Abstract
Objectives
To estimate the frequency of symptoms of obesity hypoventilation syndrome (OHS) in patients with obstructive sleep apnoea (OSA) and to evaluate comorbidities associated with OHS.
Design
Retrospective study based on patients' medical records and on further sleep tests performed in the study centre during the inclusion visit.
Setting
Respiratory Care Unit and Sleep Disorder Centre of the Zayed Military Hospital United Arab Emirates.
Participants
All patients referred to the study centre for a suspicion of sleep-disordered breathing.
Main outcome measures
Prevalence of OSA and OSA + OHS and comorbidities in patients with OSA and OHS.
Results
A total of 212 adult patients participated in the study. Of these, 107 patients (50.5% [43.8–57.1% CI 95%]) fulfilled diagnostic criteria for OSA, and the majority were men (79.4%). Among patients with OSA, 18 patients (16.8% [10.8–25.1% CI 95%]) fulfilled diagnostic criteria for OHS. In this group, women were more frequently affected than men (31.8% [7/22] vs. 12.9% [11/85], respectively; p = 0.03) and tended to be older than affected men, with a mean age of 55 ± 10.6 years versus 46 ± 13 for men. After adjustment for gender, OHS was significantly associated with hypertension (OR = 3.5; p = 0.03), diabetes mellitus (OR = 4.6; p = 0.02), ischaemic heart disease (OR = 5.1; p = 0.04) and pulmonary hypertension (OR = 16.1; p = 0.001).
Conclusion
OHS is a common condition in obese patients in the UAE and is associated with an increased risk of cardiovascular comorbidities and diabetes.
Keywords: obesity, Pickwickian syndrome, obstructive sleep apnoea, hyperpnoea syndrome, pulmonary hypertension, obstructive lung disease, restrictive lung disease
Introduction
Over the last decades, the prevalence of obesity, diabetes and related diseases has increased in the Gulf region as the consequence of the substantial economic growth of this region which has led to sedentarization and major changes in diet and lifestyle. Obesity (body mass index [BMI] ≥ 30 kg/m2) now affects around one-third of the adult population of this region.1 For example, in the UAE, it has been reported that 32.7% of adults and 12.1% of adolescents (10–19 years) were obese. An even higher prevalence rate was reported in Kuwait (42.1% of adults).1 With respect to gender, obesity is more frequent in women than in men (39.6% vs. 30.0%).1
Obesity is associated with many other medical conditions including sleep-disordered breathing. The role of obesity as a risk factor for the development of obstructive sleep apnoea (OSA) is well established.2 For example, two studies performed in South America reported that over 80% of morbidly obese patients had OSA.2,3 In addition, a recent study performed in primary care in the UK in adults older than 50 years reported that subjects with a BMI of 40 kg/m2or over were 27.39 times (95% CI 24.64–30.46) more likely to have OSA (p < 0.0001).4 Male gender and increasing age are also considered as risk factors for OSA.5 OSA is one of the most common sleep disorders which can occur on a similar incidence to that of type II diabetes and twice than that of asthma.6 In the UAE, the prevalence of OSA in the adult population has been estimated to be around 7%.6
Another sleep-disordered breathing condition related to obesity is obesity hypoventilation syndrome (OHS) or Pickwickian syndrome. This condition is characterized by the presence of hypoventilation while awake and is defined as the presence of obesity (BMI ≥ 30 kg/m2) associated with daytime hypercapnia (pCO2 > 45 mmHg) in the absence of other causes of hypoventilation.7 This syndrome is distinguished from classical OSA, in which patients have normal alveolar ventilation when awake, although the two conditions may frequently overlap. It has been reported that patients with OSA have a higher mortality rate than those similarly obese patients without OHS (23% vs. 9%; hazard ratio: 4.0).8,9
The prevalence of OHS in the general population is very poorly documented. A study performed in the USA estimated the prevalence of OHS in the general population to be between 0.15 and 0.30%.10 Another study in the USA performed in patients with OSA estimated the prevalence of OHS in this population to be between 10 and 20%.8 To our knowledge, there are no data on the prevalence of OHS in the Middle East either in the general population or in patients with OSA. As obesity is of growing concern in this region,11–13 more information on OHS in the Middle East would clearly be useful. For this reason, we performed a retrospective survey of patients with suspected sleep-related breathing disorders who attended our sleep disorder clinic in the Zayed Military Hospital, an urban tertiary level teaching hospital providing healthcare to the population of UAE. The objective of the study was to estimate the frequency of symptoms of OHS in patients with OSA and to evaluate comorbidities associated with OHS.
