Summary
Stem cells reside in niches that regulate the balance between self-renewal and differentiation. The identity of a stem cell is linked with the ability to interact with its niche through adhesion mechanisms. To identify targets that disrupt cancer stem cell (CSC) adhesion, we performed a flow cytometry screen on patient derived glioblastoma (GBM) cells and identified junctional adhesion molecule-A (JAM-A) as a CSC adhesion mechanism essential for self-renewal and tumor growth. JAM-A was dispensable for normal neural stem/progenitor cell (NPC) function and JAM-A expression was reduced in normal brain versus GBM. Targeting JAM-A compromises the self-renewal of CSCs. JAM-A expression negatively correlated to GBM patient prognosis. Our results demonstrate that novel GBM targeting strategies can be identified through screening adhesion receptors and JAM-A represents a novel mechanism for niche driven CSC maintenance.
Introduction
Stem cells are essential for the growth and homeostasis of organs and, more broadly, an organism. While stem cells have an unlimited proliferation capacity and can differentiate into any cell type in a given organ, they reside in specific anatomical locations or niches that ensure the correct balance of self-renewal and differentiation. Stem cells must be simultaneously maintained for long term tissue preservation yet constrained to avoid oncogenesis. Stem cells that exit a niche undergo differentiation and fate determination. Niche signaling via secreted factors, cell to cell, and cell to extracellular matrix (ECM) interactions represent a powerful control mechanism for stem cell regulation. Many niche factors have also been implicated in the progression of advanced cancers in the context of maintaining a self-renewing, tumorigenic cancer stem cell (CSC) population at the top of a cellular hierarchy. Like normal stem cells, CSCs also reside in distinct anatomical locations and rely on niche interactions to regulate the balance between self-renewal and differentiation, and these interactions provide pro-survival and therapeutic resistance mechanisms (Gilbertson and Rich, 2007; Spradling et al., 2001).
While the CSC hypothesis remains controversial, CSCs have been well documented in many advanced cancers including glioblastoma (GBM, World Health Organization grade IV glioma). Current therapeutic approaches for GBM remain only palliative and the standard care of surgical resection followed by aggressive radiation and chemotherapy has extended median survival to between 12–15 months while the 5-year survival remains approximately 2% (Stupp et al., 2009). GBM is refractory to the current standard therapies partly due to invasion into the normal brain and cellular heterogeneity (Bonavia et al., 2011). The identification of CSCs in GBM (Galli et al., 2004; Hemmati et al., 2003; Ignatova et al., 2002; Singh et al., 2003; Singh et al., 2004) has generated enthusiasm for the integration of CSCs into models of cancer (Visvader and Lindeman, 2012) and the development of anti-CSC therapies. CSCs in GBM are contained within hypoxic (Li et al., 2009) and perivascular niches (Calabrese et al., 2007). The perivascular niche is readily identifiable in vivo and efforts are underway to characterize the components regulating this niche which include local mitogens, cell-cell communication mechanisms, and unique structural components, such as ECM proteins (Hjelmeland et al., 2011). We previously demonstrated that integrin α6, an ECM receptor, can be used to enrich and target CSCs (Lathia et al., 2010). Additionally, interaction with the specialized ECM of the perivascular niche, which is provided by niche components, also promotes CSC growth (Lathia et al., 2012). These studies provide a paradigm for CSC maintenance whereby adhesion status is a determining factor for the position of a cell within the tumor hierarchy, with CSCs displaying an enhanced adhesion capacity as compared to their differentiated progeny.
While similarities exist between CSCs and non-neoplastic stem cells, specific targets for CSCs have been generated by comparing the molecular machinery between the cell types and have been validated in preclinical models (Eyler et al., 2011; Guryanova et al., 2011). However, niche adhesion targets have yet to be developed. The interaction between cell adhesion mechanisms, including integrins, generates diverse signaling responses based on cell type, location, and the cluster of receptors present in a signaling complex. Defining adhesion specific programs unique to the CSC niche compartment is difficult as many of these programs exist both in the normal and neoplastic niches (Hale et al., 2012). For example, integrin expression and function in organs such as the brain and the breast are similar in the normal and neoplastic context, with both normal stem cells and CSCs being enriched on the basis of integrin α6 (Ali et al., 2011; Hall et al., 2006; Lathia et al., 2010; Shackleton et al., 2006; Stingl et al., 2006), which plays a key role in regulating their growth (Kazanis et al., 2010; Lathia et al., 2010; Loulier et al., 2009). Given the importance of niche adhesion to stem cell maintenance (Niola et al., 2012; Niola et al., 2013), identification of unique CSC niche mechanisms is an immediate priority to facilitate the development of more effective GBM therapies. To identify CSC niche adhesion mechanisms, we performed an unbiased flow cytometry screen of cell adhesion receptors expressed in patient derived GBM cells with a link to patient survival. Combining additional biochemical, molecular, and biological analyses, we identified junctional adhesion molecule-A (JAM-A) as a critical maintenance factor for CSCs.
Results
Flow cytometry screen of adhesion receptors reveals GBM cells express JAM-A
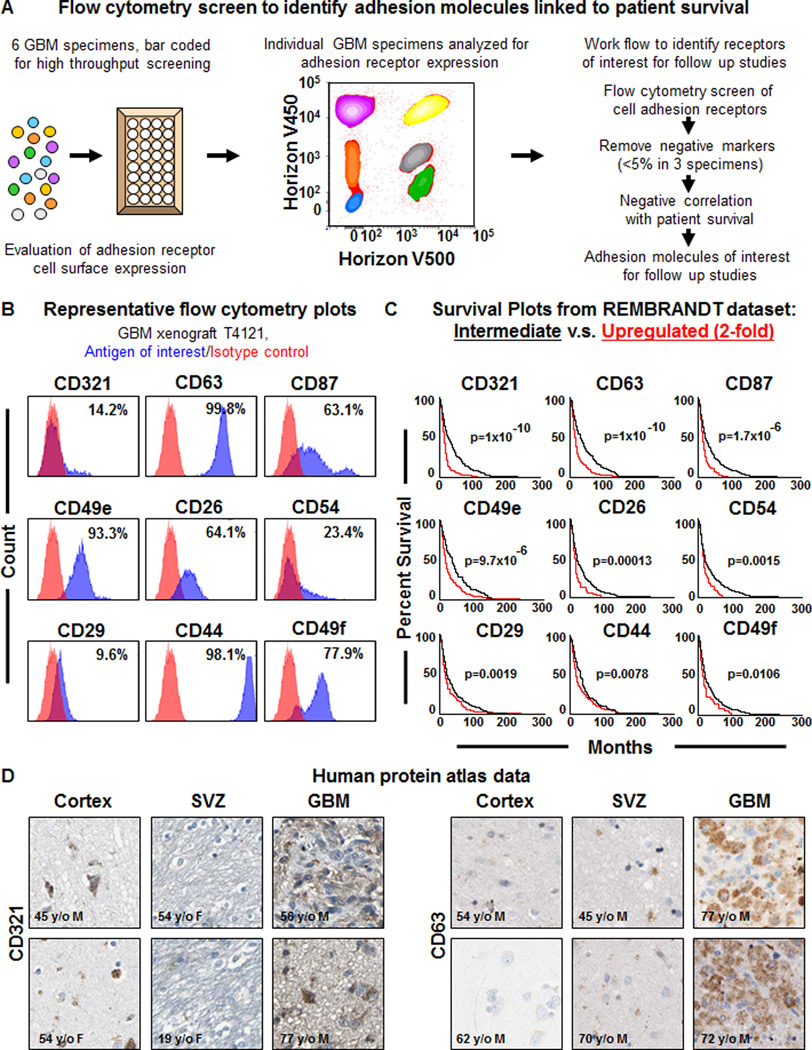
Unbiased screening approaches present an opportunity to understand the complexity of a system as well as identify new regulatory nodes that can be then further developed for targeting purposes. In the context of GBM, a variety of genetic (RNA interference) and pharmacological (small molecule) screens have been performed that have identified targets for further investigation (Hubert et al., 2013; Visnyei et al., 2011; Wurdak et al., 2010). While these approaches have revealed the complexity of GBM and provided novel targets, they have provided limited information with respect to niche interaction. Flow cytometry enables the prospective enrichment of CSCs and can be utilized to study intact cells as well as specific cell surface interactions. To identify cell adhesion receptors expressed on the surface of GBM cells, we performed a high throughput flow cytometry screen, which informed the identification of cell surface antigens differentially expressed during neural differentiation (Yuan et al., 2011). To perform the screen, we utilized 6 different GBM specimens that had validated differences in self-renewal both in vitro (Supplemental Fig. 1A) and in vivo based on previously reported cell surface markers (Eyler et al., 2011; Guryanova et al., 2011; Lathia et al., 2010; Singh et al., 2003). Each specimen was uniquely identified using a bar coding approach and then all xenografts were pooled together for the screening procedure (Fig. 1A). Along with interrogating the cell surface expression of adhesion receptors, we combined the analysis with a bioinformatics evaluation of patient survival to identify adhesion receptors that were expressed on GBM cells and informative for patient prognosis (Fig. 1A). Screening was performed on cell adhesion molecules for which antibodies were commercially available and any that were expressed above a level of 5% for 3 of the 6 GBM specimens were subsequently evaluated for a negative correlation to glioma patient survival based on the National Cancer Institute REpository for Molecular BRAin Neoplastic Data (NCI REMBRANDT) database (Fig. 1B, C). Our screen identified 9 cell adhesion receptors that met the selection criteria and were ranked based on negative correlation to glioma patient survival (Supplemental Table 1) with varying correlation between individual targets (Supplemental Fig. 1B). The screen revealed the following cell surface adhesion receptors: CD321 (JAM-A), CD63 (a tetraspannin family member), CD87 (urokinase receptor), CD49e (integrin α5), CD26 (dipeptidyl peptidase-4), CD54 (ICAM1), CD29 (integrin β1), CD44 (a hyaluronic acid receptor), and CD49f (integrin α6). Among the list were 3 previously published CSC markers, CD49f and CD29 (Lathia et al., 2010) as well as CD44 (Anido et al., 2010; Jijiwa et al., 2011), providing evidence that our approach was capable of identifying functionally relevant adhesion receptors. CD26, a regulator of colon CSC metastasis that interacts with integrin β1 (Pang et al., 2010), was also identified and CD87, which is linked to GBM survival (Hu et al., 2011) and invasion (Raghu et al., 2011) was likewise identified. The top 2 targets, CD321 and CD63, were further validated with respect to tumor specific expression using the human protein atlas (Fig. 1D).
