Abstract
Microglia are crucial for the pathogenesis of multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis (EAE). Here, we show that the E3 ubiquitin ligase Peli1 is abundantly expressed in microglia and serves as a pivotal mediator of microglial activation during the course of EAE induction. Peli1 mediates the induction of chemokines and proinflammatory cytokines in microglia and, thereby, promotes recruitment of T cells into the central nervous system. Peli1-deficient mice are refractory to EAE induction despite their competent production of inflammatory T cells in the peripheral lymphoid organs. Notably, Peli1 regulates a novel signaling axis of the toll-like receptor pathway that mediates degradation of Traf3, a potent inhibitor of MAP kinase activation and gene induction. Ablation of Traf3 restores the microglial activation and EAE sensitivity of Peli1-deficient mice. These findings establish Peli1 as a microglia-specific mediator of autoimmune neuroinflammation and suggest a novel signaling mechanism of Peli1 function.
Keywords: Peli1, Ubiquitination, CNS inflammation, EAE, Traf3, c-IAP
INTRODUCTION
Multiple sclerosis (MS) is an inflammatory disorder of the central nervous system (CNS), characterized by demyelination and axonal damage1. A widely used animal model for MS studies is experimental autoimmune encephalomyelitis (EAE)2. Initiation of EAE involves peripheral priming of myelin-specific autoimmune T helper 1 (Th1) and Th17 cells and their subsequent migration into the CNS, where they become reactivated by antigen-presenting cells (APCs) displaying myelin-derived peptides and engage in inflammatory processes. These pathological events culminate in disseminated CNS inflammation, leading to the destruction of oligodendrocytes and neurons, and the development of disease symptoms characterized by progressive paralysis3. The pathogenesis of EAE and MS also critically involves microglia4-6, innate immune cells that seed the CNS early during embryonic development and differ in ontogeny from bone marrow (BM)-derived monocytes and macrophages7,8. Microglia sense microbial invasion and tissue damages, and upon activation, they secrete chemokines and proinflammatory cytokines, thereby mediating leukocyte recruitment into the CNS and the induction of inflammation9,10. Activated microglia also function as APCs in the reactivation of T cells within the CNS9,11.
Microbial infections serve as an environmental trigger for the onset and persistence of MS1,12 as well as the induction of EAE13-15. Consistently, receptors that detect pathogen-associated molecular patterns, such as toll-like receptors (TLRs), have an important role in the regulation of MS and EAE16. In particular, the TLRs expressed on microglia are thought to mediate microglial activation and CNS inflammation16-19. TLRs respond to both microbial components and endogenous damage-associated molecular patterns released from apoptotic cells or wounded tissues during the course of autoimmune inflammations. Upon stimulation by their ligands, TLRs elicit cascades of signaling events that lead to activation of the IκB kinase (IKK) and MAP kinases (MAPKs) as well as their downstream transcription factors, NF-κB, AP-1, and related DNA-binding proteins20. These transcription factors act cooperatively to induce the expression of a plethora of genes involved in leukocyte migration and inflammation20.
The MyD88-dependent TLR pathway is particularly important for the pathogenesis of EAE16,17. An E3 ubiquitin ligase, c-IAP, is required for the activation of MAPKs, although not IKK, by the MyD88-dependent TLRs21. Upon activation by the TLR signals, c-IAP mediates K48 ubiquitination and degradation of Traf3, an action required for MAPK activation21,22. However, the mechanism mediating this c-IAP-Traf3 axis of TLR signaling is obscure. The Peli (also called Pellino) family of proteins has been implicated in the regulation of TLR and IL-1 receptor (IL-1R) signaling in innate immune cells23-25. Mammalian cells express three highly homologous Peli members, which complicates the study of their in vivo functions due to potential functional redundancies. Our recent study suggests that in innate immune cells and mouse embryonic fibroblasts (MEFs), Peli1 is dispensable for signal transduction by IL-1R and the MyD88-dependent TLRs, although Peli1 has a nonredundant role in mediating NF-κB activation by the TRIF-dependent TLR pathway26. Whether Peli1 is required for MyD88 TLR signaling in specific cell types and how Peli1 regulates TLR signaling in vivo in pathological processes remain elusive. In the present study, we discovered a crucial role for Peli1 in regulating microglial activation and EAE pathogenesis. We provide molecular and genetic evidence that Peli1 mediates the c-IAP-Traf3 signaling axis and, thereby, controls TLR-stimulated Traf3 degradation and MAPK activation.
