpaired T test Fig. 2B) at all timepoints at which responses were measured. Two out of four of the mice infected with MP7-NH2 treated VSV did not develop detectable antibodies at any of the time-points tested (Supplemental Fig. 1). The other two mice in the MP7-NH2 treated VSV group developed low titer anti-B5 Ab, which suggested that most but not all of the virions in the inoculum were inactivated by peptide pretreatment and were not capable of replication in an animal host. To test the idea that the inoculum likely had residual infectious virions we incubated the same number of VSV particles (106) as were used to infect the mice with 50 μM MP7-NH2 in 500 μl total volume. After 30 min, we transferred the entire inoculum to a 10 cm plate of BHK cells and allowed the inoculum to adsorb for 30 min in 5 ml serum free media. We then added 5 ml complete media and allowed the infection to proceed overnight (total of 16 h). As shown in Fig. 2C, when we titered the supernatant collected from the cells we did detect infectious virus, albeit in significantly reduced amount vs. that produced when cells were infected with non-treated virus. This supported the idea that there was a small amount of infectious virus remaining in the inoculum used to infect the mice (Fig. 2A and B), and that that infectious virus gave rise to the immune responses observed.
Fig. 2.
VSV inactivated by MP7-NH2 treatment does not cause disease in vivo. Groups of C57BL/6 mice (n = 4–5 mice per group) were challenged intranasally with 106 PFU of treated or untreated VSV in a total volume of 30 μl. Mice were weighed daily after inoculation. Graph (Panel A) represents average percent initial weight ±SEM for each group. Stars indicate timepoints at which the average weight for mice receiving untreated VSV was significantly lower (P ≤ 0.05, two-tailed T test) than the average weight of mice receiving MP7-NH2 treated VSV. Panel B shows serum antibody titers by group at 7, 14, and 28 days after infection as measured by ELISA with recombinant CPXV-B5 protein as the coating antigen. Bars on graph represent the geometric mean titer for each immunization group, with error bars denoting the upper and lower limit of the 95% confidence interval. Titers were significantly lower in mice immunized with peptide treated vs. untreated virus at all three timepoints (P = 0.005 at day 7, P = 0.0143 at day 14, and P = 0.045 at day 28, via unpaired T test). This study was performed twice with consistent results. (Panel C) 106 PFU of VSV was incubated with 50 μM MP7-NH2 at room temperature for 30 min. After 30 min the whole inoculum was plated on BHK cells and allowed to grow overnight (in a total volume of 10 ml). The amount of virus in the cell culture supernatants after 16 hrs was determined by plaque assay. Graph shows the total number of infectious virions detected in the supernatants and represents the average ±SEM of three experiments performed independently.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.peptides.2013.07.014.
3.4. MP7-NH2 has broad-spectrum antiviral activity
The preceding data showed that MP7-NH2 could inactivate VSV, but did not address whether MP7-NH2 might have broad-spectrum activity against other viruses. To test that idea, we performed direct inactivation studies using 104–105 PFU of each of the viruses shown in Table 1. In these studies the peptide concentration was kept constant at 50 μM, the reaction volume was 500 μl, and peptide and virus were incubated at room temperature for 30 min. After the 30 min incubation residual infectivity was assayed via plaque assay (all viruses except for influenza, for which residual infectivity was determined by TCID50) on an appropriate mammalian cell line as specified in the Methods. The limit of detection for infectious virus in all plaque assays was 50 PFU/ml and each experiment was conducted at least three times to confirm results. As shown in Table 1, MP7-NH2 was able to reduce or eliminate infectivity of eight out of ten enveloped viruses tested, but did not reduce infectivity of the non-enveloped aden-ovirus.
Table 1.
Peptide treatment reduces infectivity ofenveloped viruses.
| Virus | Virus family | Percent reduction in titer (%) |
|---|---|---|
| Vesicular stomatitis virus | Rhabdoviridae | ≥99.9a |
| Vaccinia virus Western Reserve | Poxviridae | 67.8 ± 11.6 |
| Cowpox | Poxviridae | 82.7 ± 5.1 |
| West Nile virus strain NY99 | Flaviviridae | ≥99.9a |
| WNV/DENV Type 4 Delta 30 virus chimera | Flaviviridae | ≥99.9a |
| Yellow fever virus 17D | Flaviviridae | 99.5 ± 0.3 |
| Respiratory syncytial virus | Paramyxoviridae | 70.0 ± 0.5 |
| Herpes simplex virus 1 | Herpesviridae | 99.8 ± 0.03 |
| Influenza A/Brisbane/59/2007 | Orthomyxoviridae | 0.0 |
| Influenza A/Puerto Rico/8/34 | Orthomyxoviridae | 0.0 |
| Adenovirus | Adenoviridae | 0.0 |
All experiments were performed three times. Percent reduction in titer shown is the average ± SEM from the three independent experiments. Limit of detection for Influenza virus quantification was 102.5TCID50/ml.