Figure 1. High throughput screening on GBM specimens using a multiplex flow cytometry identifies cell adhesion receptors negatively correlated to GBM patient survival.
Schematics depicting the work flow of the high throughput flow cytometry screening method (A) based on bar coding multiple GBM specimens and screening cell surface marker expression to identify cell adhesion receptors linked to GBM patient survival. Representative flow cytometry plots (B) for adhesion receptors from GBM xenograft T4121, antigen of interest shown in blue, isotype control shown in red, and percentage of positive cells indicated on each individual plot. Representative Kaplan-Meier survival curves (C) for candidate adhesion molecular generated from the NCI REpository for Molecular BRAin Neoplasia DaTa (REMBRANDT) bioinformatics database (https://caintegrator.nci.nih.gov/rembrandt/) for the 2-fold upregulated (red) and intermediate (black) groups. Log-rank p-value for survival curves between upregulated and intermediate groups provided for each adhesion molecule. Representative immunohistochemical data (D) generated for CD321 and CD63 from the human protein atlas (http://www.proteinatlas.org) for the cortex, subventricular zone (SVZ), and glioblastoma (GBM) tumor. Age and sex of each individual indicated directly on the micrographs. See also Table S1, Figure S1.
JAM-A, which had a median survival of 13.4 months, was of interest based on its reported role in cell-to-cell adhesion and facilitation of integrin signaling (McSherry et al., 2011; Severson et al., 2009). These functions have not been previously tested in GBM and are likely to be an important mechanism by which cells communicate with niche’s ECM. In the nervous system, integrin α6 predominantly uses integrin β1 as a binding partner to initiate signaling events upon activation (Lathia et al., 2007; Loulier et al., 2009). We previously demonstrated that integrin α6 expression can enrich for CSCs (Lathia et al., 2010). Further evaluation of the activation state of integrin β1 revealed that it was also informative for sphere formation potential and indeed blocking integrin β1 attenuated sphere formation (Supplemental Fig. 1C–E). Integrin β1 is regulated by a variety of co-receptors that are cell type and context dependent. In the mammary epithelium, where integrins α6 and β1 are also elevated in normal and neoplastic stem cells (Ali et al., 2011; Shackleton et al., 2006; Stingl et al., 2006), JAM-A interacts with integrin β1 and correlates with poor patient prognosis (McSherry et al., 2011; Murakami et al., 2011). Given the relationship between integrin β1 and JAM-A in the mammary gland, we wanted to determine the role of JAM-A in GBM and its role in regulating CSC maintenance.
JAM-A is elevated in CSCs
To confirm that JAM-A was expressed on GBM cells and exclude the contribution of non-neoplastic cells, we performed flow cytometry analyses on xenografted (T4121, T387) and a primary patient (CCF2418) derived specimens in combination with known endothelial cell markers (CD105 and CD31 (data not shown)). JAM-A was expressed in xenografted and primary GBM specimens on both endothelial and non-endothelial cells (Supplemental Fig. 2A). To verify that JAM-A-positive/endothelial cell marker-negative cells were tumor cells, we isolated these cells from GBM xenografts and confirmed their tumor cell origin by FISH (Supplemental Fig. 2B, C). Taken together, these results confirm that JAM-A is expressed in GBM cells.
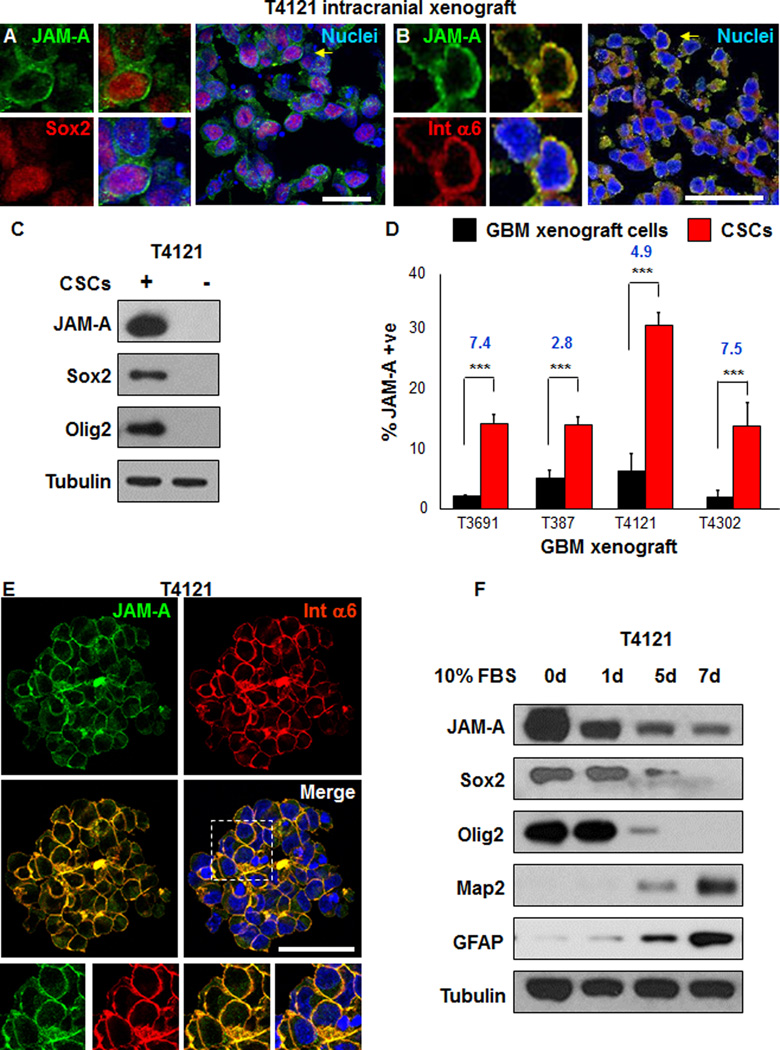
To validate our flow cytometry screen findings, we evaluated JAM-A expression by immunofluorescence analysis on cryosections from intracranial GBM xenografts. JAM-A was expressed in a fraction of tumor cells (T387: 28.8%; T3691: 28.1%; T4121: 32.7%; data not shown) and co-labeling revealed that a large fraction of JAM-A positive cells expressed CSC markers Sox2 or integrin α6 (Figure 2A, B; T387: 97.0%; T3691: 92.6%; T4121: 97.3%; data not shown). The co-expression of JAM-A with integrin α6 was further confirmed by flow cytometry analysis of xenografted patient specimens (T4121, T387) as well as primary GBM specimens (1217, 1221) and found to be at least 70% or higher (data not shown). These data suggest that JAM-A may be elevated in CSCs. To directly evaluate this possibility, we evaluated JAM-A expression by immunoblotting, flow cytometry, and immunostaining analysis. Immunoblotting analysis of matched CSCs and non-CSCs from a GBM patient xenograft enriched on the basis of a cell surface marker previously shown to distinguish tumor formation potential (Eyler et al., 2011; Guryanova et al., 2011; Lathia et al., 2010; Li et al., 2009) revealed that JAM-A was elevated in the CSC marker positive population as compared with the matched CSC marker negative population from the same GBM xenograft (Fig. 2C). The differential expression of JAM-A between CSCs and non-CSCs was also confirmed by flow cytometry analysis comparing parental xenografted GBM cells to CSCs enriched from the resulting xenografts and propagated as CSC-enriched sphere cultures (Fig. 2D). The co-expression between JAM-A and integrin α6 in CSC-enriched sphere cultures was confirmed by immunofluorescence analysis (Fig. 2E). To evaluate if JAM-A expression changed as a result of loss of stemness, we placed CSC-enriched sphere cultures under a 7 day serum induced differentiation paradigm. During differentiation, JAM-A expression was reduced as was the expression of CSC markers Sox2 and Olig2 while the differentiation markers Map2 and GFAP were increased (Fig. 2F). These data verify that the expression of JAM-A is enriched in CSCs as compared to their non-CSC counterparts and is lost during differentiation.