RESULTS
Peli1-deficient mice are refractory to EAE induction
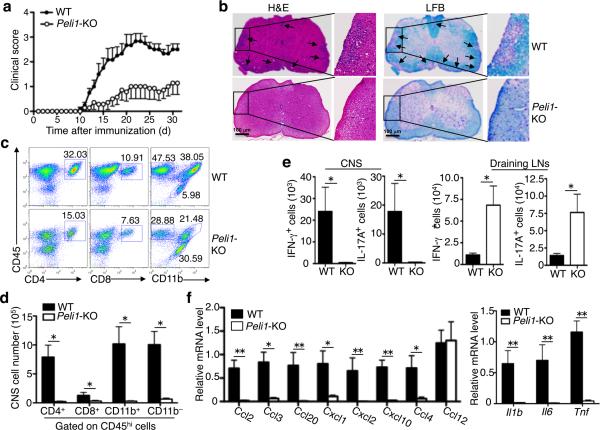
To study the role of Peli1 in CNS inflammation, we induced EAE in wild-type and Peli1-KO mice using a myelin oligodendrocyte glycoprotein (MOG) peptide (MOG35–55). As expected, wild-type mice developed severe clinical symptoms that were characterized by a gradual increase in the severity of paralysis (Fig.1a), inflammatory cell infiltration into the CNS (Fig. 1b, left), and demyelination (Fig. 1b, right). Despite the competent responses of the Peli1-KO T cells to antigen stimulation (Supplementary Fig. 1), these mutant mice were highly resistant to EAE induction (Fig. 1a,b). This phenotype of the Peli1-KO mice was associated with reduced frequency and number of CNS-infiltrating T cells (CD4+ and CD8+), myeloid cells and activated resident microglial cells (CD11b+CD45hi), and total lymphocytes (CD11b–CD45hi), with concomitant increase in resting microglial cells (CD11b+CD45lo) (Fig. 1c,d).
Figure 1. Peli1-KO mice are resistant to EAE induction.
(a) Mean clinical scores of age- and sex-matched wild-type (WT) and Peli1-KO mice subjected for MOG35-55-induced EAE (n=5/group). (b) H&E and Luxol Fast Blue (LFB) staining of spinal cord sections from MOG35-55-immunized wild-type and Peli1-KO EAE mice for visualizing immune cell infiltration and demyelinization, respectively (arrows). Scale bar, 100 μm. (c,d) Flow cytometry analysis of immune cell infiltration into the CNS (brain and spinal cord) of MOG35-55-immunized wild-type and Peli1-KO mice (n = 5, day 15 post-immunization). Data are presented as a representative plot (c) and summary graph (d). (e) Absolute number of Th1 and Th17 cells in the CNS (brain and spinal cord) and draining LNs of MOG35-55-immunized wild-type and Peli1-KO mice quantified by flow cytometry (n = 4, day 15 post-immunization). Data are presented as summary graphs. (f) QPCR analysis to determine the relative mRNA expression level of genes encoding chemokines (left panel) and proinflammatory cytokines (right panel) in spinal cords of MOG35-55-immunized wild-type and Peli1-KO mice (n = 4, day 15 post-immunization). Data were normalized to a reference gene, β-actin. *P<0.05 and **P<0.01.
The CNS of Peli1-KO mice also had a greatly reduced number of Th1 and Th17 cells, coupled with their accumulation in the draining lymph nodes (LNs) (Fig. 1e). Notably, the CNS of EAE-induced Peli1-KO mice had defective expression of various chemokines and inflammatory cytokines known to mediate immune cell recruitment and inflammation (Fig. 1f). These results suggest that the Peli1-KO mice may have impaired recruitment of T cells into the CNS.
Peli1 regulates EAE induction in radioresistant cells
To examine the cellular mechanism by which Peli1 regulates EAE pathogenesis, we performed passive EAE induction by adoptively transferring activated MOG-specific T cells into sublethally irradiated wild-type and Peli1-KO recipient mice. Transfer of wild-type T cells to wild-type recipient mice (WT to WT), but not to the Peli1-KO recipient mice (WT to KO), led to severe EAE disease, suggesting a CNS-specific role for Peli1 in mediating EAE pathogenesis (Supplementary Fig. 2a). On the other hand, Peli1 was dispensable for the EAE-inducing function of T cells, since the MOG-specific Peli1-KO T cells were fully competent in EAE induction in wild-type recipients (Supplementary Fig. 2b).
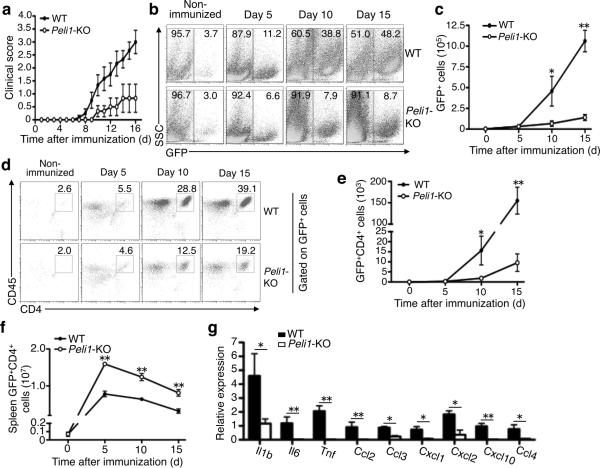
We next generated radiation BM chimeric mice by reconstituting lethally irradiated wild-type or Peli1-KO mice with wild-type BM cells isolated from a GFP-transgenic mouse27. The wild-type GFP-chimeric mice were highly susceptible, while the KO GFP-chimeric mice were refractory, to EAE induction (Fig. 2a). A reverse BM transfer experiment revealed that Peli1 was dispensable in BM cells for mediating EAE pathogenesis (Supplementary Fig. 3). These data further support a CNS-specific function for Peli1 in mediating EAE pathogenesis.