No residual virus was detected. The limit of detection was 50 PFU/ml.
The viruses for which infectivity was reduced by MP7-NH2 treatment do not enter cells via a common mechanism or via a common receptor. That result suggested that the mechanism of action was not inactivation via binding of the peptide to a specific molecule (e.g. the viral attachment protein(s)) but rather was via interference with some process common to all enveloped viruses (e.g. fusion of the viral envelope with the host cell plasma or endosomal membrane).
3.5. MP7-NH2 disrupts virus envelope integrity
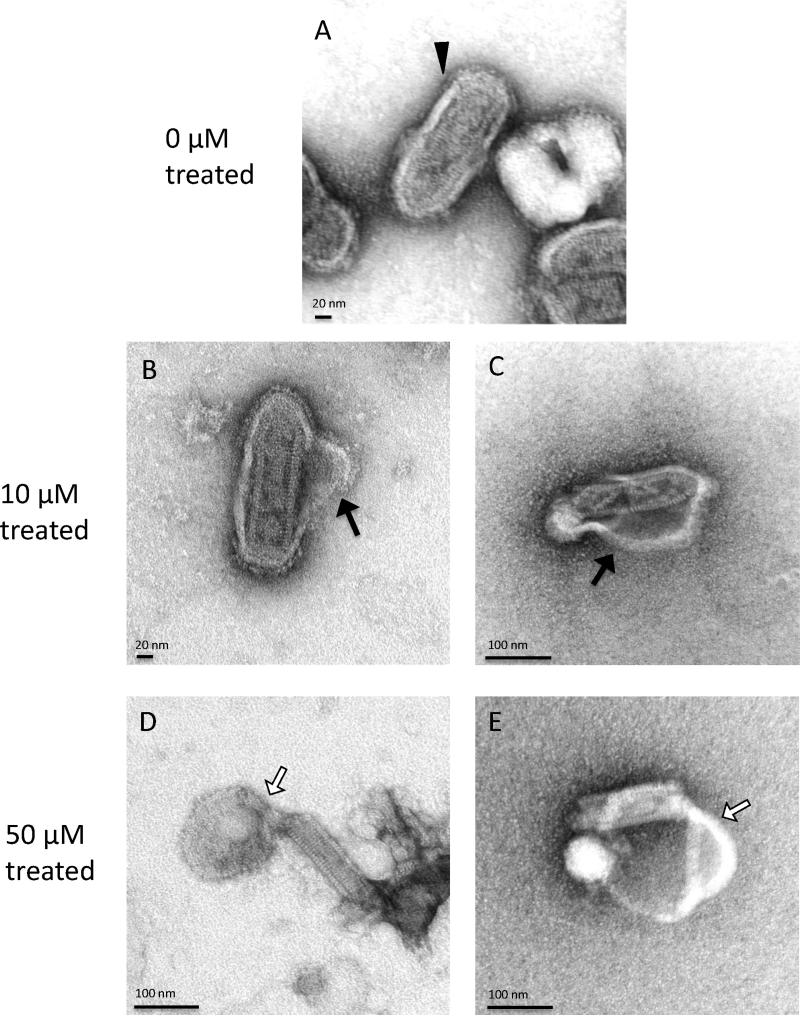
The parent peptide mastoparan has been shown to bind and penetrate cell membranes and synthetic lipid bilayers (e.g. liposomes) [23,53,55]. We therefore hypothesized that MP7-NH2 might be inactivating enveloped viruses by inserting into the virus envelope, thereby disrupting envelope structure and interfering with virus binding and/or fusion processes. To begin to test this idea, we used transmission electron microscopy (TEM) to examine the morphology of VSV virions after incubation with MP7-NH2. As shown in Fig. 3, MP7-NH2 treatment caused a significant disruption of virion structure. At low concentrations of peptide (10 μM), the envelope appeared to separate from the capsid but remain attached at either end of the virion (Fig. 3B and C). At higher peptide concentrations (50 μM, Fig. 3D and E), peptide treatment caused complete dissociation of the envelope, leaving the naked capsid intact. In some cases, the viral particle was completely broken apart, and the viral RNA extended from the end of the disrupted virion. Although virus used for TEM analysis could not be directly assayed for infectivity, the morphological changes observed would be expected to render the virus non-infectious. These data supported our model, and confirmed that MP7-NH2 could affect virion integrity in a cell-independent manner.
Fig. 3.
MP7-NH2 treatment disrupts envelope structure of VSV. Virions were treated with MP7-NH2 at the indicated concentrations for 30′ before negative staining and TEM. Untreated VSV virions had a typical “bullet” morphology in which the virus core was surrounded by an intact envelope (panel A, filled arrowhead). The envelope of virions treated with 10 μM MP7-NH2 was disrupted and separated from the virus core (Panels B and C, filled arrows). The envelope of virions treated with 50 μM MP7-NH2 was completely dissociated from the core and in many cases the envelope aggregated at one end of the core (Panels D and E, empty arrows). Images shown are from two independent experiments.