Figure 2. JAM-A is elevated in CSCs.
Confocal micrographs show that JAM-A is co-expressed with CSC markers Sox2 (A) and integrin α6 (B) on GBM cells within an intracranial xenograft (T4121) using antibodies against JAM-A (green) and co-stained with Sox2 (red) or integrin α6 (red). Areas of interest indicated by yellow arrow and shown at higher power, and scale bar represents XX um JAM-A is elevated in CSCs (enriched by cell surface expression of CD133 from GBM xenograft tumor T4121) as compared with matched non-CSCs as shown in representative immunoblots that show separation between populations with respect to Sox2 and Olig2 (C). Bar graphs summarizing flow cytometry analyses between parental cells and CSCs demonstrates that CSCs have significantly higher expression of JAM-A as compared to parental unenriched tumor cells from GBM xenografts (T3691, T387, T4121, T4302, (D). CSCs grown in sphere culture (from tumor T4121) co-express JAM-A (green) and integrin α6 (red) as shown in confocal micrographs. An area of interest indicated by white box is shown with enlarged images below, nuclei were counterstained with Hoechst 33342, and scale bar represents 50 µm (E). Upon CSC differentiation (enriched from GBM xenograft tumor T4121) using a 10% FBS differentiation paradigm over a 7 day period, JAM-A expression is lost with concomitaant loss in CSC markers Sox2 and Olig2 and an increase in differentiation markers GFAP and Map2 as shown in representative immunoblots (F). Data for bar graphs displayed as mean values +/− standard deviation, blue value represents fold change, and ***p<0.001 as assessed by one-way ANOVA. See also Figure S2.
JAM-A is not expressed by neural progenitor cells (NPCs) and is not required for NPC proliferation
Our results suggest that JAM-A is expressed on a fraction of GBM cells positive for CSC markers and encourage the evaluation of the expression profile of JAM-A in normal tissue in order to facilitate therapeutic approaches. To evaluate the expression and importance of JAM-A in normal neural function, we evaluated both human and mouse normal brain tissues for JAM-A expression. To evaluate the expression of JAM-A in human brain, we utilized both cultured fetal NPCs as well as an immunohistochemistry approach of adult brain tissue. Immunoblotting of NPCs and GBM cells (matched CSCs and non-CSCs) confirmed that JAM-A expression was elevated in CSCs and low in NPCs (Supplemental Fig. 2D). Using an immunohistochemistry analysis, we observed limited staining in the cortex or white matter areas of human adult brain (Supplemental Fig. 2E, 2F). JAM-A staining in the subependymal/subventricular zone (SEZ/SVZ) was limited to the ependymal cell layer (Supplemental Fig. 2G). To determine relative levels of JAM-A expression, we also utilized grade II astrocytoma and GBM tissue and observed low but detectable staining in grade II tumors and strong staining in GBM tissue (Supplemental Fig. 2H–J).
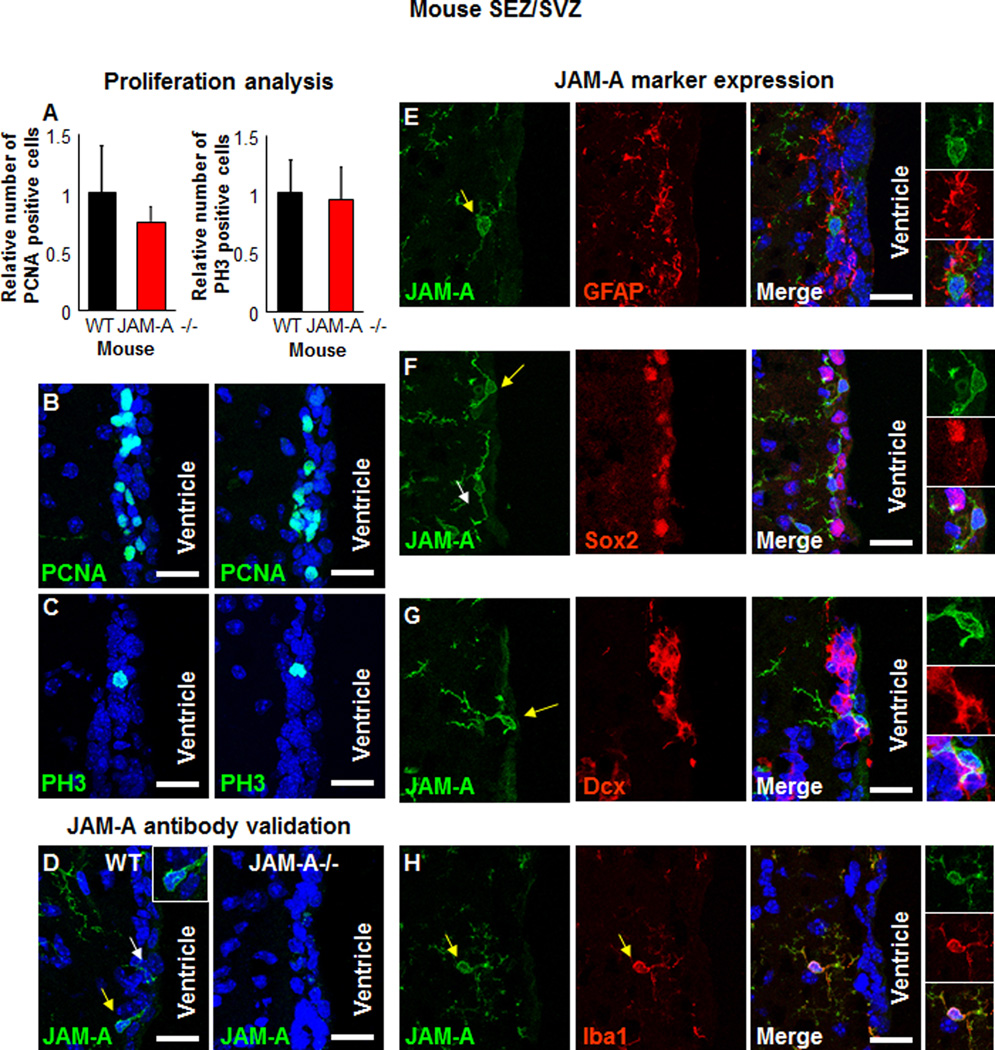
JAM-A knockout mice are viable, although the birth ratios are not Mendelian (1 (+/+): 1.7(+/−): 0.6 (−/−)) and their endothelial cells display a FGF2-induced angiogenesis defect (Cooke et al., 2006). However, the impact of JAM-A deletion in the brains of these mice is yet to be determined. To determine the role of JAM-A in NPCs, we evaluated proliferation in key a neurogenic niche (Alvarez-Buylla and Lim, 2004) in the brains of wild type and JAM-A −/− mice, the SVZ adjacent to the lateral ventricles. Analysis of SVZ proliferation using two molecular markers (proliferating cell nuclear antigen (PCNA) and phospho-histone H3 (PH3)) revealed no differences between adult wild-type (WT) and JAM-A −/− mice (Fig. 3A–C) suggesting that JAM-A is not essential for NPC proliferation.
Figure 3. JAM-A is not required for neurogenesis.
No detectable difference was observed in SEZ/SVZ proliferation between wild-type (WT) and JAM-A −/− mice as shown in summary bar graphs (A) and confocal micrographs of proliferation markers PCNA (B) and PH3 (C). Confocal micrographs show specific staining for JAM-A antibody in the SEZ/SVZ of WT mice but no staining in JAM-A−/− mice (D). Analysis of the mouse SEZ/SVZ reveals that JAM-A (green) is present in the SEZ/SVZ and not on NSCs/astrocytes (stained with an antibody against GFAP, red, E), NSC/NPCs (stained with an antibody against Sox2, red, F), neuroblasts (stained with an antibody against Dcx, red, G), but on microglia and macrophages (stained with an antibody against Iba1, red, H) as shown in confocal micrographs. Areas of interest indicated by yellow arrow and areas shown as insets. In panel F, another example of JAM-A positive, Sox2 negative cell is indicated by a white arrow. Nuclei were counterstained with Hoechst 33342 and scale bar represents 25 µm. Data for bar graphs displayed as mean values +/− standard deviation. See also Figure S3.