Methods
This was a retrospective cross-sectional study performed from March 2008 to June 2009 in the Respiratory Care Unit and Sleep Disorder Centre of the Zayed Military Hospital in UAE. This hospital is one of the largest public hospitals providing tertiary care in the UAE. Its catchment area is the Abu Dhabi region of the UAE and it attracts patients from both urban and rural areas and provides care to both military and civilian patients and to national and foreign residents. The hospital was visited by 651,981 outpatients in 2012.
Study sample
The study population consisted of all patients referred to the centre for the investigation of possible sleep-disordered breathing. These patients were referred from the respiratory clinics, or other specialties such as cardiology, ENT, endocrinology and family medicine or from outside private clinics. Patients aged younger than 18 years were excluded.
Data collection
Data on demographics, clinical symptoms and comorbidities (diabetes mellitus, arterial hypertension, ischaemic heart disease or pulmonary hypertension) were documented from the medical records. Results of spirometry, echocardiography and measurement of arterial blood gases (ABG)14 were documented from the patient's medical records, as well as the results of evaluations performed during dedicated visits to the internal medicine, cardiology and endocrinology departments.
All patients underwent overnight polysomnography (PSG) using Alice5 (Philips –respironics) with EEG (C3–A2, C4–A1, O1–A2, O2–A1) ROC, LOC, 2 Chin EMG, both legs tibialis anterior EMG for leg movements, continuous ECG and oxygen saturation monitoring. Respiration was monitored by oro-nasal thermister and piezoelectric belts for thoracic and abdominal movements. Transcutaneous CO2 monitoring was performed if the ABG showed hypercapnea.
Polysomnographic data were scored on the basis of standard criteria15,16 by an experienced registered PSG technologist. Sleep-disordered breathing parameters were obtained from the polysomnogram. For each patient, the apnoea-hypopnea index (AHI) was determined as the total number of apnoeas + hypopneas × 60/total sleep time (min). Apnoea was defined as complete cessation of breathing for >10 s. Hypopnoea was defined as a reduction in amplitude of airflow by at least 30% of baseline for duration of at least 10 s accompanied by an oxygen desaturation ≥ 4%. Patients with OSA were defined as those with an AHI > 5/h and those with OHS as having a BMI > 30 kg/m2 and day time hypoventilation with pCO2 > 45 mmHg.
Statistical analysis
For descriptive statistics, data were expressed as mean ± SD, or as percentages for categorical variables. Potential associations between incidence rates for OHS and other categorical variables were evaluated with Fisher's exact test or the Mantel–Haenszel test, as appropriate. The strength of association was expressed in terms of odds ratios (OR).
Results
Study sample
At baseline, a total of 212 patients participated in the study. The mean age was 45.6 ± 13.2 and the majority were men 164 (77.4%). The mean BMI was 33.8 ± 8.9 kg/m2 and over half of the study population (57.1%) was obese (BMI > 30 kg/m2).
Frequency of OSA and OHS
Of the 212 patients enrolled in the study and evaluated for potential sleep-disordered breathing, 107 (50.5% [43.8–57.1% CI 95%]) fulfilled the criteria for OSA according to the definition used in the study. The mean age of these patients was 48.3 ± 12.5 and the majority (n = 85; 79.4%) were men. The mean BMI was 35.0 ± 5.7 kg/m2 (Table 1).
Table 1.