3.6. Pretreatment of host cells with MP7-NH2 does not significantly reduce production of viral progeny in vitro
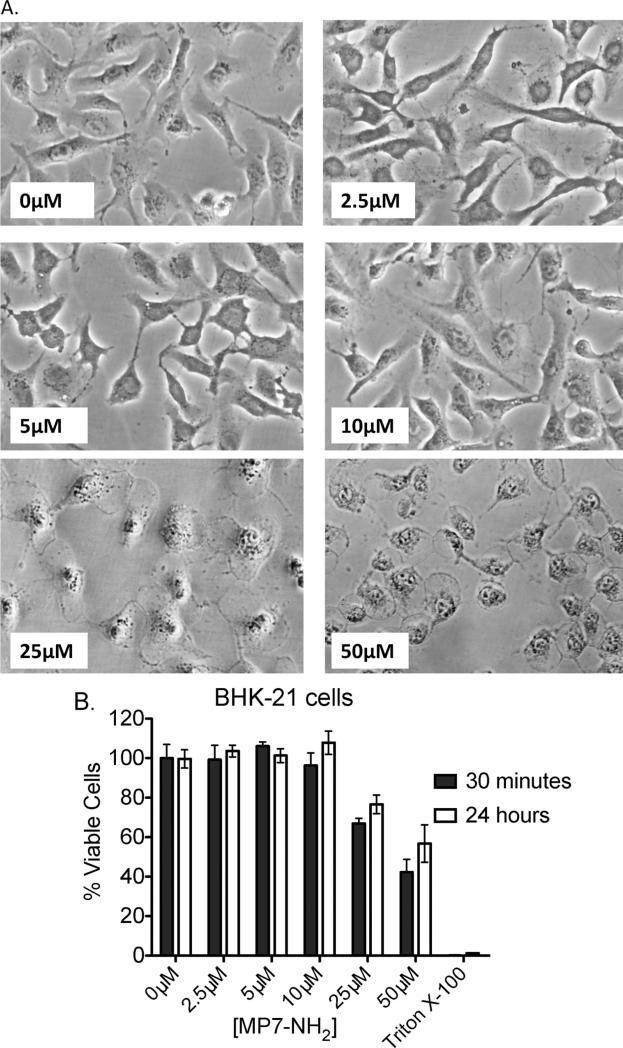
The forgoing studies demonstrated that MP7-NH2 could alter virus infectivity in a cell free environment, but did not exclude the possibility that the peptide could also act upon host cells to make them resistant to infection or less permissive for viral replication. One barrier to addressing this question directly was that mastoparan and mastoparan-derived peptides are cytotoxic at high concentrations [26], in part because mastoparan triggers the release of pro-apoptotic mediators from mitochondria [18,43,54]. Because of this, we began by determining the maximum concentration of MP7-NH2 that did not cause morphological changes or loss of viability in our cell line of interest (BHK-21 cells). As shown in Fig. 4A, incubation of BHK-21 cells with relatively high concentrations of MP7-NH2 (≥10 μM) caused rapid morphological changes such as blebbing of the plasma membrane and cell rounding in BHK cells that could be readily visualized by light microscopy. Incubation of cells with concentrations of MP7-NH2 at or below 10 μM did not cause visible morphological changes even after 24 h of culture (data not shown). To confirm that lower concentrations of MP7-NH2 were not causing toxicity not evident by visual inspection we performed an assay to measure mitochondrial activity. As shown in Fig. 4B, incubation of cells with MP7-NH2 concentrations at or below 10 μM resulted in no significant difference in cell viability between treated and untreated samples, even when the cells were incubated with MP7-NH2 for 24 h. This meant that we could pre-treat cells with concentrations of MP7-NH2 ≤ 10 μM without compromising cell viability in our assays.
Fig. 4.
MP7-NH2 is non-toxic to cells in vitro at concentrations at or below 10 μM. Monolayers of BHK cells were incubated with the indicated concentrations of MP7-NH2. Volume for all incubations was 200 μl (in a single well of a 12-well plate) and the diluent was 1× PBS. Cells were observed continually after application of the peptide solution and morphological changes were noted. Photographs show representative changes after 30 min. (Panel B) BHK cells were incubated with increasing concentrations of MP7-NH2 peptide and viability was measured via MTS assay for mitochondrial activity. Graph shows average percent viable cells in the MP7-NH2 treated samples after 30 min (filled bars) and 24 h (open bars) ±SD, where percent viability is expressed as [(OD490 treated sample/mean OD490 untreated samples) × 100]. Cells incubated in media containing 0.2% Triton X-100 served as a negative control for viability. Each condition was tested in three independent samples. There was no significant decrease in the viability of treated vs. untreated samples at concentrations of peptide at or below 10 μM. When cells were treated with 25 μM or 50 μM MP7-NH2, viability was significantly reduced relative the untreated control (P < 0.05 via unpaired T test).