JAM-A has previously been reported to be expressed in the mouse corpus callosum on NG2 expressing cells (Stelzer et al., 2010), likely to be glial progenitors and pericytes. However, the expression in the neurogenic niches or NPCs is yet to be determined. The JAM-A antibody used only stained JAM-A expression in WT mice but not in JAM-A−/− mice confirming the specificity of the JAM-A antibody (Fig. 3D). JAM-A expression did not overlap with NSCs and astrocytes (marked by GFAP, Fig. 3E), NPCs (marked by Sox2, Fig. 3F), or neuroblasts (marked by doublecortin, Dcx, Fig. 3G). JAM-A expression, however, was detected in the SVZ and was found to overlap with Iba1 positive cells, reported to be putative microglia and macrophages (Fig. 3H). Iba1 positive cells were detected in both WT and JAM-A −/− mice, suggesting that the genesis of microglia and macrophages is not dependent on JAM-A (Supplemental Fig. 3A,B). JAM-A was not detected on additional SVZ components (ependymal cells, blood vessels, glial progenitors) or proliferating cells (Supplemental Fig. 3C–F). JAM-A was also not detected on additional cell types in another neurogenic niche, the subgranular zone (SGZ) of the dentate gyrus in the hippocampus, with the exception of microglia and macrophages (Supplemental Fig. 3G–J). Our analysis of the neurogenic regions in the mouse brain revealed that in the SVZ and SGZ, JAM-A was only co-expressed with Iba1 positive cells (Supplemental Fig. 3K, L) and that JAM-A −/− does not affect lba1+ cell genesis. Taken together, our human and mouse analysis suggests that JAM-A is not highly expressed on NPCs and not likely to be essential for their function.
Targeting JAM-A by blocking antibody attenuates CSC growth, self-renewal, and adhesion but does not affect non-CSC or NPCs
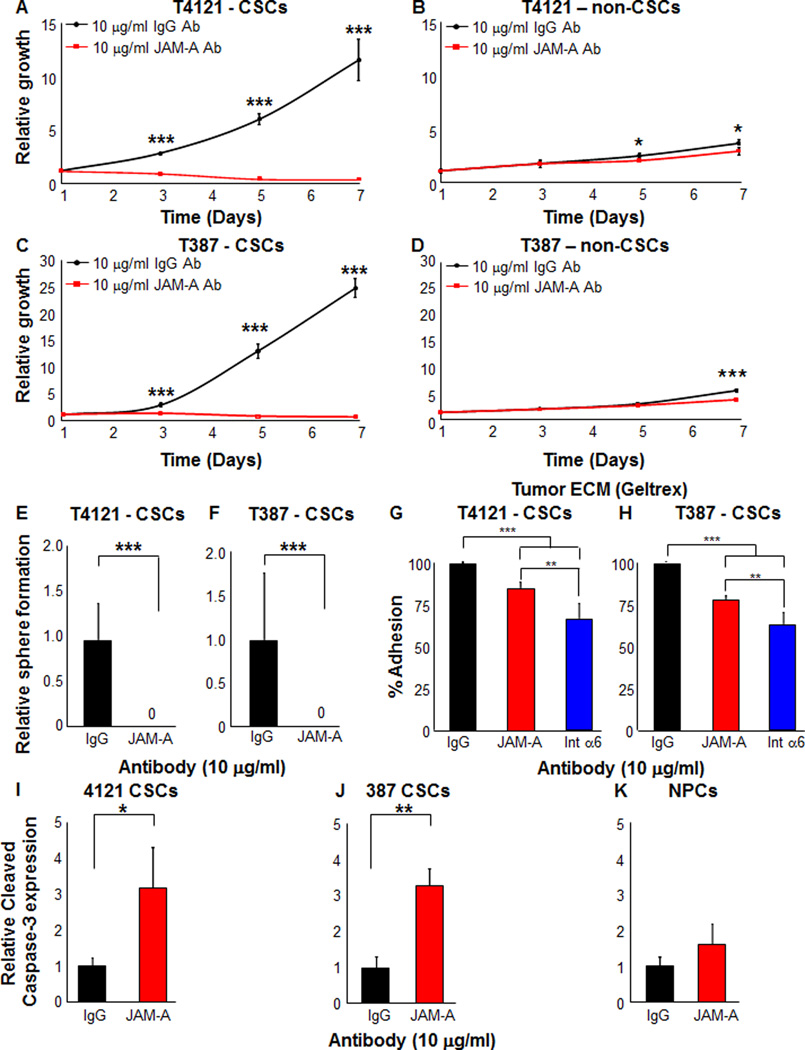
Our analysis confirmed the expression of JAM-A on CSCs and the very low or non-detectable levels of expression in matched non-CSC or NPCs, supporting the hypothesis that JAM-A is primarily utilized by CSCs. To evaluate the impact of disrupting JAM-A on CSC growth, we utilized a blocking antibody. CSCs treated with the JAM-A blocking antibody at previously reported concentrations of 1 and 10 µg/ml (Severson et al., 2009) showed significant reduction in growth over a 7 day time course (Fig. 4A, C, Supplemental Fig. 4A, C), which was not observed to the same extent in the non-CSC population (Fig. 4B, D, Supplemental Fig. 4B, D). A longer time course (days 10–14) did reveal a significant reduction in non-CSC growth at the 10 µg/ml but not the 1 µg/ml antibody concentration (data not shown). Sphere formation, a surrogate of self-renewal, was also significantly attenuated in the presence of the JAM-A blocking antibody at both 10 µg/ml (Fig. 4E, F) and 1 µg/ml (data not shown). To determine if the effects of the JAM-A blocking antibody on CSCs are due, in part, to apoptosis, we evaluated Caspase 3/7 activity and found a significant increase in CSCs at both concentrations of the JAM-A blocking antibody (Supplemental Fig. 4E–H). For equivalent conditions tested for non-CSCs, only a significant induction of caspase activity was observed from GBM specimen T4121 at 10 µg/ml (Supplemental Fig. 4F) but this was 4.8 fold lower when compared to CSCs from the same specimen. Unlike non-CSCs from GBM specimen T4121 over a longer time course, Caspase 3/7 activity was not induced by the JAM-A blocking antibody at both concentrations in non-CSCs from T387 (Supplemental Fig. 4G, H). A key function of JAM-A is to stabilize integrins and thereby facilitate adhesion and to evaluate if blocking JAM-A compromised this function, we performed short-term adhesion assays. Using a representative tumor ECM enriched in laminins (Geltrex), we found that the JAM-A blocking antibody significantly inhibited cell adhesion to the matrix (Fig. 4G, H), which was phenocopied with an integrin α6 blocking antibody (Fig. 4G, H). These differences in adhesion were not observed when assays were performed on fibronectin, another ECM protein, suggesting that JAM-A mediated adhesion is laminin specific. Finally, to determine if the JAM-A blocking antibody induced apoptosis in NPCs, we evaluated CSCs and NPCs treated with 10 µg/ml of the JAM-A blocking antibody and observed a significant induction of cleaved Caspase 3 by flow cytometry only in CSCs but not in NPCs (Fig. 4I–K) measured after 3 days. Taken together, these data suggest that blockade of JAM-A in CSCs attenuates growth, self-renewal, and adhesion and induces cell death while leaving non-CSCs and NPCs intact.
Figure 4. Targeting JAM-A by blocking antibodies decreases growth, self-renewal, adhesion, and increases apoptosis of CSCs but does not impact survival of non-stem tumor cells or NPCs.
JAM-A blocking antibody (red line/red bar) was used to treat CSCs (enriched from GBM xenografts T4121 and T387) and matched non-CSCs at 10 µg/ml and as compared to a control antibody (black line/black bar). A strong decrease was seen in CSC growth over time (A, C) but was not observed in non-CSCs (B, D). An attenuation in self-renewal as assessed by sphere formation was observed in CSCs in the presence of the JAM-A blocking antibody (E, F). A significant reduction in CSC adhesion onto a tumor ECM (Geltrex) was also observed with the JAM-A blocking antibody as well as an integrin a6 blocking antibody (G, H). Apoptosis induced by the JAM-A blocking antibody in CSCs (I,J) was not observed in NPCs (K). Data for all graphs displayed as mean values +/− standard deviation, *p<0.05, ** p<0.01, *** p<0.001 as assessed by one-way ANOVA. See also Figure S4.