Demographics and patients' characteristics during the admission phase.
| Number of patients (Total N = 212) | |
|---|---|
| Gender (men, n%) | 164 (77.4%) |
| Age (year) | 45.6 ± 13.2 |
| BMI (kg/m2) | N = 212 |
| Mean ± SD | 33.8 ± 8.9 |
| Median | 32 |
| <20 kg/m2 | 0 (0%) |
| 20–25 kg/m2 | 33 (15.6%) |
| 5–30 kg/m2 | 58 (27.3%) |
| 30–35 kg/m2 | 70 (33%) |
| >35 kg/m2 | 51 (24.1%) |
| Pulmonary function test | n = 60 |
| FEV1 (L) | 2.4 ± 1.0 |
| FEV1, % predicted | 79.5 ± 20.9 |
| FVC, L | 3.0 ± 1.2 |
| FVC, % predicted | 80.7 ± 18.5 |
| FEV1/FVC ratio | 78.3 ± 10.0 |
| Comorbidities | N = 212 |
| Diabetes mellitus | 18 (8.5%) |
| Arterial hypertension | 29 (13.7%) |
| Ischaemic heart disease | 12 (5.7%) |
| Pulmonary hypertension | 9 (4.2%) |
| Dyslipidemia | 6 (2.8%) |
| Any identified comorbidity | 107 (50.5%) |
Data were collected from the patient's medical records. All data are represented as mean ± SD or number (%). Data on pulmonary function tests (FEV1 and FVC) concerned 60 patients.
BMI: body mass index.
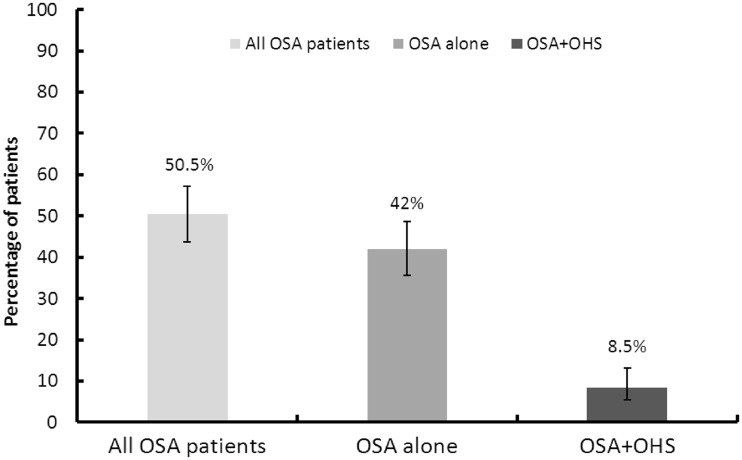
Of the 107 patients with OSA, 18 patients (16.8% [10.8–25.1%; CI 95%]) fulfilled criteria for OHS according to the definition used. This corresponds to 8.5% [5.4–13.1%; CI 95%] of the total population involved in the study (Figure 1). The mean BMI in this population was 45.4 ± 13.1 kg/m2 and the mean daytime pCO2 was 57.4 ± 11.69 mmHg.
Figure 1.
Prevalence of OSA and OSA + OHS in the population referred to the sleep centre (n = 212).
Although the absolute number of subjects with OSA and OHS was higher in men than in women, the proportion of patients with OSA who also presented OHS was higher in women (31.8% [7/22] vs. 12.9% [11/85], respectively; p = 0.03) (Table 2). Patients with OHS were significantly older than those with OSA but without OHS (54.7 ± 15.1 years vs. 47.0 ± 12.0 years) (Table 2).
Table 2.