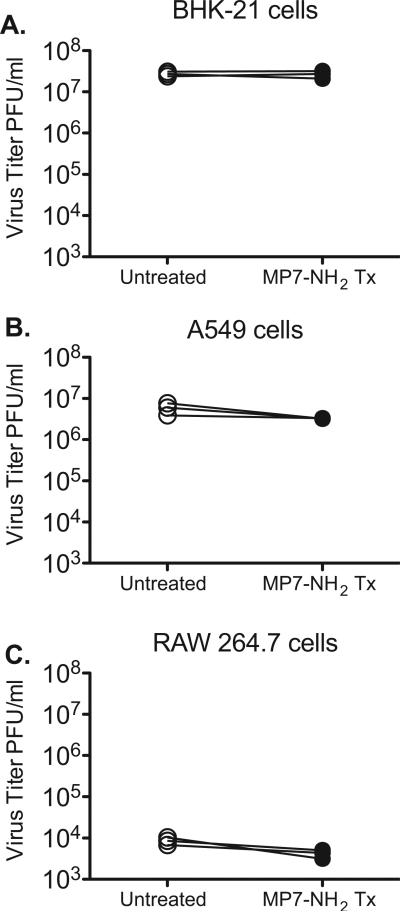
To determine whether MP-7 NH2 could promote cell-mediated antiviral activity, we pre-incubated cells with 5 μM MP7-NH2 for 1 h, then infected them with 105 PFU of VSV and measured the amount of virus in the supernatant 8 h after infection. As shown in Fig. 5A, pretreatment of BHK cells with MP7-NH2 for 1 h before infection did not significantly reduce the amount of virus recovered from the supernatant of infected cells (graph shows results of three independent experiments). These data showed that MP7-NH2 did not promote robust cell-mediated antiviral activity in BHK-21 cells, but did not exclude the possibility that MP7-NH2 might act on other cell types to render them more resistant to viral infection.
Fig. 5.
Pretreatment of cells with MP7-NH2 does not significantly reduce the amount of viral progeny released by infected cells. 1 × 105 of the indicated cell type were either pretreated with 5 μM MP7-NH2 for 1 h (filled circles) or incubated in serum-free medium without peptide (open circles) in a total volume of 1 ml. After the 1 h incubation the media was removed, cells were washed 3×, and infected with 105 PFU VSV (MOI 1) in SF-DMEM. After 1 h the inoculum was removed and replaced with DMEM-5%FBS. Supernatants were collected after 6 h and titered via plaque assay on BHK-21 cells to measure the amount of virus released. Graphs show the results of three independent studies for each cell line. The difference in the amount of virus released by the treated vs. untreated cells was not significantly different for any of the three cell types tested.
BHK-21 cells do not produce Type I interferons [3,36], and have not been shown to express pathogen recognition receptors such as TLRs or NLRs. To determine whether an interferon competent cell line that expressed pathogen recognition receptors might be more able to restrict viral entry/replication after MP7-NH2 treatment we repeated the above studies using A549 lung epithelial cells and RAW264.7 macrophage cells. As shown in Fig. 5B and C, peptide pretreatment of A549 and RAW264.7 cells did not significantly decrease the amount of virus released by those cells (P = 0.142 and P = 0.094 respectively, via paired two-tailed T test) although treated cells always produced slightly reduced amounts of virus relative to untreated cells.
4. Discussion
We undertook this study with the goal of determining whether a cationic peptide (MP7-NH2) derived from the wasp venom peptide mastoparan had antiviral activity. The characterization of such peptides is important because natural host defense peptides (NHP) have the potential to serve as broad-spectrum antiviral agents. Antiviral drugs with broad reactivity are urgently needed; there are only two such drugs currently licensed for human use (ribavirin and interferon), both of which cause significant side effects in treated individuals [47] and neither of which are effective against emerging viruses such as West Nile virus [13], avian influenza [49], or the henipaviruses [51].
We found that MP7-NH2 potently inactivated enveloped viruses of five different families in a cell-free system in vitro but did not measurably reduce infectivity of non-enveloped adenovirus. Those results confirmed that MP7-NH2 had potent and broad-spectrum antiviral activity and supported the idea that MP7-NH2 acted by interfering with a shared process or structure that was essential for infectivity of enveloped but not non-enveloped viruses. When we used a prototype virus (VSV) to determine the minimum virucidal concentration for MP7-NH2, we found that MP7-NH2 still retained activity at concentrations as low as 5 μM. Although it is difficult to directly compare those results to the reports of other groups, it appears that the activity of MP7-NH2 compares favorably to that of other invertebrate peptides that have been tested. For example, the modified NHP mucroporin-M1 used at 35 μM reduced the infectious titer of measles virus by 50% [31], while 10 μM MP7-NH2 reduced the infectious titer of VSV by between 2 and ≥3 logs depending upon the amount of input virus (Fig. 1A). In addition, we found that MP7-NH2 was active at physiologic temperatures and retained activity (albeit at reduced levels) in the presence of serum proteins. This was important because many mammalian antimicrobial peptides that have potent activity in serum-free media are either partially or completely inactive in the presence of serum [14].
