Abstract
In many growing tissues, slowly dividing stem cells give rise to rapidly proliferating progenitors that eventually exit the cell cycle and differentiate. Growth rates are limited by nutrient availability, but it is unclear which steps of the proliferation-differentiation programme are particularly sensitive to fuel supplies. We examined how nutrient deprivation (ND) affects stem and progenitor cells in the ciliary marginal zone (CMZ) of the amphibian retina, a well-characterised neurogenic niche. We show that ND specifically blocks the proliferation and differentiation of progenitor cells through an mTOR-mediated mechanism. By contrast, the identity and proliferation of retinal stem cells are insensitive to ND and mTOR inhibition. Re-feeding starved retinas in vitro rescues both proliferation and differentiation, and activation of mTOR is sufficient to stimulate differentiation even in ND retinas. These results suggest that an mTOR-mediated restriction point operates in vivo to couple nutrient abundance to the proliferation and differentiation programme in retinal progenitor cells.
Keywords: Differentiation, mTOR, Nutrient deprivation, Proliferation, Restriction point, Retina, Xenopus laevis
INTRODUCTION
Adequate nutrition is vital for growth (Hietakangas and Cohen, 2009), and nutrient deprivation (ND) has been implicated in disease (Jackson, 2002) and aging (Jasper and Jones, 2010). Despite the fact that growth control is exercised through the precise regulation of stem and progenitor cell lineages, little is known about how this regulation is affected by impaired nutrition.
Studies in Drosophila show that ND slows cell proliferation (Drummond-Barbosa and Spradling, 2001; McLeod et al., 2010) and that feeding induces quiescent neuroblasts to re-enter the cell cycle (Chell and Brand, 2010; Sousa-Nunes et al., 2011). Although cell cycle control of proliferating populations operates in tandem with the control of differentiation, it is not fully understood how the latter is affected by nutrient availability. In Drosophila, ND promotes stem and progenitor cell differentiation in the germline (Hsu and Drummond-Barbosa, 2009) and the lymph gland (Benmimoun et al., 2012; Shim et al., 2012). If stem and progenitor cells deprived of nutrients are forced to differentiate without dividing, tissues could become depleted of stem cells and thus be unable to grow even if food becomes available again. However, in most systems it seems that there are mechanisms in place to allow the re-initiation of growth with the return of nutrient availability, suggesting that a particular step in the proliferation-differentiation programme might be sensitive to nutrient loss, so that stem cells are maintained even if other cells are lost.
To investigate the effects of ND on the proliferation-differentiation programme, we used amphibian and fish retinas, which grow throughout life by the addition of new cells from the ciliary marginal zone (CMZ), a neurogenic niche at the retina periphery (supplementary material Fig. S1A) (reviewed by Agathocleous and Harris, 2009). Within the CMZ, slowly dividing retinal stem cells reside at the extreme periphery (supplementary material Fig. S1B,C) (Xue and Harris, 2012). Just central to these cells are rapidly proliferating retinal progenitor cells that express markers of neuronal commitment, and at the central edge of the CMZ are cells that are poised to exit the cell cycle and terminally differentiate into neurons and glial cells (Perron et al., 1998; Xue and Harris, 2012) (supplementary material Fig. S1B,C). The CMZ thus recapitulates in space from periphery to centre the temporal sequence of development from uncommitted stem cells, to committed rapidly dividing progenitors, to differentiating neurons (Harris and Perron, 1998). Several studies have elucidated the transcriptional and signalling mechanisms behind this differentiation cascade in the retina (Borday et al., 2012; Cerveny et al., 2010; Denayer et al., 2008; Wehman et al., 2005; El Yakoubi et al., 2012). Here, we examine how nutrient availability regulates the proliferation-differentiation programme in the CMZ. We find that ND induces an mTOR-dependent block in proliferation and differentiation specifically in progenitor but not stem cells, defining a nutrient-sensitive restriction point that operates in the differentiation programme of retinal stem and progenitor cells.