Retrospective data of patient with OSA alone and OSA + OHS.
| OSA alone (n = 89) | OSA + OHS (n = 18) | p | |
|---|---|---|---|
| Gender | |||
| Men (n (%)) | 74 (83.1%) | 11 (61.1%) | 0.03 |
| Age (year) | |||
| Mean ± SD | 47.0 ± 12.0 | 54.7 ± 15.1 | 0.02 |
| Median | 47 | 55.5 | – |
| BMI (kg/m2) | |||
| Mean ± SD | 35.0 ± 5.7 | 45.4 ± 13.1 | 0.0001 |
| Median | 36.5 | 45.55 | – |
| AHI (events/h) | |||
| Mean ± SD | 35.5 ± 27.5 | 48.4 ± 27.9 | 0.07 |
| Median | 25.9 | 48.35 | – |
| pCO2 (mmHg) | |||
| Mean ± SD | 40.7 ± 3.2 | 57.4 ± 11.7 | 0.0001 |
| Median | 41.6 | 53.05 | – |
Data on pCO2 were missing for 82 patients.
OHS: obesity hypoventilation syndrome; OSA: obstructive sleep apnoea; BMI: body mass index; AHI: apnoea-hypopnea index.
The occurrence of apnoea or hypopnoea episodes was quite higher in the OSA + OHS group and the median AHI score was >30 events/h reflecting a severe OSA.
Comorbidities
Comorbidities were frequent in patients with OHS, with 55.6% having arterial hypertension and 44.4% having type II diabetes. These comorbidities were somewhat more frequent in women than in men, but between-gender differences were not significant (Table 3).
Table 3.
Comorbidities in patients with OSA and OHS.
| Patients with OHS (n = 18) | |
|---|---|
| Diabetes mellitus | 8 (44.4% [95% CI: 24.6 − 66.3]) |
| Men, n (%)* | 4 (36.4%) |
| Women, n (%)* | 4 (57.1%) |
| Arterial hypertension | 10 (55.6% [95% CI: 33.7–75.4]) |
| Men, n (%)* | 6 (54.5%) |
| Women, n (%)* | 4 (57.1%) |
| Ischaemic heart disease | 4 (22.2% [95% CI: 9.0–45.2]) |
| Men, n (%)* | 4 (36.4%) |
| Women, n (%)* | 0 |
| Pulmonary hypertension | 6 (33.3% [95% CI: 16.3−56.3]) |
| Men, n (%)* | 3 (27.3%) |
| Women, n (%)* | 3 (42.9%) |
*The denominator was the total number of men and women with-OHS group, respectively, n = 11 and n = 7.
OHS: obesity hypoventilation syndrome; OSA: obstructive sleep apnoea.
The association between OHS and the presence of comorbibities was analysed using the Mantel–Haenszel test. The presence of OHS in OSA patients was significantly associated with all comorbidities documented in the study: hypertension (OR = 3.5; [95% CI: 1.2 – 10.3]; p = 0.03), diabetes mellitus (OR = 4.6; [95% CI: of 1.4 – 15.4]; p = 0.02), ischaemic heart disease (OR = 5.1; [95% CI: 1.2 – 21.3]; p = 0.04) and pulmonary hypertension (OR = 16.1; [95% CI: 3.2 – 82.0]; p = 0.001). The results of this analysis are presented as a Forest plot in Figure 2.
Figure 2.
Risk of selected cardiovascular and diabetes comorbidities in patients with OHS. The reference group in each case corresponded to patients with OSA who did not fulfill criteria for OHS. Data are presented in the form of a Forest plot showing odds ratios with their 95% confidence intervals. Data are adjusted for sex, using the Mantel–Haenszel test.
Discussion
This retrospective study was performed in the UAE in adult patients admitted to the Respiratory Care Unit and Sleep Disorder Centre of Zayed Military Hospital for suspicion of sleep-disordered breathing. The primary objective of the study was to estimate the frequency of symptoms of OHS in patients with OSA. In this study, we found that the proportion of patients with OSA referred to the sleep centre who also fulfilled criteria for OHS was 16.8%.
The main limitation of our study is missing data due to the retrospective design of the study, which could have led to incomplete case ascertainment. In addition, the study sample is relatively small and came from a single tertiary care centre in the UAE, albeit a large one. Without a complete description of the hospital's patient base, we are unable to ascertain the representativeness of the sample included, which may not portray accurately the OSA population in the UAE. The results of this study should thus not be generalized to the entire population of the UAE.