These results were encouraging, and led us to investigate the mechanism by which MP7-NH2 exerted its antiviral effect. Because the viruses inactivated by MP7-NH2 do not share a common cellular receptor, do not enter the host cell by the same mechanism, and do not utilize the same type of nucleic acid as their genome (RNA vs. DNA), we reasoned that physical disruption of the envelope itself might be contributing to direct virus inactivation by MP7-NH2. Indeed, when we subjected MP-7 NH2 treated VSV virions to electron microscopy, we found that the envelope had separated from the core of the virion, and in some cases had completely detached from the nucleocapsid. That result agreed with those of Howell et al., who reported that vaccinia virus treated with mammalian host defense peptide LL-37 had a perturbed envelope structure [25].
Those results fit with our predicted model of peptide-mediated virus inactivation, but raised the question of why MP7-NH2 failed to inactivate influenza viruses, and inactivated RSV less effectively than VSV, HSV-1, and the flaviviruses. Our influenza virus stocks were grown in eggs, and one possibility was that trace amounts of allantoic fluid in the virus preparations could have inhibited the activity of MP7-NH2. However, when we added allantoic fluid to a direct-inactivation reaction with VSV and MP7-NH2, we did not observe any reduction in efficacy of MP7-NH2, which suggested that presence of allantoic fluid proteins was not a factor in the resistance of influenza to MP7-NH2 mediated inactivation. An alternate possibility is that differences in relative plasticity of the virus envelopes made some of the viruses more resistant to inactivation than others. For example, VSV and the flaviviruses have highly ordered envelope structures, in which the virus attachment and/or fusion proteins are anchored in regular patterns among the lipids of the virus envelope [15,35]. By contrast, influenza and RSV virions are relatively more pleomorphic [6,21], with the attachment and fusion proteins assuming a less ordered array within the lipid bilayer. This plasticity might render flu and RSV more resistant to perturbations of the envelope structure; i.e. if insertion of the peptide altered the shape or curvature of the virion, attachment and fusion might still be possible.
Another possibility is that differences in the lipid composition of the various virus envelopes may have rendered some viruses more resistant than others to inactivation. It has been shown that the selective cytotoxicity of Polybia mastoparan MP-1 depends on the lipid composition of the plasma membrane, with phosphatidylserine being protective and phosphatidylcholine rich membranes being more susceptible to damage [16]. Testing of these hypotheses will be a priority for future studies, with the ultimate goal of being able to predict the activity of MP derivatives against other viruses based on structure and fluidity of the virus envelope.
A second question was why inactivation of cowpox and vaccinia virus was less effective than that of VSV and the flaviviruses. Unlike the other enveloped viruses tested, which have a single type of infectious particle surrounded by a single bilaminar envelope, poxviruses exist in four different infectious forms. Intracellular mature virions (IMV) account for the majority of infectious particles and have a single lipid envelope [24]. However intracellular enveloped virions (IEV) which are usually the second most numerous particle in laboratory preparations of virus, acquire additional envelope layers as they migrate toward the plasma membrane via the Golgi and/or endosome [38,48]. Presumably the envelopes of IEV would be more difficult for MP7-NH2 to penetrate and disrupt. Indeed, in the work of Howell et al. [25], the disruption of the vaccinia virus envelope appears to be much less extensive than that observed here with MP7-NH2 and VSV. Although those differences could be due to other factors, they are consistent with the idea that a more substantial envelope structure might render viruses more resistant to inactivation by peptides with this shared mode of action.
Another goal for our study was to determine whether MP7-NH2 could trigger cell-mediated antiviral activity. Many other NHP have been shown to induce and/or enhance innate immune responses in the host. For example, pretreatment of cells with human defensin HNP-1 renders cells less permissive for viral replication by inducing expression of IFN-responsive gene Mx3, [19], and by suppressing upregulation of cellular PKC [44], which is required for influenza virus replication and nuclear trafficking [46]. In our hands, pre-treatment of cells of three different lineages with MP7-NH2 did not prevent infection of those cells with VSV, nor did it reduce the amount of virus produced by infected cells. One consideration in interpreting those results however is that our system in vitro used a single cell type in each culture. In the whole animal, where a range of cell types would be expected to respond to peptide treatment, indirect cell-mediated anti-viral activity might be induced by MP7-NH2. That prediction is supported by the results of King et al. who examined the immunological response to wasp venom and found that mice which were immunized with mastoparan and another venom protein had enhanced anti-venom IgE responses vs. those receiving venom protein alone [27].