Figure 2. Peli1 deficiency in radioresistant cells inhibits immune cell recruitment into the CNS and ameliorates EAE pathogenesis.
(a) EAE induction of wild-type and Peli1-KO mice adoptively transferred with GFP+ wild-type BM cells. (b–e) Flow cytometry analysis of total CNS-infiltrating cells (GFP+, b,c) and CNS-infiltrating CD4 T cells (GFP+CD4+, d,e) of the MOG35-55-immunized wild-type and Peli1-KO GFP-chimeric mice described in a, showing a representative plot (b,d) and a summary graph (c,e). (f) Flow cytometry analysis of GFP+CD4+ cell number in the spleen of MOG35-55-immunized wild-type and Peli1-KO GFP-chimeric mice. (g) QPCR analysis of the indicated genes. *P<0.05 and **P<0.01.
Taking advantage of the GFP-chimeric mice described above, we determined whether Peli1 functions in CNS-resident cells to mediate the recruitment of immune cells into the CNS during EAE induction. On day 5 following EAE induction, the CNS of both the wild-type and Peli1-KO chimeras had a low frequency of immune cells (GFP+) (Fig. 2b,c). Over time, the frequency of the CNS-infiltrating immune cells was substantially increased in the wild-type chimeras, but not in the Peli1-KO chimeras (Fig. 2b,c). Similarly, the Peli1-KO chimeras had a severe defect in recruiting CD4+ T cells into the CNS (Fig. 2d,e). This was not due to a defect in donor T-cell activation, since the spleen of these mutant chimeras contained a considerably higher number of donor CD4+ T cells (Fig. 2f). The CNS of Peli1-KO chimeric mice had considerably reduced expression of proinflammatory cytokine and chemokine genes (Fig. 2g). Thus, Peli1 appears to function within the CNS-resident cells to mediate the induction of genes involved in immune cell recruitment and inflammation.
Peli1 has a microglia-specific role in EAE regulation
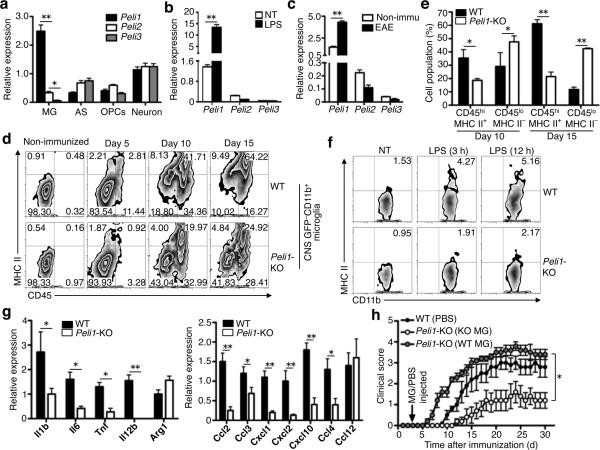
The brain and spinal cord were among the tissues with abundant Peli1 expression (Supplementary Fig. 4a). In particular, Peli1 was the predominant Peli family member expressed in microglia, whereas at least two of the three Peli family members were comparably expressed in astrocytes, oligodendrocyte precursor cells (OPCs), and neurons (Fig. 3a, Supplementary Fig. 4b) or innate immune cells28. Furthermore, Peli1, but not the other Peli family members, was induced in microglia upon in vitro stimulation by LPS (Fig. 3b, Supplementary Fig. 4c,d) or during in vivo EAE induction (Fig. 3c). The Peli1 induction along with EAE was transient (Supplementary Fig. 4e).
Figure 3. Peli1-mediated microglial activation contributes to EAE pathogenesis.
(a,b) QPCR analysis of relative mRNA expression for Peli family members in non-treated primary microglia (MG), astrocytes (AC), oligodendrocyte precursor cells (OPC), and neurons (a) or in non-treated (NT) and LPS-stimulated microglia (b). (c) QPCR analysis of Peli family member expression in FACS-sorted GPF–CD11b+ microglia isolated from the non-immunized or EAE-induced (15 day after MOG35-55 immunization) GFP-chimeric mice described in Fig. 2a. (d) Flow cytometry analysis of activation markers (CD45 and MHC class II) on gated GFP−CD11b+ microglia isolated from the CNS of wild-type or Peli1-KO GFP-chimeric mice, which were either non-immunized or immunized for the indicated days of EAE induction. Data are representative of 4 animals per group and time points. (e) Summary graph of d, showing the mean ± S.D. of the frequency value of resting (GFP−CD11b+CD45loMHC II−) and activated (GFP−CD11b+CD45hiMHC II+) microglia at the two indicated time points. (f) Flow cytometry analysis of MHC II expression on wild-type and Peli1-KO primary microglia that were either not treated (NT) or stimulated with LPS in vitro for the indicated times. (g) QPCR analysis of the indicated genes in FACS-sorted GPF–CD11b+ microglia isolated from EAE-induced (day 15 post-immunization) wild-type or Peli1-KO GFP-chimeric mice. (h) EAE induction in wild-type or Peli1-KO mice that were stereotaxically injected, on day 3 of MOG35-55 immunization, with PBS, Peli1-KO microglia (KO MG), or wild-type microglia (WT MG). Mean clinical scores were determined based on 5 mice per group. *P<0.05 and **P<0.01.