The frequency of OHS observed in this study is somewhat lower than those reported from previous prospective and retrospective studies performed in the USA, Western Europe and Japan, which have estimated the prevalence of OHS to be between 10 and 20% of all patients referred to sleep centres and between 20 and 30% of all patients with OSA.15,17 In the Middle East and Gulf region, there are no comparable data and our study is the first to address this issue. Nonetheless, our study may underestimate the true prevalence of OHS in patients with OSA as the study sample represented a pre-selected population referred to the study centre for a suspicion of a sleep-disordered breathing, which may not correspond to those most at risk for OHS. On the other hand, we could overestimate the true prevalence of OHS as we attributed all pCO2 > 45 mmHg measured in obese patients to OHS, whereas in reality some patients may have been hypercapnic due to other conditions such as chronic obstructive pulmonary disease. Prospective studies should be able to avoid this by excluding other comorbidities in patients with hypercapnia by performing thyroid function tests, pulmonary function testing, and chest imaging.
The relationship between OHS and OSA has been investigated in previous studies. For example, in a prospective study performed in France, it was found that in patients with sleep-disordered breathing, there was a strong association between OHS and OSA given that around 90% of patients with OHS have OSA.18 This result suggests that the association of comorbid OHS and OSA was much more frequent than OHS alone.
Our patients with OSA were predominantly men and obese (BMI > 30 kg/m2), consistent with other studies.5 In contrast, we did not observe a clear gender difference with respect to OHS which is again consistent with previous studies reporting a similar prevalence of OHS in women and in men. For example, Nowbar et al.8 showed that around half (48.9%; 23/47 patients) of patients with obesity associated hypoventilation were men. This suggests that risk factors for OHS and possibly OSA may differ between women and men.
The secondary objective of this study was to identify comorbidities associated with OHS. The strong association between OHS, diabetes cardiovascular comorbidities and pulmonary hypertension is consistent with findings from other studies. Nevertheless, the relative weight of these comorbidities is somewhat different to studies performed elsewhere in the world. For example, a prospective study performed in France estimated that 58% of OHS patients have pulmonary hypertension defined as a mean pulmonary artery pressure > 20 mmHg,18 which is nearly twice that observed in our study (33.3%). However, the limited precision of comorbidity rates due to the small sample size should also be born in mind when interpreting such differences. Incomplete documentation of comorbidities or ethnic differences in vulnerability may also contribute to inconsistencies in reported comorbidity rates between studies.
As in other regions of the world, this study reveals a relatively high proportion of patients with OSA in Abu Dhabi who also present OHS. In light of fast-rising obesity in the Middle East region, the prevalence of OHS is also likely to rise in the near future as obese patients enter the at-risk age bracket. Since this condition is associated with serious life-threatening comorbidities, it is important to build awareness about OHS in healthcare professionals in the UAE and to encourage systematic diagnosis of OHS through systematic measurement of blood gases in subjects who are obese or have OSA. Early diagnosis leading to timely treatment may improve long-term prognosis, especially since access to treatment with positive airway pressure devices is free in the UAE. In addition, patient education programmes to encourage weight loss in obese subjects are clearly important. Further prospectively designed studies would be useful in order to measure the magnitude of the problem at the general population level and to monitor patients' response to therapy.
Declarations
Competing Interests
None declared
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors and was funded out of the budget of the Respiratory Care Unit and Sleep Disorder Centre of the Zayed Military Hospital.
Ethical approval
The study was performed in accordance with the Declaration of Helsinki and relevant national guidelines. Ethical approval was obtained from the Ethical Committee at Zayed Military Hospital, Abu Dhabi, UAE. All patients were informed of the goals of the study and required to provide informed consent before inclusion. All patient data were rendered anonymous before registration in the study database.