RESULTS
Nutrient deprivation causes a decrease in CMZ proliferation
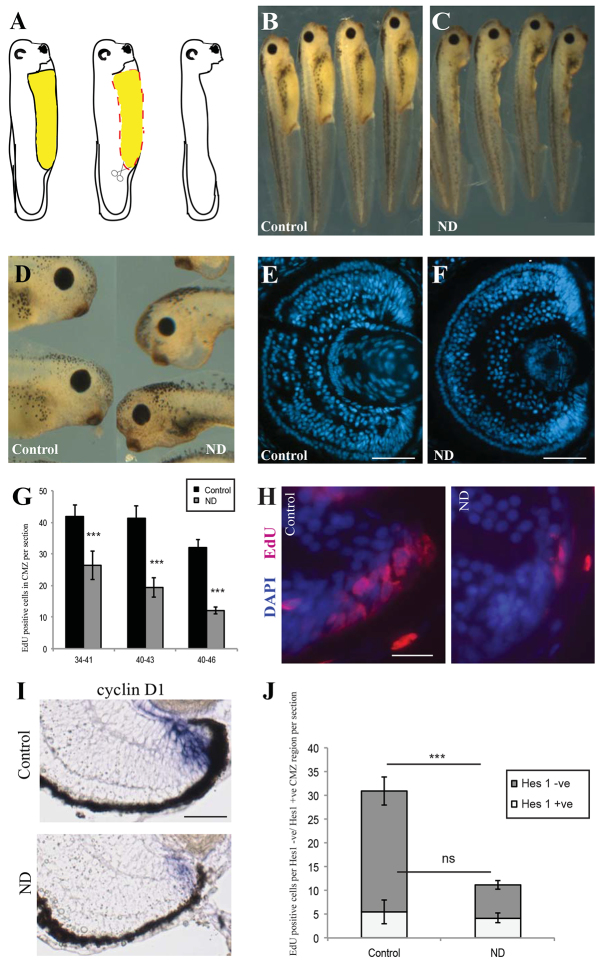
We deprived Xenopus laevis larvae of their physiological internal nutrients (Jorgensen, 2008; Jorgensen et al., 2009) by dissection of the yolk deposits in the gut, starting at stage 34/35 (Fig. 1A), ∼36 hours post-fertilisation (hpf). From this stage onwards, cells in the central retina are mostly postmitotic, with proliferating cells confined to the CMZ (Holt et al., 1988). These ND embryos survived well and 2 days later, at stage 41, were morphologically normal (Fig. 1B,C), although smaller than controls with proportionally smaller eyes (Fig. 1D-F). The CMZs of ND larvae were smaller and had fewer cells than controls (Fig. 1E,F). ND caused a significant drop in incorporation of the nucleotide analogue EdU in the CMZ (Fig. 1G,H). There was no conspicuous increase in CMZ cell apoptosis (supplementary material Fig. S2A-D). These results suggest that ND prevents proliferation in the CMZ. The loss of proliferation was due to ND rather than injury to the embryo, as no reduction in proliferation was observed when less than a quarter of the yolk was removed (supplementary material Fig. S3).
Fig. 1.
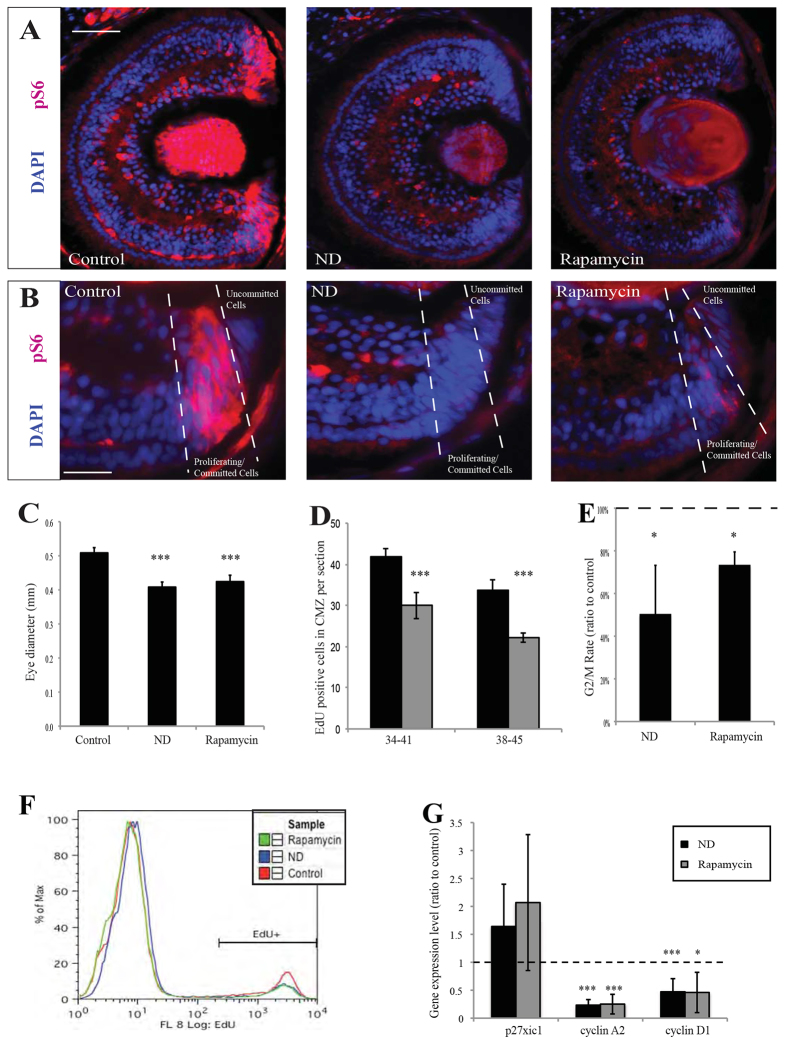
Nutrient deprivation causes a decrease in CMZ proliferation. (A) The nutrient deprivation (ND) procedure. The yolk-rich gut (yellow area), which feeds the embryo, was removed by dissection (red dashed line), leaving the animal in a nutrient-deprived state. Xenopus ND embryos appeared developmentally and morphologically normal 2 days after yolk dissection when compared with control animals (B), but were smaller (C) with smaller eyes (D; control on left, ND embryo on right). (E,F) The cross-sectional area of the whole retina was reduced after ND. ND also reduced the size of the ciliary marginal zone (CMZ) (H) and decreased CMZ proliferation as measured by EdU incorporation after a 2-hour pulse (G,H). In G, embryos were nutrient-deprived during the stages shown. n=10-20 retinas per condition. (I) ND decreases both the domain and intensity of cyclin D1. (J) ND significantly decreased EdU incorporation in progenitor (Hes1 negative) cells but did not affect proliferation in the Hes1-positive stem cell population. n=3, 12 embryos/condition. (G,J) Error bars show 95% confidence intervals; ***P≤0.001 (unpaired t-test); ns, not significant. Scale bars: 100 μm (E,F); 50 μm (H,I).
To test whether the effects of ND on the CMZ were specific to Xenopus, we also deprived zebrafish larvae of nutrients by removing the majority of their yolk at 56 hpf. Both the number of CMZ cells (supplementary material Fig. S4A) and CMZ proliferation (supplementary material Fig. S4B,C) were decreased in ND fish at 84 and 112 hpf. CMZ proliferation is therefore dependent on abundant nutrients in both fish and frogs.