To examine whether Peli1 is required for microglial activation during EAE induction, we took the advantage of the GFP-chimeric mice to distinguish between the infiltrating macrophages (CD11b+GFP+) and CNS-resident microglia (CD11b+GFP–). We detected microglial activation based on their well-defined induction of CD45 and MHC class II (MHC II)5. Following EAE induction, the wild-type chimeras substantially increased the frequency of activated microglia (CD45hiMHC II+GFP–) and decreased the frequency of resting microglia (CD45loMHC II–GFP–), whereas the Peli1-KO chimeras contained predominantly the resting microglia (Fig. 3d,e). Purified Peli1-deficient microglia were also defective in LPS-stimulated MHC II expression in vitro (Fig. 3f). The Peli1-deficient microglia, isolated from EAE-induced mice, also had impaired expression of proinflammatory cytokine and chemokine genes (Fig. 3g). These results suggest the requirement of Peli1 for EAE-associated microglial activation.
As a more direct approach to examine the microglia-specific function of Peli1 in mediating EAE pathogenesis, we stereotaxically injected purified microglia (Supplementary Fig. 4f) into the cerebrospinal fluid of Peli1-KO mice. Injection with the wild-type microglia, but not with the Peli1-KO microglia, rendered the Peli1-KO mice highly sensitive to EAE induction (Fig. 3h). These data further emphasize a microglia-specific role for Peli1 in mediating CNS inflammation and EAE induction.
Peli1 mediates TLR-stimulated gene expression in microglia
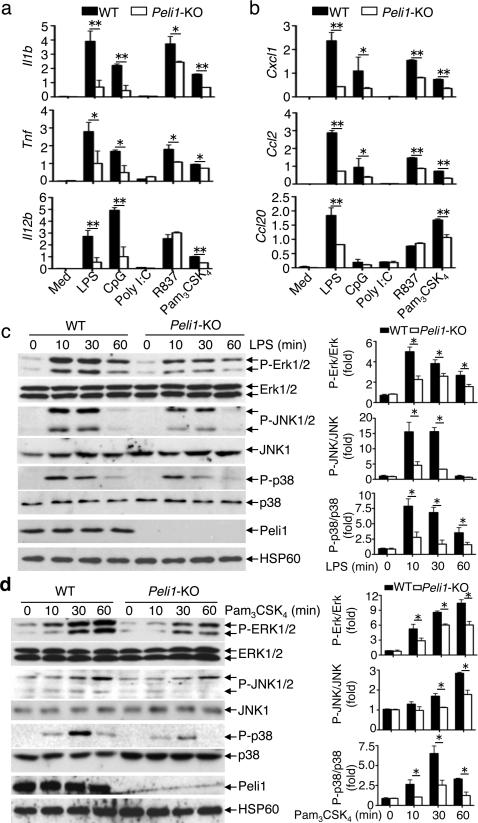
Although microglia did not appreciably respond to the TLR3 ligand, poly(I:C), they responded to several ligands of MyD88-dependent TLRs, including LPS (TLR4), CpG (TLR9), R837 (TLR7), and Pam3CSK4 (TLR1/2), leading to induction of several proinflammatory cytokine and chemokine genes (Fig. 4a,b). The Peli1 deficiency severely attenuated the gene induction by these MyD88-dependent TLR ligands (Fig. 4a,b, Supplementary Fig. 5).
Figure 4. Peli1 mediates TLR-stimulated gene expression and MAPK activation in microglia.
(a,b) QPCR analysis of relative mRNA expression for the indicated proinflammatory cytokine genes (a) and chemokine genes (b) in wild-type and Peli1-KO microglia stimulated with ligands of different TLRs: TLR4 (LPS, 100 ng ml–), TLR9 (CpG, 2.5 μM–), TLR7/8 (R837, 1 μg ml–), TLR1/2 (Pam3CSK4, 1 μg ml–), and TLR3 (poly(I:C), 10 μg ml–). (c,d) IB analysis of phosphorylated (P-) and total MAPKs in whole-cell lysates of wild-type or Peli1-KO microglia stimulated with LPS (100 ng ml–) (c) or Pam3CSK4 (1 μg ml–) (d). The protein bands were quantified using ImageJ and presented as fold of phosphorylated over total MAPKs. Data are mean±S.D. values based on three independent experiments. *P<0.05 and **P<0.01.