In sum, our findings demonstrate that MP7-NH2 has potent broad-spectrum antiviral activity in vitro. MP7-NH2 was active at physiologic conditions in a cell-free system, and treatment of virions with MP7-NH2 significantly disrupted the integrity of the virus envelope. These results demonstrate for the first time the antiviral properties of a mastoparan-derived peptide, and add to our understanding of the mechanisms by which natural host defense peptides inactivate viral pathogens.
Supplementary Material
Acknowledgements
Supported by NIH grants AI082107 (to HFS), and AI51445 (to EAR). A portion of the work was conducted in the Global Health Research Building at Duke University, which receives support from grant number UC6 AI058607. Electron microscopy studies were funded by an Institutional grant to EAR.
References
- 1.Albiol Matanic VC, Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. International Journal of Antimicrobial Agents. 2004;23:382–9. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Alekseeva L, Huet D, Femenia F, Mouyna I, Abdelouahab M, Cagna A, et al. Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiology. 2009;9:33. doi: 10.1186/1471-2180-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andzhaparidze OG, Bogomolova NN, Boriskin YS, Bektemirova MS, Drynov ID. Comparative study of rabies virus persistence in human and hamster cell lines. Journal of Virology. 1981;37:1–6. doi: 10.1128/jvi.37.1.1-6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbuzova A, Schwarz G. Pore kinetics of mastoparan peptides in large unilamellar lipid vesicles. Progress in Colloid and Polymer Science. 1996;100:345–50. [Google Scholar]
- 5.Ariki S, Koori K, Osaki T, Motoyama K, Inamori K, Kawabata S. A serine protease zymogen functions as a pattern-recognition receptor for lipopolysaccharides. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:953–8. doi: 10.1073/pnas.0306904101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachi T, Howe C. Morphogenesis and ultrastructure of respiratory syncytial virus. Journal of Virology. 1973;12:1173–80. doi: 10.1128/jvi.12.5.1173-1180.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barefoot B, Thornburg NJ, Barouch DH, Yu JS, Sample C, Johnston RE, et al. Comparison of multiple vaccine vectors in a single heterologous prime-boost trial. Vaccine. 2008;26:6108–18. doi: 10.1016/j.vaccine.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barefoot BE, Athearn K, Sample CJ, Ramsburg EA. Intramuscular immunization with a vesicular stomatitis virus recombinant expressing the influenza hemagglutinin provides post-exposure protection against lethal influenza challenge. Vaccine. 2009;28:79–89. doi: 10.1016/j.vaccine.2009.09.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boman HG, Hultmark D. Cell-free immunity in insects. Annual Review of Microbiology. 1987;41:103–26. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- 10.Chang TL, Vargas Jr J, DelPortillo A, Klotman ME. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. Journal of Clinical Investigation. 2005;115:765–73. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen HQ, Veluthakal R, Palanivel R, Kowluru A. GTP-binding protein-independent potentiation by mastoparan of IL-1beta-induced nitric oxide release from insulin-secreting HIT-T15 cells. Apoptosis. 2004;9:145–8. doi: 10.1023/B:APPT.0000018796.52262.b3. [DOI] [PubMed] [Google Scholar]
- 12.Cho H, Kehrl JH. Regulation of immune function by G protein-coupled receptors, trimeric G proteins, and RGS proteins. Progress in Molecular Biology and Translational Science. 2009;86:249–98. doi: 10.1016/S1877-1173(09)86009-2. [DOI] [PubMed] [Google Scholar]
- 13.Chowers MY, Lang R, Nassar F, Ben-David D, Giladi M, Rubinshtein E, et al. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerging Infectious Diseases. 2001;7:675–8. doi: 10.3201/eid0704.010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. Journal of Virology. 1986;60:1068–74. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doms RW, Ruusala A, Machamer C, Helenius J, Helenius A, Rose JK. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. Journal of Cell Biology. 1988;107:89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dos Santos Cabrera MP, Arcisio-Miranda M, Gorjao R, Leite NB, de Souza BM, Curi R, et al. Influence of the bilayer composition on the binding and membrane disrupting effect of Polybia-MP1, an antimicrobial mastoparan peptide with leukemic T-lymphocyte cell selectivity. Biochemistry. 2012 doi: 10.1021/bi201608d. [DOI] [PubMed] [Google Scholar]