Genetic disruption of JAM-A results in decreased CSC growth, self-renewal, and tumor formation
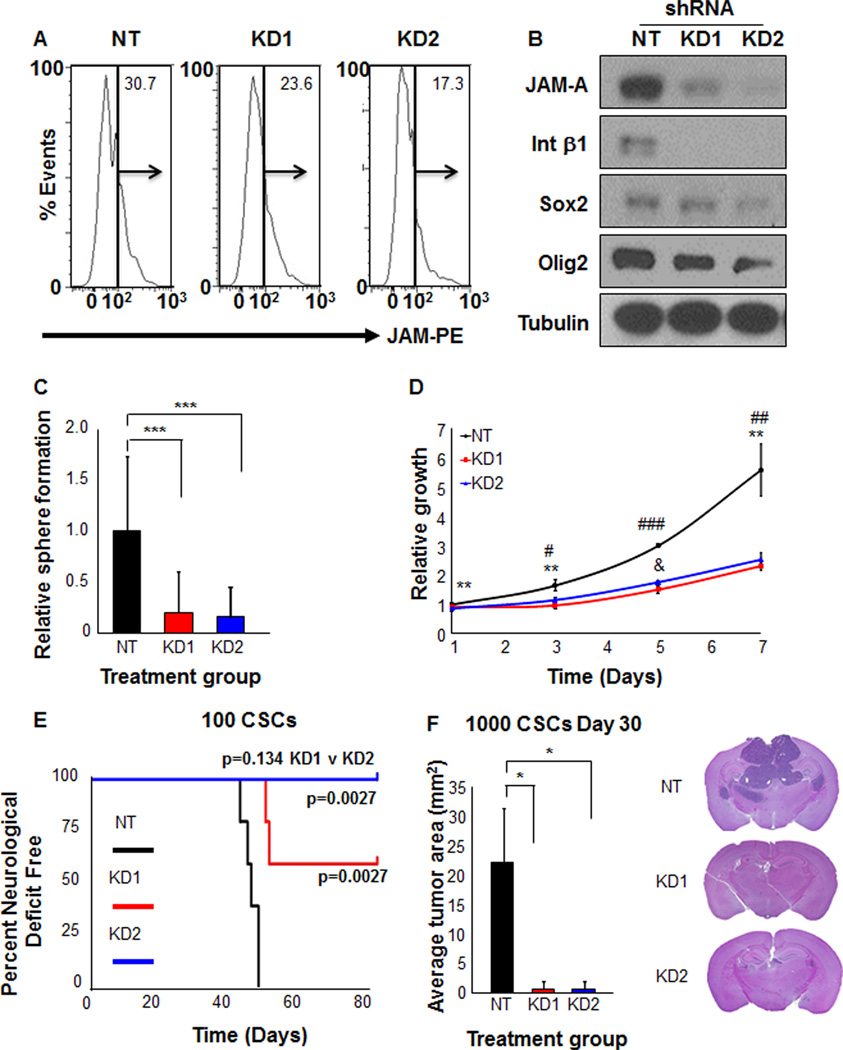
Acute disruption of JAM-A by a blocking antibody compromised CSC adhesion, growth, and self-renewal. To confirm the role of JAM-A in CSC maintenance, we also utilized a genetic approach to attenuate JAM-A expression by RNA interference. We developed two independent, non-overlapping shRNA constructs to knockdown JAM-A (KD1, KD2). When compared to a non-targeting (NT) control construct, both JAM-A knockdown constructs reduced JAM-A expression levels as evaluated by qPCR (data not shown), flow cytometry (Fig. 5A), and immunoblotting (Fig. 5B). JAM-A plays a major role in integrin stabilization (Severson et al., 2009) and knockdown of JAM-A resulted in reduced expression of integrin β1 (Fig. 5B). In addition, flow cytometry analysis revealed a reduction in integrin α6 cell surface levels after JAM-A knockdown (data not shown), further supporting a role for JAM-A in integrin stabilization. CSCs with attenuated JAM-A also displayed deficiencies in sphere formation (Fig. 5C) and proliferation (Fig. 5D). To evaluate if JAM-A was required for tumor formation, the key functional criteria for a CSC, we transplanted NT or KD CSCs into the brain of immunocompromised mice and observed significantly less tumor formation with JAM-A deficient CSCs (Fig. 5E). Additionally, analysis of mice 30 days after CSC transplantation revealed significantly smaller tumor area in the mouse brains receiving JAM-A knock down CSCs than in the ones receiving CSCs with NT construct (Fig. 5F). These results indicate that JAM-A is critical for integrin stabilization and that attenuation of JAM-A results in a deficiency of CSC phenotypes including growth, self-renewal, and tumor formation.
Figure 5. Targeting JAM-A by RNA interference decreases stem cell expression, growth, self-renewal, and tumor formation capacity of CSCs.
Two independent shRNA constructs (knockdown (KD) 1 and 2) were generated against JAM-A and reduced JAM-A expression as compared to non-targeting controls was verified in CSCs from GBM xenograft T4121 by flow cytometry (A). Attenuation of JAM-A in CSCs (enriched from GBM xenograft T4121) results in a decrease in integrin β1 expression as well as other core CSC markers (Sox2, Olig2) as shown by immunoblots (B). Reduction of JAM-A in CSCs (enriched from GBM xenograft T4121) also resulted in decreased self-renewal (C) and CSC growth (D). Slower Tumor growth after grafting CSCs (enriched from GBM xenograft T4121) was also observed when JAM-A expression was attenuated by shRNA (E) and overall tumor size was reduced as shown in bar graphs and images from brain sections 30 days after transplantation of 1000 CSCs (F). Data for all graphs displayed as mean values +/− standard deviation, *p<0.05, ** p<0.01, *** p<0.001 as assessed by one-way ANOVA. For multiple groups, # p < 0.05, ## p < 0.01, ### p < 0.001 - NT vs KD1; * p < 0.05, ** p < 0.01, *** p < 0.001 - NT vs KD2; & p < 0.05 - KD1 vs KD2 as assessed by one-way ANOVA.
JAM-A expression is informative for tumor grade and patient prognosis
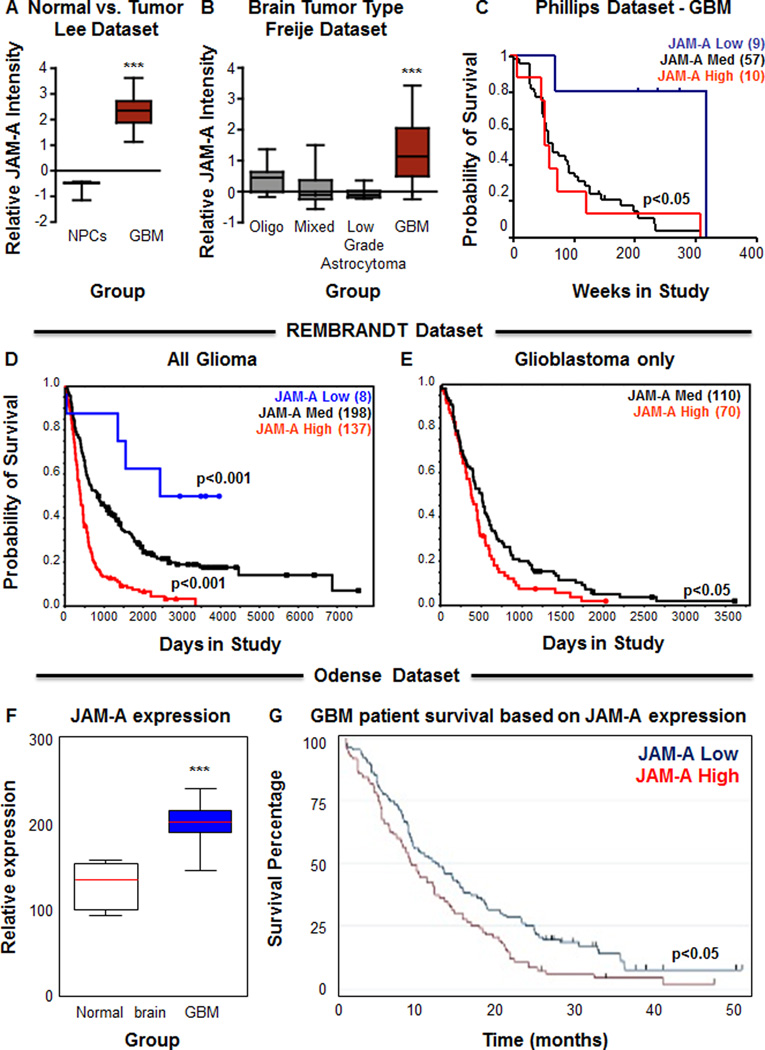
The molecular genetics of GBM have been extensively investigated permitting the association between gene profiles and patient outcome (Brennan et al., 2013; Cancer Genome Atlas Research, 2008; Frattini et al., 2013; Phillips et al., 2006; Verhaak et al., 2010). To evaluate the relationship between JAM-A and GBM patient survival and determine if JAM-A was informative for tumor grade and patient prognosis, we interrogated brain tumor bioinformatics databases. To determine if elevated JAM-A expression was restricted to GBM or also present in other neural cell types, such as NPCs, we evaluated the Lee dataset (Lee et al., 2006) from Oncomine and observed a significantly higher level of JAM-A in GBM as compared to NPCs (Fig. 6A). Evaluation of JAM-A expression in relation to tumor grade from the Freije dataset (Freije et al., 2004) in the Oncomine database indicated a significant elevation of JAM-A expression in GBM as compared to lower grade tumors (Oligodendroglioma, mixed glioma, Grade III Astrocytoma, Fig. 6B). Kaplan-Meier analysis also indicated that high JAM-A expression (at least one standard deviation above the mean) correlated with poor survival, while low JAM-A expression (at least one standard deviation below the mean) correlated with better survival in the Phillips dataset (Phillips et al., 2006) from Oncomine. (data not shown). Likewise JAM-A informed poor patient prognosis in GBM from the same dataset (Fig. 6C). We further confirmed the association between JAM-A expression and glioma patient survival using the NCI Rembrandt database and found that high JAM-A expression (>2-fold) correlated to poor glioma patient survival while low JAM-A expression (<2-fold) correlated to increased patient survival (Fig. 6D). The correlation between elevated JAM-A expression and poor patient prognosis was observed in GBM patients as well (Fig. 6E). To determine if these differences in survival were specific to JAM-A or shared by the other JAM family members, JAM-B and JAM-C, we evaluated the REMBRANDT database and Phillips dataset in Oncomine and found neither isoform was informative for glioma or GBM patient prognosis (Supplemental Fig. 5). Taken together, our bioinformatics analysis reveals that JAM-A is associated with increased malignancy and poor patient prognosis.