EAE pathogenesis also involves the CNS-resident astrocytes, which respond to proinflammatory cytokines, such as IL-17, IFN-γ, and TNF-α. However, the Peli1 deficiency did not appreciably affect such responses in astrocytes (Supplementary Fig. 6). This result suggests a dispensable role for Peli1 in IL-17R signaling or functional redundancy of Peli1 with other Peli family members that are also expressed in astrocytes (Fig. 3a).
Peli1 mediates TLR-stimulated MAPK activation in microglia
We have previously shown that in MEFs, Peli1 mediates NF-κB activation by the TRIF-dependent TLR pathway, but is dispensable for the MyD88-dependent TLR pathway26. Similarly, we found that the loss of Peli1 in microglia impaired the activation of NF-κB stimulated by the TLR3 ligand poly(I:C) (Supplementary Fig. 7a). In contrast, Peli1 was dispensable for NF-κB activation by the MyD88-dependent TLR ligand Pam3CSK4 (Supplementary Fig. 7b). The LPS-induced NF-κB activation in Peli1-deficient microglia was partially defective (Supplementary Fig. 7c), reflecting the fact that LPS stimulates both TRIF- and MyD88-dependent pathways20. These results further emphasize that Peli1 regulates NF-κB activation specifically in the TRIF-dependent TLR pathway.
The requirement of Peli1 in gene induction by MyD88-dependent TLRs in microglia (Fig. 4a,b) suggested a novel signaling function of Peli1. Indeed, the Peli1 deficiency severely attenuated the LPS-stimulated activation of three major families of MAPKs: ERK, JNK, and p38 (Fig. 4c). This function of Peli1 appeared to involve the MyD88-dependent pathway, since Peli1 was also required for MAPK activation by Pam3CSK4 (Fig. 4d).
Peli1 mediates TLR-stimulated c-IAP ubiquitination and TRAF3 degradation
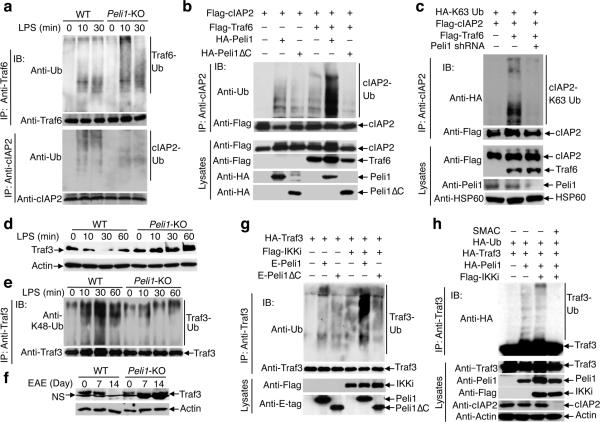
Upon LPS stimulation, activated Traf6 undergoes autoubiquitination23,24 and mediates MAPK activation by inducing K63 ubiquitination of c-IAP, a modification that triggers the ubiquitin ligase activity of c-IAP21,22. We found that the Peli1 deficiency did not inhibit Traf6 ubiquitination but severely attenuated c-IAP2 ubiquitination in LPS-stimulated microglia (Fig. 5a). Since Traf6 is required for Peli1 activation29, we surmised that Peli1 might mediate Traf6-induced c-IAP2 ubiquitination. We found that although both Traf6 and Peli1 induced K63 ubiquitination of c-IAP2, the wild-type Peli1 markedly promoted the Traf6-mediated c-IAP2 ubiquitination (Fig. 5b, Supplementary Fig. 8a). Conversely, the Traf6-induced c-IAP2 ubiquitination was blocked by expression of the Peli1 RING-deletion mutant (Peli1ΔC) (Fig. 5b) and by knocking down endogenous Peli1 by shRNA (Fig. 6c). Moreover, the LPS-stimulated K63 ubiquitination of c-IAP2 was attenuated in the Peli1-deficient microglia (Supplementary Fig. 8b). These results suggest that Peli1 is essential for the induction of c-IAP ubiquitination by LPS and Traf6.
Figure 5. Peli1 regulates TLR-stimulated c-IAP2 ubiquitination and Traf3 degradation.
(a) Ubiquitination of Traf6 (upper) and c-IAP2 (lower) in LPS-stimulated wild-type or Peli1-KO microglia. (b) Ubiquitination of c-IAP2 (upper) and expression of the indicated proteins (lower) in HEK293 cells transfected with (+) or without (–) the indicated expression vectors. (c) Ubiquitination of c-IAP2 (upper) and expression of the indicated proteins (lower) in HEK293 cells transfected with (+) or without (–) the indicated cDNA expression vectors or Peli1 shRNA. (d) IB analysis of Traf3 and actin in whole-cell lysates of LPS-stimulated wild-type and Peli1-KO microglia. (e) Analysis of Traf3 K48 ubiquitination in wild-type or Peli1 KO microglia stimulated with LPS in the presence of a proteasome inhibitor, MG132. (f) IB analysis of Traf3 and actin in whole-cell lysates of FACS-sorted GPF–CD11b+ microglia isolated from non-immunized (0 d) or EAE-induced (7 and 14 d) GFP-chimeric mice (as described in Fig. 2a). NS indicates a nonspecific band. (g) Ubiquitination of Traf3 (upper) and expression of the indicated proteins (lower) in HEK293 cells transfected with the indicated expression vectors. (h) Ubiquitination of Traf3 (upper) and expression of the indicated proteins (lower) in HEK293 cells transfected with (+) or without (–) the indicated expression vectors and subsequently (after 10 h of transfection) cultured in the absence (–) or presence (+) of Smac mimetic (SM, 1 μM) for another 24 h.