Detection of cell cycle gene expression by in situ hybridisation showed that ND inhibited the expression levels of cyclin A2 and cyclin D1. ND did not significantly change the expression levels of the cyclin-dependent kinase inhibitor p27Xic1, although it appeared to expand its expression domain (Fig. 1I; supplementary material Fig. S5A). These data indicate that the reduction in CMZ proliferation after ND might be ultimately mediated by changes in the expression of specific cell cycle regulators.
The reduction in CMZ proliferation is due to a specific effect on retinal progenitor cells
To test whether all CMZ cells are similarly impaired in proliferation by nutrient withdrawal, or whether stem and progenitor cells respond differently, we measured EdU incorporation in cells positive for the stem cell marker Hes1 (M. Perron, personal communication). We saw a significant decline in the proliferation of EdU-positive progenitors in the Hes1-negative compartment. Surprisingly, however, there was no reduction in the number of EdU/Hes1 double-positive cells (Fig. 1J). This argues that progenitor but not stem cell proliferation is particularly sensitive to nutrient abundance.
Nutrients are necessary for the acquisition of the committed neural progenitor fate but not for the maintenance of the most immature stem/progenitor cells
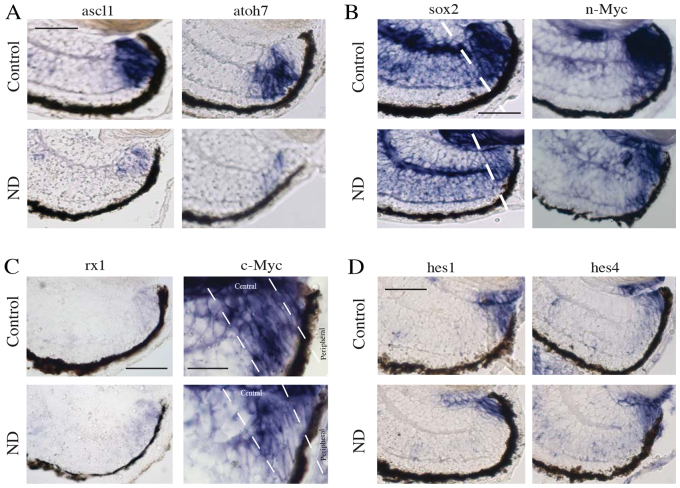
We next investigated whether the reduced size and cell number of the CMZ after ND in the absence of cell death is solely due to changes in proliferation or whether impairments in the differentiation programme are also involved. Early stage progenitor cells express n-Myc (Xue and Harris, 2012), Sox2 (Agathocleous et al., 2009; Van Raay et al., 2005) and several proneural basic helix-loop-helix (bHLH) genes (Perron et al., 1998). These transcription factors define the progenitor fate and are necessary for neurogenic competence. We assayed the CMZ for their expression by in situ hybridisation. The domain of expression of these progenitor genes was reduced, suggesting fewer progenitors, in agreement with our data for a smaller CMZ and specific deficits in progenitor proliferation (Fig. 2A,B; supplementary material Fig. S5B), Crucially, however, we also found that the levels of expression of the bHLH genes ascl1, atoh7, neurogenin2, neuroD and ath3 and of sox2 and n-Myc were all strongly reduced by ND, as judged by the intensity of in situ hybridisation staining (Fig. 2A,B; supplementary material Fig. S5B; see Fig. 6A,B for quantification). As these genes are crucial for differentiation, these findings suggest that ND not only depletes the CMZ of progenitors, but also blocks the progenitor differentiation programme upstream of proneural genes.