Figure 6. JAM-A correlates with glioma malignancy and is informative for patient survival.
Box and whisker plots of expression level for JAM-A using Oncomine (https://www.oncomine.org/) demonstrates significantly higher expression (p<0.001 as assessed by one-way ANOVA) of JAM-A in glioblastoma tissue than in neural progenitor cells (A) and significantly higher expression (p<0.001 as assessed by one-way ANOVA) of JAM-A in glioblastoma tissue as compared with oligodendroglia (Oligo) tumors, low grade astrocytoma, or mixed glioma (oligodendroglia (Oligo) and astrocytoma (Astro)) (B). JAM-A is also informative for GBM patient survival as demonstrated by Kaplan-Meier survival plots (C). The red line indicates the high expression group (10 patients) with greater than one standard deviation above the mean of all patients. The blue line indicates the low expression group (9 patients) with greater than one standard deviation below the mean of all patients. The black line indicates the group (57 patients) within one standard deviation of mean. Kaplan-Meier survival plots for glioma (D) and GBM (E) patients with differential tumor JAM-A expression using the NCI REMBRANDT database at a level of two-fold upregulation indicates that JAM-A levels inversely correlates with patient survival (red line indicates two-fold upregulation (137 glioma patients, 70 GBM patients), blue line indicates two-fold downregulation (8 glioma patients), and black line represents intermediate expression (198 glioma patients, 110 GBM patients). Analysis of JAM-A using histochemical staining in a patient dataset (Odense dataset, Odense University Hospital Denmark) indicates that JAM-A is elevated in GBM as compared to normal brain controls (F) and that high JAM-A expression correlates with poor patient prognosis (G; 102 patients in the JAM-A high group and 83 patients in the JAM-A low group). Data for all graphs displayed as mean values +/− standard deviation, ** p < 0.01 and *** p < 0.001 as assessed by one-way ANOVA. For survival analysis, the log-rank p-value for significance of difference between groups is indicated on each individual graph. See also Figure S5.
The ability for a biomarker to provide informative molecular diagnosis is useful and is further enhanced by its capacity to inform survival based on protein expression. Using immunohistochemical approaches, we observed that JAM-A expression increased with malignancy and was elevated in GBM tissue as compared to non-neoplastic cortical tissue (Fig. 1D, Supplemental Fig. 2E–J, Fig. 6F). We examined the capacity of JAM-A protein expression to predict GBM patient outcome in a clinically annotated dataset (Dahlrot et al., 2013). High JAM-A protein level also significantly correlated to poor GBM patient prognosis as compared with low JAM-A expression (Fig. 6G). Taken together, our findings demonstrate that JAM-A is increased with malignancy and is a negative indicator of patient prognosis.
Discussion
The ability for a stem cell to interact with its niche through adhesion mechanisms enables it to maintain a physical location as well as receive specialized signals from the niche (Spradling et al., 2001). Adhesion receptors are essential transducers of this process and facilitate niche adhesion as well as stem cell maintenance signaling. Adhesion receptor expression has been used to prospectively enrich them from normal and neoplastic tissues including the breast (Ali et al., 2011; Shackleton et al., 2006; Stingl et al., 2006; Vaillant et al., 2008) and brain (Hall et al., 2006; Lathia et al., 2010). Stem cell niches, themselves, also contain unique ECM expression, which for the brain includes elevated laminin expression (Lathia et al., 2012; Lathia et al., 2007). The close association of adhesion to stem cell maintenance is further supported by functional studies targeting niche adhesion that results in stem cell detachment and subsequent differentiation and /or death (Georges-Labouesse et al., 1998; Graus-Porta et al., 2001; Loulier et al., 2009; Niola et al., 2012). While the majority of niche adhesion studies involve adhesion receptors such as integrins or CD44, here we provide evidence that JAM-A promotes CSC maintenance in GBM and our results pose several outstanding questions. Why is JAM-A elevated in GBM? Why might JAM-A be useful to CSCs rather than other adhesion molecules? Possible explanations include the necessity to form cell-cell contacts within a GBM to promote tumor progression and the need for CSCs to directly interact with other cells within the niche.
JAM-A is considered to be an epithelial marker as it is expressed at tight junctions and regulates cell-cell adhesion (Severson and Parkos, 2009). The JAM family members play a variety of roles in normal and neoplastic processes including angiogenesis (Naik et al., 2008), leukocyte migration (Wojcikiewicz et al., 2009), platelet activation (Naik et al., 2012), and the regulation of cell morphology and polarity (Rehder et al., 2006). JAMs can execute these programs by several methods including adjacent cell JAM to JAM interaction, stabilization of integrins on the same cell, or the direct interaction of integrins on an adjacent cell (Severson and Parkos, 2009). There are conflicting reports as to the role of JAM-A in breast cancer progression, with evidence to suggest that loss of JAM-A increases migration and invasion (Naik and Naik, 2008), while others suggest that JAM-A is increased during tumor cell migration and adhesion (McSherry et al., 2011). Our work suggests that in CSCs, JAM-A is instrumental in regulating stabilization of integrins on the same cell, although interaction of JAM-A dimers between adjacent CSCs as well as JAM-A – integrin interactions with adjacent cells may also be possible and may contribute to the regulation of CSC maintenance by JAM-A. Future studies expanding the signaling network of JAM-A regulation in CSCs is likely to connect it to other key GBM pathways as well as other stem cell processes such as epithelial to mesenchymal transition. In addition, future studies focusing on the transcriptome, cell cycle, and cell division mode changes as a result of JAM-A targeting are likely to identify additional mechanisms governing JAM-A function in CSCs. Further interrogation of JAM-A function in CSCs from other advanced cancer may also demonstrate a conserved role for JAM-A in CSC maintenance.
Given the importance of adhesion in stem cell maintenance, the question arises whether this process can be targeted in CSCs. This therapeutic paradigm is not without complexity and challenges as many adhesion mechanisms, including integrins, are present in both normal and neoplastic stem cells (Hale et al., 2012). Alternative strategies to develop CSC anti-adhesion therapies include the identification of co-receptors and/or other signaling pathway members unique to the CSC population. With this goal in mind, our studies demonstrate that JAM-A is specifically used by CSCs to maintain integrin β1 activation and promote stem cell maintenance. Moreover, targeting of JAM-A compromises CSC growth but does not adversely impact non-CSCs or NPCs, suggesting that only tumor cells at the top of the cellular hierarchy are sensitive to JAM-A inhibition. These data provide a rationale for further evaluation of JAM-A in a clinical setting. Taken together, our data demonstrate that CSCs possess specific cell adhesion mechanisms to regulate their self-renewal and can be targeted with limited disruption to the normal tissue, representing a promising anti-GBM therapeutic paradigm.
Experimental Procedures
CSC derivation, culture, and analysis
Human GBM cells were derived under written informed consent and approved IRB protocols from Cleveland Clinic (CCF2352, CCF2418), University Hospitals-Seidman Cancer Center at Case Western Reserve University (1217, 1221) for experimental analysis and from Duke University to establish xenografts in BalbC nu/nu (athymic nude) mice for maintenance of the tumor hierarchy as previously published (Bao et al., 2006; Eyler et al., 2011; Guryanova et al., 2011; Lathia et al., 2010; Li et al., 2009). GBM xenografts for 4 tumors (T3691, T387, T4121, T4302) were used for experimental studies. Only low (<5) passage cells were used for analysis to minimize cellular drift and the majority of cells were used immediately after dissociation. Dissociation and culture of cells was done as previously described (Bao et al., 2006; Eyler et al., 2011; Guryanova et al., 2011; Lathia et al., 2010; Li et al., 2009) and detailed in the Supplemental Information.