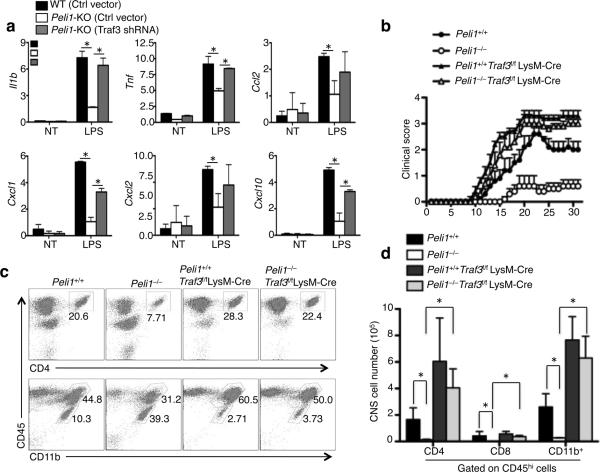
Figure 6. Traf3 ablation restores proinflammatory gene induction in Peli1-deficient microglia and EAE induction in Peli1-deficient mice.
(a) QPCR analysis of the indicated mRNAs in nontreated (NT) or LPS-stimulated wild-type and Peli1-KO microglia infected with control vector or Traf3 shRNA. *P<0.05. (b) Mean clinical scores of the indicated mice subjected for MOG35-55-induced EAE (n=5/group). (c,d) Flow cytometry analysis of immune cell infiltration into the CNS (brain and spinal cord) of the indicated mice at 30 days following MOG35-55-mediated EAE induction (n = 4). Data are presented as a representative plot (c) and summary graph (d).
An important function of c-IAP in the TLR pathway is to mediate K48 ubiquitination and degradation of Traf3, thereby triggering MAPK activation21,22. We found that LPS stimulated rapid degradation of Traf3 in wild-type microglia (Fig. 5d), although such a signaling event was much less prominent in BM-derived macrophages (data not shown). The LPS-stimulated Traf3 degradation was blocked in the Peli1-deficient microglia (Fig. 5d), which could be rescued by reconstitution with exogenous Peli1 (Supplementary Fig. 9a). Consistently, LPS stimulated Traf3 K48-ubiquitination in wild-type microglia, which was diminished in the Peli1-deficient microglia (Fig. 5e). A parallel experiment revealed that the TLR2 ligand Pam3CSK4 also induced Traf3 degradation in wild-type, but not Peli1-deficient, microglia (Supplementary Fig. 9b). Of note, Traf3 was gradually depleted in the microglia of wild-type mice but was accumulated in the microglia of Peli1-KO mice along with EAE induction (Fig. 5f). Thus, Peli1 mediates the ubiquitin-dependent Traf3 degradation both in vitro upon TLR stimulation and in vivo along with EAE induction.
Consistent with a recent report that IKKi (also known as IKKε) activates Peli130, we found that overexpressed Peli1 induced Traf3 ubiquitination, which was promoted by IKKi (Fig. 5g). Because c-IAP is known as an E3 of Traf321, we asked whether Peli1-induced Traf3 ubiquitination required c-IAP. We found that the Peli1-induced Traf3 ubiquitination was blocked in cells treated with the c-IAP inhibitor Smac mimetic31-33 (Fig. 5h) or transfected with the c-IAP1,2 shRNAs (Supplementary Fig. 10). Collectively, these results suggest that Peli1 activates c-IAP and participates in c-IAP-mediated Traf3 ubiquitination and degradation.
Traf3 ablation restores microglial activation and EAE sensitivity in Peli1-KO mice
To assess the functional significance of Traf3 degradation in mediating microglial activation, we knocked down Traf3 in Peli1-deficient microglia. Infection of the Peli1-KO microglia with a Traf3-specific shRNA, but not a control shRNA vector, largely rescued their defect in LPS-stimulated gene induction (Fig. 6a). Consistent with this in vitro finding, the Traf3fl/flLysM-Cre mice, which had specific Traf3 ablation in microglia and other myeloid-lineage cells, were evidently more sensitive to EAE induction than the control Traf3+/+LysM-Cre mice (Supplementary Fig. 11a). This phenotype was not due to elevation in the production of Th1 or Th17 cells (Supplementary Fig. 11b) or in the recall responses of MOG-specific T cells (Supplementary Fig. 11c). On the other hand, the microglia derived from the Traf3fl/flLysM-Cre mice were hyper-responsive to LPS-stimulated expression of proinflammatory cytokine and chemokine genes (Supplementary Fig. 11d).