Guarantor
AA
Contributorship
This study was initiated by the Respiratory Care Unit and Sleep Disorder Centre of the Zayed Military Hospital in UAE. AA, SF and BM were responsible for the conception and design of the study, MR was responsible for data collection and NN was responsible for analysis and interpretation of the data. The present article was prepared by the corresponding author (AA) with the editorial support of a medical writing agency (Foxymed, Paris, France). This agency received honoraria for this support. All authors had full access to the data and contributed to the revision of the different drafts of the manuscript. The corresponding author had final responsibility for the decision to submit the manuscript for publication. There was no financial compensation for participant patients.
Acknowledgements
The authors would like to thank all the staff members of the sleep disordered breathing laboratory at Zayed Military Hospital for their support throughout the study.
Provenance
Not commissioned; peer-reviewed by José Morales-Rull.
References
- 1. Ng SW, Zaghloul S, Ali HI, Harrison G, Popkin BM. The prevalence and trends of overweight, obesity and nutrition-related non-communicable diseases in the Arabian Gulf States. Obes Rev 2011; 12(1): 1–13 [DOI] [PubMed] [Google Scholar]
- 2. Valencia-Flores M, Orea A, Castano VA, Resendiz M, Rosales M, Rebollar V, et al. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res 2000; 8(3): 262–269 [DOI] [PubMed] [Google Scholar]
- 3. Daltro C, Gregorio PB, Alves E, Abreu M, Bomfim D, Chicourel MH, et al. Prevalence and severity of sleep apnea in a group of morbidly obese patients. Obes Surg 2007; 17(6): 809–814 [DOI] [PubMed] [Google Scholar]
- 4. Wall H, Smith C, Hubbard R. Body mass index and obstructive sleep apnoea in the UK: a crosssectional study of the over-50s. Prim Care Respir J 2012; 21(4): 371–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328(17): 1230–1235 [DOI] [PubMed] [Google Scholar]
- 6. Sankri-Tarbichi AG. Obstructive sleep apnea-hypopnea syndrome: etiology and diagnosis. Avicenna J Med 2012; 2(1): 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005; 118(9): 948–956 [DOI] [PubMed] [Google Scholar]
- 8. Nowbar S, Burkart KM, Gonzales R, Fedorowicz A, Gozansky WS, Gaudio JC, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116(1): 1–7 [DOI] [PubMed] [Google Scholar]
- 9. Carden KA, Fogel RB. Obesity-associated hypoventilation – a “growing” concern. Am J Med 2004; 116(1): 58–59 [DOI] [PubMed] [Google Scholar]
- 10. Littleton SW, Mokhlesi B. The Pickwickian syndrome – obesity hypoventilation syndrome. Clin Chest Med 2009; 30(3): 467–478 [DOI] [PubMed] [Google Scholar]
- 11. Roberts WC. Facts and ideas from anywhere. Proc (Bayl Univ Med Cent) 2006; 19(1): 73–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheikh-Ismail LI, Henry CJ, Lightowler HJ, Aldhaheri AS, Masuadi E, Al Hourani HM. Prevalence of overweight and obesity among adult females in the United Arab Emirates. Int J Food Sci Nutr 2009; 60(Suppl 3): 26–33 [DOI] [PubMed] [Google Scholar]
- 13. Malik M, Bakir A. Prevalence of overweight and obesity among children in the United Arab Emirates. Obes Rev 2007; 8(1): 15–20 [DOI] [PubMed] [Google Scholar]
- 14. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005; 26(2): 319–338 [DOI] [PubMed] [Google Scholar]
- 15. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification, Westchester, IL, USA: American Academy of Sleep Medicine, 2007 [Google Scholar]
- 16. Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects, Los Angeles, CA: BIS/BRI, UCLA, 1968 [DOI] [PubMed] [Google Scholar]
- 17. Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest 2007; 132(4): 1322–1336 [DOI] [PubMed] [Google Scholar]
- 18. Kessler R, Chaouat A, Schinkewitch P, Faller M, Casel S, Krieger J, et al. The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest 2001; 120(2): 369–376 [DOI] [PubMed] [Google Scholar]