Flow cytometry screening
Flow cytometry screening was done as previously described using the BD lyoplate system (Yuan et al., 2011) and additional parameters are provided in the Supplemental Information.
Database mining and processing
To further refine the flow cytometry screen and interrogate the association of JAM-A to GBM patient survival, the following databases were used: NCI REpository for Molecular BRAin Neoplasia DaTa bioinformatics database (https://caintegrator.nci.nih.gov/rembrandt/, REMBRANDT), human protein atlas (http://proteinatlas.org), TCGA data (accessed at https://tcga-data.nci.nih.gov/tcga/tcgaHome2.jsp), Oncomine (Compendia Bioscience, Ann Arbor, MI http://www.oncomine.org). Relevant analytical parameters are provided in the Supplemental Information.
Flow cytometry analysis and fluorescence in situ hybridization (FISH)
For flow cytometry analysis, GBM cells were dissociated from a patient specimen or xenograft, recovered overnight in supplemented neurobasal media, and sorted on a BD FACS Aria II as previously described (Lathia et al., 2010) using the following antibodies: JAM-A-PE (BD Biosciences), CD31-FITC (BD Biosciences), CD105-FITC (BD Biosciences). Appropriate isotype control antibodies were used to set gates and live/dead determination was based on 7AAD exclusion (BD Biosciences). Additional flow cytometry analysis was done with JAM-A and an integrin α6-FITC antibody (BD Biosciences). JAM-A positive/CD105 negative cells were collected for fluorescence in situ hybridization of Chromosome 7 and epidermal growth factor (EGFR) using a Vysis EGFR/CEP 7 FISH Probe Kit (Abbott Molecular) as per the manufacturers’ protocol. Images were taken with a 63× oil immersion objective using a Leica widefield microscope and images were processed and assembled in Photoshop (Adobe).
Immunoblotting analysis
Protein analysis was done by immunoblotting as previously described (Eyler et al., 2011; Guryanova et al., 2011; Li et al., 2009). Briefly, 10 µg of total protein was loaded per condition and probed using the following antibodies: JAM-A (Santa Cruz, 1:1000), Sox2 (Sigma, 1:1000), integrin α6 (Cell signaling, 1:1000), integrin β1 (Millipore, 1:1000), Olig2 (Millipore, 1:1000), GFAP (Sigma, 1:1000), Map2 (Covance, 1:1000), Actin (Santa Cruz, 1:2500), and α-Tubulin antibody (Sigma, 1:500) was used as a loading control. Species specific horseradish peroxidase (HRP) conjugated secondary antibodies were used for detection (Invitrogen, 1:2000).
Immunostaining and immunohistochemical analysis
Immunostaining analysis on xenografted GBM specimens (generated by intracranial injection of GBM specimen T4121) or tumorspheres was done as previously described (Lathia et al., 2010) using 10 µm frozen sections obtained from the Duke University Brain Tumor Center Tissue Bank and histologically confirmed by a neuropathologist (R.E.M.) using antibodies against integrin α6 (Millipore, 1:100), integrin β1 (Millipore, 1:100), Sox2 (R&D Systems, 1:200), and JAM-A (Santa Cruz, 1:50) and species appropriate secondary antibodies (Alexa 488, 568, or 633, Invitrogen, 1:200). Nuclei were counterstained with Hoechst 33342 (1:1000 dilution from a 5 mg/ml stock solution, Invitrogen). All confocal imaging was done using a Leica SP-5 confocal microscope as described previously (Lathia et al., 2010) and images were processed and assembled in Photoshop (Adobe). Immunohistochemical analysis was done using standard procedures (Li et al., 2009) and details are provided in the Supplemental Information
JAM-A mutant mice and mouse histological analysis
JAM-A mutant (−/−) and wild-type (WT) control mice obtained from the University of Delaware and generated as previously described (Cooke et al., 2006). For histological analysis of PCNA and PH3, 6 age-matched JAM-A −/− and WT were evaluated at 3 anatomical depths per brain for the SVZ and DG. For expression analysis, the following antibodies were used: JAM-A (Santa Cruz, 1:100), PCNA (Abcam, 1:200), PH3 (Millipore 1:500), Doublecortin (Dcx, Cell Signaling, 1:100), Sox2 (R&D Systems, 1:200), GFAP (Dako, 1:200), Iba1 (Millipore, 1:200), S100β (Dako, 1:200), Collagen IV (Millipore, 1:500), NG2 (Millipore, 1:100), NeuN (Millipore, 1:200). Nuclei were counterstained with Hoechst 33342 (1:1000 dilution from a 5 mg/ml stock solution, Invitrogen). All imaging was done using a Leica SP-5 confocal microscope as described previously (Lathia et al., 2010) and images were processed and assembled in Photoshop (Adobe).
ECM adhesion assays
To evaluate adhesion, CSCs were trypsinized, and allowed to recover for a minimum of 5 hours. Subsequently, CSCs were plated at 100,000 cells per well in a 24-well plate and treated overnight with either 10 µg/ml IgG1 control antibody (Millipore), 10 µg/ml JAM-A blocking antibody (Santa Cruz), or 10 µg/ml GoH3 integrin α6 blocking antibody (Millipore). Cells were transferred to a 6-well plate coated with Geltrex (24 µg/ml) or fibronectin (10 µg/ml), as per manufacturer’s instructions, and allowed to adhere for 30 minutes at 37°C. Following adhesion, wells were washed with neurobasal medium, and cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature. After two washes with 1x PBS, nuclei were counterstained with DAPI or propidium iodide (1:1000). Cells were imaged using the well plate acquisition tool on a Hamamtsu C9100 imaging system. The number of cells adhered to each well were quantified using ImageJ software. Values are expressed in comparison to the non-immune IgG1-treated samples, which were set to a relative value of 1.
JAM-A lentiviral short hairpin RNA (shRNA) and overexpression construct production
Lentiviral shRNA constructs were prepared as we previously reported (Eyler et al., 2011; Guryanova et al., 2011; Lathia et al., 2010; Li et al., 2009). In short, using lipofectamine 2000 (Invitrogen), 293FT cells were co-transfected with packaging vectors psPAX2 and pCI-VSVG (Addgene) and lentiviral vectors directing expression of shRNA (Sigma) specific to JAM-A (TRCN0000061649 (KD1) and TRCN0000061650 (KD2)) or a non-targeting control (NT) shRNA (SHC002) to produce virus. Media of the 293FT cell cultures were changed 18 hours after transfection and viral supernatants were collected 24 and 48 hours later and filtered for immediate use or concentrated with polyethylene glycol precipitation and stored at −80°C for future use.
Cell proliferation, caspase activity, and tumorsphere formation assays
Assessments were made as previous described (Bao et al., 2006; Eyler et al., 2011; Guryanova et al., 2011; Lathia et al., 2010; Li et al., 2009) and outlined in the Supplemental Information.
Quantitative real time PCR (qPCR)
qPCR analysis was done as previously described (Wang et al., 2008) using outlined in the Supplemental Information.
In vivo tumor formation assays
For in vivo tumor formation evaluation, CSCs were transplanted into the frontal lobe of BalbC Nu/Nu mice at 100, 1000, or 5000 cells per mouse and evaluated as previous described (Bao et al., 2006; Eyler et al., 2011; Guryanova et al., 2011; Lathia et al., 2010; Li et al., 2009). Additional information is provided in the Supplemental Information.
Statistical analysis
Reported values are mean values +/− standard deviation from studies done in at least triplicate. Unless otherwise stated, one-way ANOVA was used to calculate statistical significance with p-values are detailed in the text and figure legends.
Supplementary Material
Highlights.
