Abstract
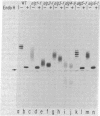
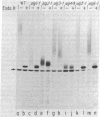
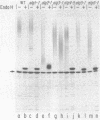
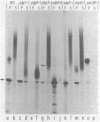
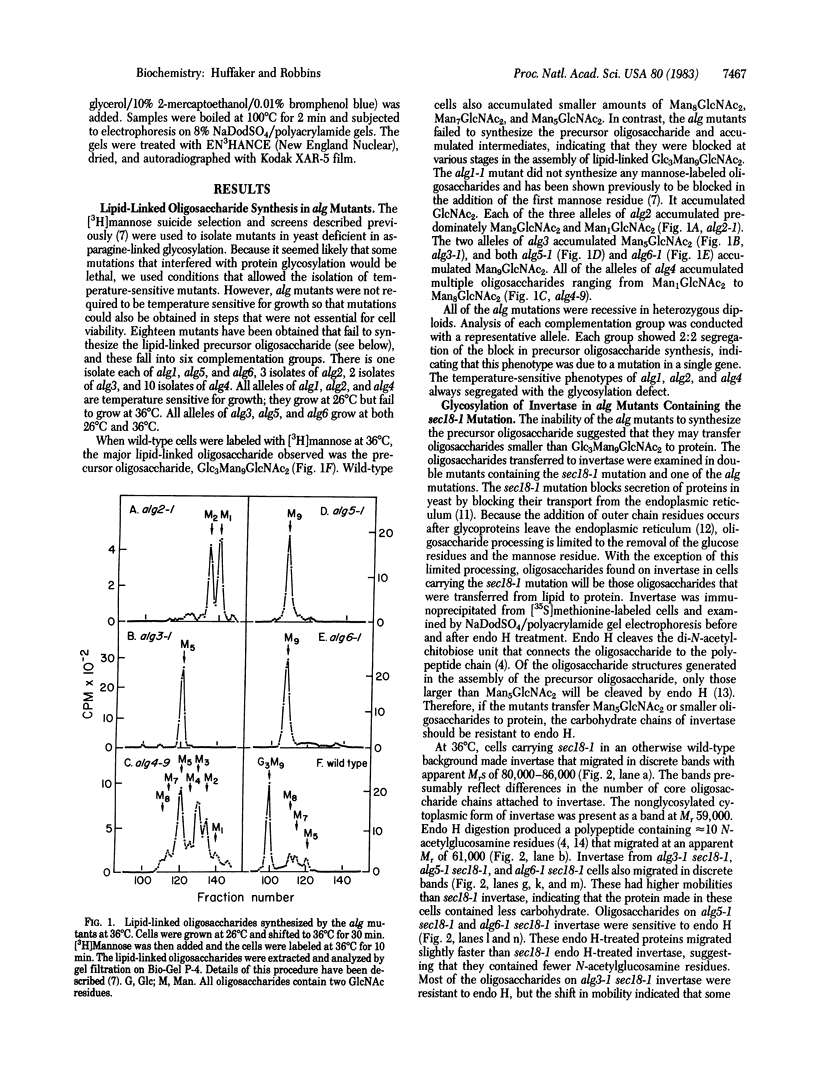
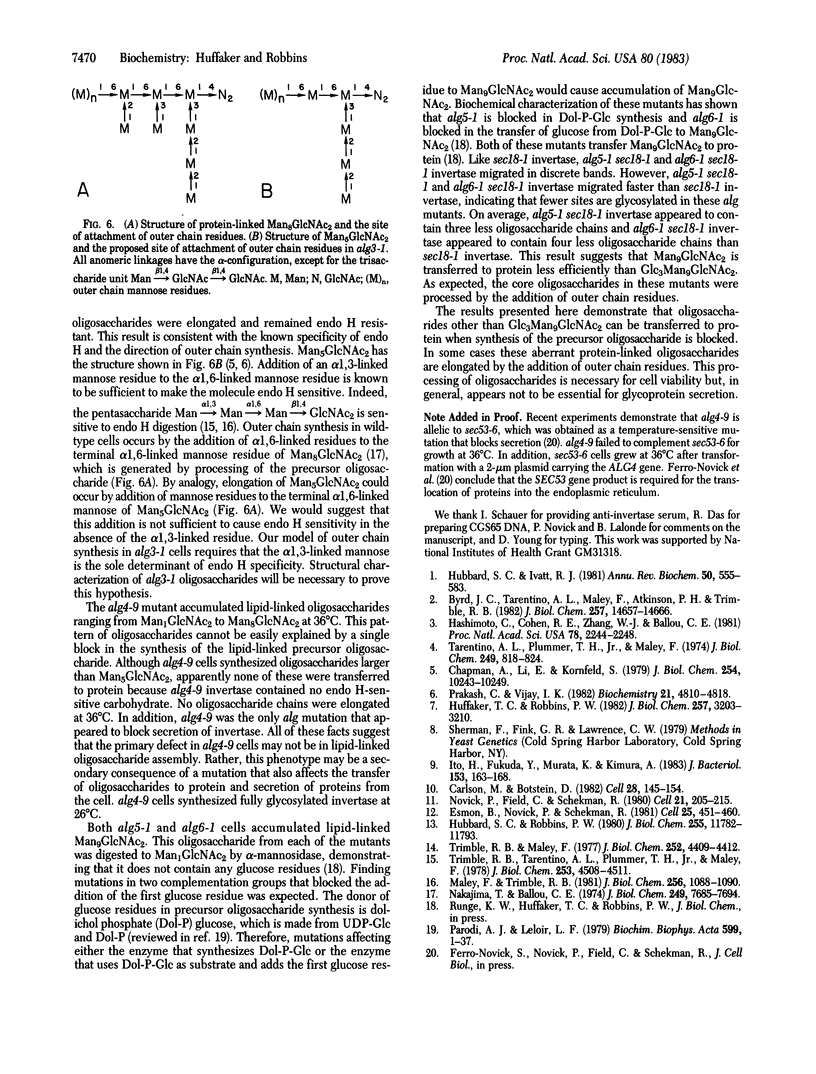
The synthesis of asparagine-linked oligosaccharides involves the formation of a lipid-linked precursor oligosaccharide that has the composition Glc3Man9GlcNAc2. We have used a [3H]mannose suicide selection to obtain mutants in yeast that are blocked in the synthesis of this precursor oligosaccharide. The alg1 mutant accumulated lipid-linked GlcNAc2, alg2 mutants accumulated Man1-2GlcNAc2, alg3 mutants accumulated Man5GlcNAc2, alg4 mutants accumulated Man1-8GlcNAc2, and alg5 and alg6 mutants accumulated Man9GlcNAc2. Some of these mutants appeared to transfer oligosaccharides other than Glc3Man9GlcNAc2 from the lipid carrier to invertase. These aberrant protein-linked oligosaccharides were processed by the addition of outer chain residues in the alg3, alg5, and alg6 mutants. There was virtually no outer chain addition in the alg2 and alg4 mutants. alg4 was the only mutant that failed to secrete invertase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrd J. C., Tarentino A. L., Maley F., Atkinson P. H., Trimble R. B. Glycoprotein synthesis in yeast. Identification of Man8GlcNAc2 as an essential intermediate in oligosaccharide processing. J Biol Chem. 1982 Dec 25;257(24):14657–14666. [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Chapman A., Li E., Kornfeld S. The biosynthesis of the major lipid-linked oligosaccharide of Chinese hamster ovary cells occurs by the ordered addition of mannose residues. J Biol Chem. 1979 Oct 25;254(20):10243–10249. [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Cohen R. E., Zhang W. J., Ballou C. E. Carbohydrate chains on yeast carboxypeptidase Y are phosphorylated. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2244–2248. doi: 10.1073/pnas.78.4.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis of the N-linked oligosaccharides of glycoproteins. Assembly of the lipid-linked precursor oligosaccharide and its relation to protein synthesis in vivo. J Biol Chem. 1980 Dec 25;255(24):11782–11793. [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Temperature-sensitive yeast mutants deficient in asparagine-linked glycosylation. J Biol Chem. 1982 Mar 25;257(6):3203–3210. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley F., Trimble R. B. Revision of the structure for an endo-beta-N-acetylglucosaminidase H substrate using a novel modification of the Smith degradation. J Biol Chem. 1981 Feb 10;256(3):1088–1090. [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Structure of the linkage region between the polysaccharide and protein parts of Saccharomyces cerevisiae mannan. J Biol Chem. 1974 Dec 10;249(23):7685–7694. [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Prakash C., Vijay I. K. Characterization of intermediates up to lipid-linked heptasaccharide implicated in the biosynthesis of Saccharomyces cerevisiae mannoproteins. Biochemistry. 1982 Sep 14;21(19):4810–4818. doi: 10.1021/bi00262a045. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Subunit structure of external invertase from Saccharomyces cerevisiae. J Biol Chem. 1977 Jun 25;252(12):4409–4412. [PubMed] [Google Scholar]
- Trimble R. B., Tarentino A. L., Plummer T. H., Jr, Maley F. Asparaginyl glycopeptides with a low mannose content are hydrolyzed by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1978 Jul 10;253(13):4508–4511. [PubMed] [Google Scholar]