- 17.Dunn MD, Rossi SL, Carter DM, Vogt MR, Mehlhop E, Diamond MS, et al. Enhancement of anti-DIII antibodies by the C3d derivative P28 results in lower viral titers and augments protection in mice. Virology Journal. 2010;7:95. doi: 10.1186/1743-422X-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellerby HM, Martin SJ, Ellerby LM, Naiem SS, Rabizadeh S, Salvesen GS, et al. Establishment of a cell-free system of neuronal apoptosis: comparison of premitochondrial, mitochondrial, and postmitochondrial phases. Journal of Neuroscience. 1997;17:6165–78. [PMC free article] [PubMed] [Google Scholar]
- 19.Falco A, Mas V, Tafalla C, Perez L, Coll JM, Estepa A. Dual antiviral activity of human alpha-defensin-1 against viral haemorrhagic septicaemia rhabdovirus (VHSV): inactivation of virus particles and induction of a type I interferon-related response. Antiviral Research. 2007;76:111–23. doi: 10.1016/j.antiviral.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Fang XM, Shu Q, Chen QX, Book M, Sahl HG, Hoeft A, et al. Differential expression of alpha- and beta-defensins in human peripheral blood. European Journal of Clinical Investigation. 2003;33:82–7. doi: 10.1046/j.1365-2362.2003.01076.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris A, Cardone G, Winkler DC, Heymann JB, Brecher M, White JM, et al. Influenza virus pleiomorphy characterized by cryoelectron tomography. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19123–7. doi: 10.1073/pnas.0607614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashijima T, Uzu S, Nakajima T, Ross EM. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). Journal of Biological Chemistry. 1988;263:6491–4. [PubMed] [Google Scholar]
- 23.Higashijima T, Wakamatsu K, Takemitsu M, Fujino M, Nakajima T, Miyazawa T. Conformational change of mastoparan from wasp venom on binding with phospholipid membrane. FEBS Letters. 1983;152:227–30. doi: 10.1016/0014-5793(83)80385-8. [DOI] [PubMed] [Google Scholar]
- 24.Hollinshead M, Vanderplasschen A, Smith GL, Vaux DJ. Vaccinia virus intra-cellular mature virions contain only one lipid membrane. Journal of Virology. 1999;73:1503–17. doi: 10.1128/jvi.73.2.1503-1517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. Journal of Immunology. 2004;172:1763–7. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 26.Jones S, Howl J. Charge delocalisation and the design of novel mastoparan analogues: enhanced cytotoxicity and secretory efficacy of [Lys5, Lys8, Aib10]MP. Regulatory Peptides. 2004;121:121–8. doi: 10.1016/j.regpep.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 27.King TP, Jim SY, Wittkowski KM. Inflammatory role of two venom components of yellow jackets (Vespula vulgaris): a mast cell degranulating peptide mastoparan and phospholipase A1. International Archives of Allergy and Immunology. 2003;131:25–32. doi: 10.1159/000070431. [DOI] [PubMed] [Google Scholar]
- 28.Konrad RJ, Young RA, Record RD, Smith RM, Butkerait P, Manning D, et al. The heterotrimeric G-protein Gi is localized to the insulin secretory granules of beta-cells and is involved in insulin exocytosis. The Journal of Biological Chemistry. 1995;270:12869–76. doi: 10.1074/jbc.270.21.12869. [DOI] [PubMed] [Google Scholar]
- 29.Krisanaprakornkit S, Weinberg A, Perez CN, Dale BA. Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infection and Immunity. 1998;66:4222–8. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SI, Park KH, Kim SJ, Kang YG, Lee YM, Kim EC. Mechanical stress-activated immune response genes via Sirtuin 1 expression in human periodontal ligament cells. Clinical and Experimental Immunology. 2012;168:113–24. doi: 10.1111/j.1365-2249.2011.04549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Zhao Z, Zhou D, Chen Y, Hong W, Cao L, et al. Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides. 2011;32:1518–25. doi: 10.1016/j.peptides.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maisetta G, Di Luca M, Esin S, Florio W, Brancatisano FL, Bottai D, et al. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human beta defensin 3. Peptides. 2008;29:1–6. doi: 10.1016/j.peptides.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Morimoto M, Mori H, Otake T, Ueba N, Kunita N, Niwa M, et al. Inhibitory effect of tachyplesin I on the proliferation of human immunodeficiency virus in vitro. Chemotherapy. 1991;37:206–11. doi: 10.1159/000238855. [DOI] [PubMed] [Google Scholar]
- 34.Mousli M, Bronner C, Bueb JL, Tschirhart E, Gies JP, Landry Y. Activation of rat peritoneal mast cells by substance P and mastoparan. Journal of Pharmacology and Experimental Therapeutics. 1989;250:329–35. [PubMed] [Google Scholar]
- 35.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nature Reviews Microbiology. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 36.Nagai Y, Ito Y, Hamaguchi M, Yoshida T, Matsumoto T. Relation of interferon production to the limited replication of Newcastle disease virus in L cells. Journal of General Virology. 1981;55:109–16. doi: 10.1099/0022-1317-55-1-109. [DOI] [PubMed] [Google Scholar]
- 37.O'Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. Journal of Immunology. 1999;163:6718–24. [PubMed] [Google Scholar]
