Abstract
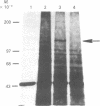
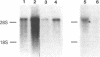
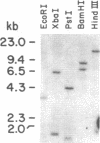
The low density lipoprotein (LDL) receptor belongs to a class of migrant cell surface proteins that mediate endocytosis of macromolecular ligands. No cDNAs for this class of proteins have been isolated to date. In the current paper, we report the isolation of a cDNA clone for the LDL receptor from a bovine adrenal cDNA library. The library was constructed by the Okayama-Berg method from poly(A)+ RNA that had been enriched in receptor mRNA by immunopurification of polysomes. Mixtures of synthetic oligonucleotides encoding the amino acid sequence of two neighboring regions of a single cyanogen bromide fragment were used as hybridization probes to identify a recombinant plasmid containing the LDL receptor cDNA. This plasmid, designated pLDLR-1, contains a 2.8-kilobase (kb) insert that includes a sequence which corresponds to the known amino acid sequence of a 36-residue cyanogen bromide fragment of the receptor. pLDLR-1 hybridized to a mRNA of approximately equal to 5.5 kb in the bovine adrenal gland. This mRNA, like the receptor protein, was 9-fold more abundant in bovine adrenal than in bovine liver. pLDLR-1 cross-hybridized to a mRNA of approximately equal to 5.5 kb in cultured human epidermoid carcinoma A-431 cells. This mRNA was markedly reduced in amount when sterols were added to the culture medium, an observation that explains the previously observed feedback regulation of LDL receptor protein. Southern blot analysis of bovine genomic DNA with 32P-labeled pLDLR-1 revealed a simple pattern of hybridization, consistent with a single-copy gene containing introns.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Brown M. S., Goldstein J. L. Inefficient internalization of receptor-bound low density lipoprotein in human carcinoma A-431 cells. J Cell Biol. 1981 Feb;88(2):441–452. doi: 10.1083/jcb.88.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisiegel U., Kita T., Anderson R. G., Schneider W. J., Brown M. S., Goldstein J. L. Immunologic cross-reactivity of the low density lipoprotein receptor from bovine adrenal cortex, human fibroblasts, canine liver and adrenal gland, and rat liver. J Biol Chem. 1981 Apr 25;256(8):4071–4078. [PubMed] [Google Scholar]
- Beisiegel U., Schneider W. J., Goldstein J. L., Anderson R. G., Brown M. S. Monoclonal antibodies to the low density lipoprotein receptor as probes for study of receptor-mediated endocytosis and the genetics of familial hypercholesterolemia. J Biol Chem. 1981 Nov 25;256(22):11923–11931. [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog Horm Res. 1979;35:215–257. doi: 10.1016/b978-0-12-571135-7.50009-6. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Kita T., Brown M. S. Defective lipoprotein receptors and atherosclerosis. Lessons from an animal counterpart of familial hypercholesterolemia. N Engl J Med. 1983 Aug 4;309(5):288–296. doi: 10.1056/NEJM198308043090507. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Knudsen P. J., Kaufman J. F., Strominger J. L. cDNA clones for the heavy chain of HLA-DR antigens obtained after immunopurification of polysomes by monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1844–1848. doi: 10.1073/pnas.79.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Williamson V. M., Young E. T. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983 Feb 25;258(4):2674–2682. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. J., Beisiegel U., Goldstein J. L., Brown M. S. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J Biol Chem. 1982 Mar 10;257(5):2664–2673. [PubMed] [Google Scholar]
- Shapiro S. Z., Young J. R. An immunochemical method for mRNA purification. Application to messenger RNA encoding trypanosome variable surface antigen. J Biol Chem. 1981 Feb 25;256(4):1495–1498. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Goldstein J. L., Schneider W. J., Brown M. S. Posttranslational processing of the LDL receptor and its genetic disruption in familial hypercholesterolemia. Cell. 1982 Oct;30(3):715–724. doi: 10.1016/0092-8674(82)90276-8. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Hobgood K. K., Brown M. S., Goldstein J. L. The LDL receptor locus in familial hypercholesterolemia: multiple mutations disrupt transport and processing of a membrane receptor. Cell. 1983 Mar;32(3):941–951. doi: 10.1016/0092-8674(83)90079-x. [DOI] [PubMed] [Google Scholar]