The myeloid ablation of Traf3 also rescued the defect of the Peli1-KO mice in EAE induction (Fig. 6b) and in CNS-infiltration of T cells and CD45+CD11b+ myeloid cells (Fig. 6c,d). Moreover, the Traf3 ablation rescued the defect of the Peli1-KO mice in microglial activation, as evidenced by the lack of the accumulation of resting microglial population (CD45loCD11b+) (Fig. 6c). Taken together, these in vitro and in vivo studies highlight the functional significance of Peli1-mediated Traf3 degradation in mediating microglial activation and CNS inflammation.
DISCUSSION
Microglial activation is an early event in the development of CNS inflammatory disorders, and ablation or functional inhibition of microglia ameliorates EAE induction without affecting peripheral T-cell activation, emphasizing the promise of targeting microglial activation in the treatment of MS4,34. In the present study, we identified the E3 ubiquitin ligase Peli1 as a pivotal mediator of microglial activation and EAE pathogenesis. We have previously shown that peripheral innate immune cells and MEFs express different Peli family members at comparable levels28, which suggests functional redundancies in some of their functions and explains why loss of Peli1 in peripheral innate immune cells selectively affects the TRIF-dependent TLR signaling in the NF-κB pathway26. Our present study revealed that microglia predominantly expressed Peli1 and required Peli1 for their activation during the course of EAE induction and TLR stimulation. In addition to attenuated TRIF-dependent NF-κB activation, the Peli1-deficient microglia had impaired activation of MAPKs stimulated by MyD88-dependent TLRs. Like microglia, T cells predominantly express Peli1; however, Peli1 is not required, but it rather serves as a negative regulator for T-cell activation28. In contrast to microglia, several other CNS cell types, including astrocytes, OPCs, and neurons, express different Peli family members at comparable levels. Although the role of Peli1 in mediating TLR signaling in these cell types needs to be further studied, the pattern of Peli expression predicts functional redundancy.
Microglia form a major population of CNS APCs that become activated along with EAE induction10. We found that Peli1 is crucial for mediating microglial activation both in vivo along with EAE induction and in vivo by LPS stimulation. These findings suggest the requirement of Peli1 in T-cell activation within the CNS. Peli1 is also required for the induction of proinflammatory cytokines and chemokines in microglia, which explains why the Peli1 deficiency impairs immune cell infiltration into the CNS and ameliorated EAE pathogenesis.
A major signaling function of Peli1 in microglia was to mediate the activation of MAPKs by MyD88-dependent TLRs. Peli1 deficiency did not interfere with the TLR-mediated activation of Traf6 but rather appeared to prevent Traf6 from mediating the K63 ubiquitination of c-IAP. Our data are in line with the recent report that Peli1 is a downstream target of Traf6 in the MyD88 TLR pathway29. The K63 ubiquitination of c-IAP is known to trigger its K48 ubiquitin ligase activity, allowing c-IAP to engage ubiquitin-dependent degradation of Traf321,22. Consistently, Peli1 was required for Traf3 degradation in microglia both in vitro upon TLR stimulation and in vivo along with EAE induction. By different approaches, we demonstrated that the Traf3 accumulation in Peli1-deficient microglia contributes to the impaired inflammatory responses and ameliorated EAE in the Peli1-KO mice. Our data are in agreement with a previous report that Traf3 suppresses IL-17-stimulated signaling and EAE pathogenesis in radioresistant cells, likely the CNS cells35. Since IL-17 does not induce Traf3 degradation35, it is likely that the Peli1 may not be directly involved in IL-17R signaling and that the Peli1-mediated Traf3 degradation in the TLR pathway may indirectly promote the IL-17R signaling.
It is currently unclear whether Peli1 is involved in human MS pathogenesis. Nevertheless, a recently published microarray data set suggests elevated Peli1 expression in the initial white matter lesion areas of MS brains36 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE32915). We also performed microarray studies to analyze gene expression in the grey matters of several MS and non-MS brains. While Peli1 expression was largely comparable between the MS and non-MS samples, one MS sample showed a markedly higher level of Peli1 expression (Supplementary Fig. 12 and Supplementary Table 1). One technical issue was that the brain tissues contain only a small percentage of microglial cells. Nevertheless, these human study data generally suggest that the expression of Peli1 is altered in MS.
In summary, our work establishes Peli1 as a novel mediator of microglial activation and EAE pathogenesis. Based on our data, we propose a model that Peli1 functions downstream of Traf6 in the MyD88 TLR pathway to mediate the activation of c-IAP and induction of ubiquitin-dependent degradation of Traf3, thereby contributing to the activation of MAPKs and the induction of genes involved in microglial activation and CNS inflammation.