- 38.Payne LG, Kristenson K. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. Journal of Virology. 1979;32:614–22. doi: 10.1128/jvi.32.2.614-622.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perron GG, Zasloff M, Bell G. Experimental evolution of resistance to an antimicrobial peptide. Proceedings: Biological Sciences. 2006;273:251–6. doi: 10.1098/rspb.2005.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Publicover J, Ramsburg E, Robek M, Rose JK. Rapid pathogenesis induced by a vesicular stomatitis virus matrix protein mutant: viral pathogenesis is linked to induction of tumor necrosis factor alpha. Journal of Virology. 2006;80:7028–36. doi: 10.1128/JVI.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, et al. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. Journal of Virology. 1998;72:4704–11. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson Jr WE, McDougall B, Tran D, Selsted ME. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. Journal of Leukocyte Biology. 1998;63:94–100. doi: 10.1002/jlb.63.1.94. [DOI] [PubMed] [Google Scholar]
- 43.Rocha T, de Barros LL, Fontana K, de Souza BM, Palma MS, da Cruz-Hofling MA. Inflammation and apoptosis induced by mastoparan Polybia-MPII on skeletal muscle. Toxicon. 2010;55:1213–21. doi: 10.1016/j.toxicon.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Salvatore M, Garcia-Sastre A, Ruchala P, Lehrer RI, Chang T, Klotman ME. Alpha-defensin inhibits influenza virus replication by cell-mediated mechanism(s). The Journal of Infectious Diseases. 2007;196:835–43. doi: 10.1086/521027. [DOI] [PubMed] [Google Scholar]
- 45.Scott MG, Hancock RE. Cationic antimicrobial peptides and their multi-functional role in the immune system. Critical Reviews in Immunology. 2000;20:407–31. [PubMed] [Google Scholar]
- 46.Sieczkarski SB, Brown HA, Whittaker GR. Role of protein kinase C betaII in influenza virus entry via late endosomes. Journal of Virology. 2003;77:460–9. doi: 10.1128/JVI.77.1.460-469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snoeck E, Wade JR, Duff F, Lamb M, Jorga K. Predicting sustained virological response and anaemia in chronic hepatitis C patients treated with peginterferon alfa-2a (40KD) plus ribavirin. British Journal of Clinical Pharmacology. 2006;62:699–709. doi: 10.1111/j.1365-2125.2006.02741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. European Journal of Cell Biology. 1993;60:163–78. [PubMed] [Google Scholar]
- 49.Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VC, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. New England Journal of Medicine. 2004;350:1179–88. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 50.Tzeng YL, Ambrose KD, Zughaier S, Zhou X, Miller YK, Shafer WM, et al. Cationic antimicrobial peptide resistance in Neisseria meningitidis. Journal of Bacteriology. 2005;187:5387–96. doi: 10.1128/JB.187.15.5387-5396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vigant F, Lee B. Hendra and nipah infection: pathology, models and potential therapies. Infectious Disorders – Drug Targets. 2011;11:315–36. doi: 10.2174/187152611795768097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, et al. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. Journal of General Virology. 1998;79(Pt 4):731–40. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- 53.Whiles JA, Brasseur R, Glover KJ, Melacini G, Komives EA, Vold RR. Orientation and effects of mastoparan X on phospholipid bicelles. Biophysical Journal. 2001;80:280–93. doi: 10.1016/S0006-3495(01)76013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan GM, Lin SZ, Irwin RP, Paul SM. Activation of G proteins bidirectionally affects apoptosis of cultured cerebellar granule neurons. Journal of Neurochemistry. 1995;65:2425–31. doi: 10.1046/j.1471-4159.1995.65062425.x. [DOI] [PubMed] [Google Scholar]
- 55.Yandek LE, Pokorny A, Almeida PF. Wasp mastoparans follow the same mechanism as the cell-penetrating peptide transportan 10. Biochemistry. 2009;48:7342–51. doi: 10.1021/bi9008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yount NY, Yeaman MR. Emerging themes and therapeutic prospects for anti-infective peptides. Annual Review of Pharmacology and Toxicology. 2012;52:337–60. doi: 10.1146/annurev-pharmtox-010611-134535. [DOI] [PubMed] [Google Scholar]
- 57.Yu K, Kang S, Park N, Shin J, Kim Y. Relationship between the tertiary structures of mastoparan B and its analogs and their lytic activities studied by NMR spectroscopy. Journal of Peptide Research. 2000;55:51–62. doi: 10.1034/j.1399-3011.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 58.Zasloff M. Antimicrobial peptides in health and disease. The New England Journal of Medicine. 2002;347:1199–200. doi: 10.1056/NEJMe020106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.