High throughput flow cytometry can identify novel CSC targets
CSCs selectively express JAM-A which regulates self-renewal
Targeting JAM-A inhibits CSC maintenance but not NPC function
JAM-A negatively correlates to GBM patient prognosis
Acknowledgements
We thank the members of the Lathia and Rich labs for critical comments on the manuscript. We thank C. Shemo, M. Morgan, P. Barrett, and S. O’Bryant for flow cytometry assistance. We thank the Cleveland Clinic Foundation Tissue Procurement Service and S. Staugatis, R. Weil, and M. McGraw. We also thank Diane Satterfield for technical assistance. This work was also supported by National Institutes of Health grants NS083629 (JDL), CA112958 (JNR), CA154130 (JNR), CA169117 (JNR), CA151522 (ABH), CA137443 (AES), NS063971 (AES), CA128269 (AES), CA101954 (AES), CA116257 (AES), CA108786 (REM), NS20023 (REM), and a K99/R00 Pathway to Independence Award CA157948 (JDL). Work in the Rich lab is also supported by funding from the James D. McDonnell Foundation. Work in the Lathia lab is also supported by funding from a V Scholar Grant from the V Foundation for Cancer Research, Voices Against Brain Cancer, Ohio Cancer Research Associates, Grant #IRG-91-022-18 to the Case Comprehensive Cancer Center from the American Cancer Society, and the Lerner Research Institute. ML is supported by funding from Yunnan Province High-tech Talent Introduction Project and the National Natural Science Foundation of China No.30960091 and No.81271330 AES is supported by funding from the Cancer Genome Atlas (TCGA) Project, the Ben & Catherine Ivy Foundation, the Kimble Foundation, the Peter B. Cristal Endowment, and the Ohio Department of Development Technology (09-071).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali HR, Dawson SJ, Blows FM, Provenzano E, Pharoah PD, Caldas C. Cancer stem cell markers in breast cancer: pathological, clinical and prognostic significance. Breast Cancer Res. 2011;13:R118. doi: 10.1186/bcr3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sanchez RM, Barba I, Martinez-Saez E, Prudkin L, et al. TGF-beta Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Inda MM, Cavenee WK, Furnari FB. Heterogeneity maintenance in glioblastoma: a social network. Cancer research. 2011;71:4055–4060. doi: 10.1158/0008-5472.CAN-11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke VG, Naik MU, Naik UP. Fibroblast growth factor-2 failed to induce angiogenesis in junctional adhesion molecule-A-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2005–2011. doi: 10.1161/01.ATV.0000234923.79173.99. [DOI] [PubMed] [Google Scholar]
- Dahlrot RH, Kristensen BW, Hjelmborg J, Herrstedt J, Hansen S. A population-based study of high-grade gliomas and mutated isocitrate dehydrogenase 1. Int J Clin Exp Pathol. 2013;6:31–40. [PMC free article] [PubMed] [Google Scholar]
- Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, Lathia JD, Forrester MT, Lee J, Stamler JS, et al. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146:53–66. doi: 10.1016/j.cell.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattini V, Trifonov V, Chan JM, Castano A, Lia M, Abate F, Keir ST, Ji AX, Zoppoli P, Niola F, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013 doi: 10.1038/ng.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freije WA, Castro-Vargas FE, Fang Z, Horvath S, Cloughesy T, Liau LM, Mischel PS, Nelson SF. Gene expression profiling of gliomas strongly predicts survival. Cancer research. 2004;64:6503–6510. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer research. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, MacSwords J, Eyler CE, McLendon RE, Heddleston JM, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Li M, Lathia JD. The malignant social network: Cell-cell adhesion and communication in cancer stem cells. Cell Adh Migr. 2012;6 doi: 10.4161/cam.21294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PE, Lathia JD, Miller NG, Caldwell MA, ffrench-Constant C. Integrins are markers of human neural stem cells. Stem Cells. 2006;24:2078–2084. doi: 10.1634/stemcells.2005-0595. [DOI] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmeland AB, Lathia JD, Sathornsumetee S, Rich JN. Twisted tango: brain tumor neurovascular interactions. Nat Neurosci. 2011;14:1375–1381. doi: 10.1038/nn.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Jo M, Cavenee WK, Furnari F, VandenBerg SR, Gonias SL. Crosstalk between the urokinase-type plasminogen activator receptor and EGF receptor variant III supports survival and growth of glioblastoma cells. Proc Natl Acad Sci U S A. 2011;108:15984–15989. doi: 10.1073/pnas.1113416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert CG, Bradley RK, Ding Y, Toledo CM, Herman J, Skutt-Kakaria K, Girard EJ, Davison J, Berndt J, Corrin P, et al. Genome-wide RNAi screens in human brain tumor isolates reveal a novel viability requirement for PHF5A. Genes Dev. 2013;27:1032–1045. doi: 10.1101/gad.212548.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Jijiwa M, Demir H, Gupta S, Leung C, Joshi K, Orozco N, Huang T, Yildiz VO, Shibahara I, de Jesus JA, et al. CD44v6 regulates growth of brain tumor stem cells partially through the AKT-mediated pathway. PLoS One. 2011;6:e24217. doi: 10.1371/journal.pone.0024217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanis I, Lathia JD, Vadakkan TJ, Raborn E, Wan R, Mughal MR, Eckley DM, Sasaki T, Patton B, Mattson MP, et al. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci. 2010;30:9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Li M, Hall PE, Gallagher J, Hale JS, Wu Q, Venere M, Levy E, Rani MR, Huang P, et al. Laminin alpha 2 enables glioblastoma stem cell growth. Annals of neurology. 2012;72:766–778. doi: 10.1002/ana.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Patton B, Eckley DM, Magnus T, Mughal MR, Sasaki T, Caldwell MA, Rao MS, Mattson MP, ffrench-Constant C. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol. 2007;505:630–643. doi: 10.1002/cne.21520. [DOI] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loulier K, Lathia JD, Marthiens V, Relucio J, Mughal MR, Tang SC, Coksaygan T, Hall PE, Chigurupati S, Patton B, et al. beta1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS Biol. 2009;7:e1000176. doi: 10.1371/journal.pbio.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSherry EA, Brennan K, Hudson L, Hill AD, Hopkins AM. Breast cancer cell migration is regulated through junctional adhesion molecule-A-mediated activation of Rap1 GTPase. Breast Cancer Res. 2011;13:R31. doi: 10.1186/bcr2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Giampietro C, Giannotta M, Corada M, Torselli I, Orsenigo F, Cocito A, d'Ario G, Mazzarol G, Confalonieri S, et al. Abrogation of junctional adhesion molecule-A expression induces cell apoptosis and reduces breast cancer progression. PLoS One. 2011;6:e21242. doi: 10.1371/journal.pone.0021242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik MU, Stalker TJ, Brass LF, Naik UP. JAM-A protects from thrombosis by suppressing integrin alphaIIbbeta3-dependent outside-in signaling in platelets. Blood. 2012;119:3352–3360. doi: 10.1182/blood-2011-12-397398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik TU, Naik MU, Naik UP. Junctional adhesion molecules in angiogenesis. Front Biosci. 2008;13:258–262. doi: 10.2741/2676. [DOI] [PubMed] [Google Scholar]
- Naik UP, Naik MU. Putting the brakes on cancer cell migration: JAM-A restrains integrin activation. Cell Adh Migr. 2008;2:249–251. doi: 10.4161/cam.2.4.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niola F, Zhao X, Singh D, Castano A, Sullivan R, Lauria M, Nam HS, Zhuang Y, Benezra R, Di Bernardo D, et al. Id proteins synchronize stemness and anchorage to the niche of neural stem cells. Nat Cell Biol. 2012;14:477–487. doi: 10.1038/ncb2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niola F, Zhao X, Singh D, Sullivan R, Castano A, Verrico A, Zoppoli P, Friedmann-Morvinski D, Sulman E, Barrett L, et al. Mesenchymal high-grade glioma is maintained by the ID-RAP1 axis. The Journal of clinical investigation. 2013;123:405–417. doi: 10.1172/JCI63811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Raghu H, Gondi CS, Dinh DH, Gujrati M, Rao JS. Specific knockdown of uPA/uPAR attenuates invasion in glioblastoma cells and xenografts by inhibition of cleavage and trafficking of Notch-1 receptor. Mol Cancer. 2011;10:130. doi: 10.1186/1476-4598-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehder D, Iden S, Nasdala I, Wegener J, Brickwedde MK, Vestweber D, Ebnet K. Junctional adhesion molecule-a participates in the formation of apico-basal polarity through different domains. Exp Cell Res. 2006;312:3389–3403. doi: 10.1016/j.yexcr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20:1916–1925. doi: 10.1091/mbc.E08-10-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson EA, Parkos CA. Structural determinants of Junctional Adhesion Molecule A (JAM-A) function and mechanisms of intracellular signaling. Curr Opin Cell Biol. 2009;21:701–707. doi: 10.1016/j.ceb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer research. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Stelzer S, Ebnet K, Schwamborn JC. JAM-A is a novel surface marker for NG2-Glia in the adult mouse brain. BMC Neurosci. 2010;11:27. doi: 10.1186/1471-2202-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer research. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnyei K, Onodera H, Damoiseaux R, Saigusa K, Petrosyan S, De Vries D, Ferrari D, Saxe J, Panosyan EH, Masterman-Smith M, et al. A molecular screening approach to identify and characterize inhibitors of glioblastoma stem cells. Mol Cancer Ther. 2011;10:1818–1828. doi: 10.1158/1535-7163.MCT-11-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, Hjelmeland AB, Rich JN. c-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 2008;3:e3769. doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz EP, Koenen RR, Fraemohs L, Minkiewicz J, Azad H, Weber C, Moy VT. LFA-1 binding destabilizes the JAM-A homophilic interaction during leukocyte transmigration. Biophys J. 2009;96:285–293. doi: 10.1529/biophysj.108.135491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdak H, Zhu S, Romero A, Lorger M, Watson J, Chiang CY, Zhang J, Natu VS, Lairson LL, Walker JR, et al. An RNAi screen identifies TRRAP as a regulator of brain tumor-initiating cell differentiation. Cell Stem Cell. 2010;6:37–47. doi: 10.1016/j.stem.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6:e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.