METHODS
Methods and any associated references are available in the online version of the paper.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Centenary Institute of Cancer Medicine and Cell Biology for Traf3-floxed mice; Texas A&M Institute for Genomic Medicine for Peli1-KO mice; S.H. Park (Sungkyunkwan University) for HA-tagged Peli1 and Peli1ΔC; R. Beyaert (Ghent University) for E-tag-Peli1; Z. Chen (University of Texas Southwestern Medical Center) for HA-tagged K63 ubiquitin; C. Du (University of Cincinnati College of Medicine) for Flag-c-IAP2; and S. Akira (Osaka University) for Flag-IKKi, X. Qin (Sun Yat-Sen University) for lentiviral packing plasmids. We also thank the personnel from the flow cytometry, DNA analysis, animal facility, and histology core facilities at MD Anderson Cancer Center for technical assistance. This research was supported by grants from the US National Institutes of Health (AI057555, AI064639, GM84459, and T32CA009598).
Footnotes
AUTHOR CONTRIBUTIONS
Y.X. designed and did the research, prepared the figures, and wrote part of the manuscript; J.J., M.C., J.-H.C., H.H., X.Z., G.C.B., and X.C. contributed experiments; C.S. and Ø.T. performed the human microarray experiments. X.W. and R.B. contributed reagents; and S.-C.S. designed the research and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse. Curr. Protoc. Immunol. 2010;11 doi: 10.1002/0471142735.im1501s88. Chapter 15, Unit 15. [DOI] [PubMed] [Google Scholar]
- 3.Goverman J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heppner FL, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 5.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 6.Friese MA, Fugger L. T cells and microglia as drivers of multiple sclerosis pathology. Brain. 2007;130:2755–2757. doi: 10.1093/brain/awm246. [DOI] [PubMed] [Google Scholar]
- 7.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 9.Chastain EM, Duncan DS, Rodgers JM, Miller SD. The role of antigen presenting cells in multiple sclerosis. Biochim. Biophys. Acta. 2011;1812:265–274. doi: 10.1016/j.bbadis.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Z, Tsirka SE. Animal Models of MS Reveal Multiple Roles of Microglia in Disease Pathogenesis. Neurol. Res. Int. 2011;2011:383087. doi: 10.1155/2011/383087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almolda B, Gonzalez B, Castellano B. Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front. Biosci. 2011;16:1157–1171. doi: 10.2741/3781. [DOI] [PubMed] [Google Scholar]
- 12.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann. Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 13.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat. Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 14.Libbey JE, Fujinami RS. Experimental autoimmune encephalomyelitis as a testing paradigm for adjuvants and vaccines. Vaccine. 2011;29:3356–3362. doi: 10.1016/j.vaccine.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berer K, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature Epub ahead of print. 2011 doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 16.Gambuzza M, et al. Targeting Toll-like receptors: Emerging therapeutics for multiple sclerosis management. J. Neuroimmunol. 2011;239:1–12. doi: 10.1016/j.jneuroim.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Prinz M, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 19.Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain. Behav. Immun. 2008;22:140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Tseng PH, et al. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat. Immunol. 2010;11:70–75. doi: 10.1038/ni.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat. Rev. Immunol. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 23.Schauvliege R, Janssens S, Beyaert R. Pellino proteins: novel players in TLR and IL-1R signalling. J. Cell. Mol. Med. 2007;11:453–461. doi: 10.1111/j.1582-4934.2007.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moynagh PN. The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol. 2009;30:33–42. doi: 10.1016/j.it.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Jin W, Chang M, Sun SC. Peli: a family of signal-responsive E3 ubiquitin ligases mediating TLR signaling and T-cell tolerance. Cell. Mol. Immunol. 2012;9:113–122. doi: 10.1038/cmi.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang M, Jin W, Sun SC. Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat. Immunol. 2009;10:1089–1095. doi: 10.1038/ni.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 28.Chang M, et al. The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat. Immunol. 2011;12:1002–1009. doi: 10.1038/ni.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goh ET, et al. Identification of the protein kinases that activate the E3 ubiquitin ligase Pellino 1 in the innate immune system. Biochem. J. 2012;441:339–346. doi: 10.1042/BJ20111415. [DOI] [PubMed] [Google Scholar]
- 30.Smith H, et al. The role of TBK1 and IKKepsilon in the expression and activation of Pellino 1. Biochem. J. 2011;434:537–548. doi: 10.1042/BJ20101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, et al. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 32.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 34.Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–595. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu S, et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J. Exp. Med. 2010;207:2647–2662. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisele S, et al. Prospects of Transcript Profiling for mRNAs and MicroRNAs Using Formalin-Fixed and Paraffin-Embedded Dissected Autoptic Multiple Sclerosis Lesions. Brain Pathol. 2012;22:607–618. doi: 10.1111/j.1750-3639.2012.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 39.Reiley WW, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat. Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- 40.Jin W, Zhou XF, Yu J, Cheng X, Sun SC. Regulation of Th17 cell differentiation and EAE induction by the MAP3K NIK. Blood. 2009;113:6603–6610. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 42.Torkildsen O, et al. Upregulation of immunoglobulin-related genes in cortical sections from multiple sclerosis patients. Brain Pathol. 2010;20:720–729. doi: 10.1111/j.1750-3639.2009.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dysvik B, Jonassen I. J-Express: exploring gene expression data using Java. Bioinformatics. 2001;17:369–370. doi: 10.1093/bioinformatics/17.4.369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.