Fig. 2.
Nutrients are necessary for acquisition of the committed neural progenitor fate but not for the maintenance of the most immature stem/progenitor cells. (A) Proneural gene expression levels decreased following ND, as did levels of the progenitor markers sox2 and n-Myc (B). Dashed line in B indicates the central domain of the CMZ. n=8, 32 embryos/condition. (C) Expression of the eye field transcription factor gene rx1 remained unchanged. n=4, 16 embryos/condition. (C) c-Myc gene expression levels remained unchanged; however, the domain of c-Myc gene expression was reduced in the central CMZ of ND embryos. n=8, 32 embryos/condition. (D) Expression levels of the stem cell marker genes hes1 and hes2 remained unchanged in ND conditions. n=4, 16 embryos/condition. Scale bars: 50 μm (A,B,D); 50 μm (C; rx1) 25 μm (C; c-Myc).
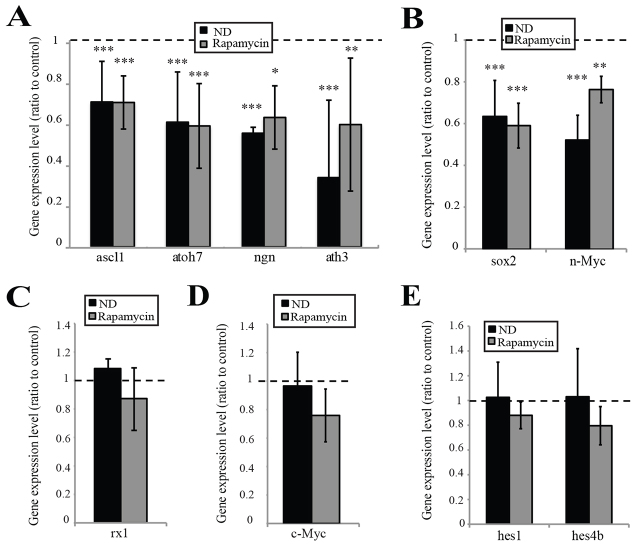
Fig. 6.
Activation of mTOR by nutrients is necessary for neural commitment and differentiation but not for the maintenance of the most immature stem/progenitor cells. (A,B) Proneural gene expression levels were decreased in ND and rapamycin-treated embryos (A), as were levels of the progenitor markers sox2 and n-Myc (B). n=8, 32 embryos/condition. (C) Expression of the eye field transcription factor gene rx-1 remained unchanged by ND. n=4, 16 embryos/condition. (D) c-Myc expression levels remained unchanged; however, the domain of c-Myc expression was reduced in the central CMZ of ND and rapamycin-treated embryos. n=8, 32 embryos/condition). (E) Expression levels of the stem cell marker genes hes1 and hes4 remained unchanged (n=4, 16 embryos/condition. In all cases, error bars show 95% confidence intervals; ***P≤0.001, **P≤0.01, *P≤0.05 (unpaired t-test).
We then examined the most peripheral population, where retinal stem cells reside. The eye field transcription factor gene rx1 is expressed throughout the CMZ, including the extreme peripheral population (Perron et al., 1998). Its expression was unaltered following ND (Fig. 2C, Fig. 6C). The retinal stem cells at the extreme periphery express the transcription factor c-Myc in the absence of n-Myc, and this gene is also expressed along with n-Myc in the more central CMZ where progenitor cells reside (Xue and Harris, 2012). c-Myc was still strongly expressed in the most peripheral domain after ND, despite its domain of expression contracting in the central CMZ (Fig. 2C, Fig. 6D). The very peripheral cells of the CMZ additionally express the hairy and enhancer-of-split (hes) genes hes1 and hes4, which are believed to be markers of retinal stem cells (Parain et al., 2012; El Yakoubi et al., 2012). Following ND, expression of hes1 and hes4 remained high in the peripheral CMZ (Fig. 2D, Fig. 6E). These results suggest that, unlike the progenitors, the stem cells in the extreme periphery of the CMZ are relatively immune to ND.
Committed progenitor cells continue to differentiate normally following ND
Retinal progenitors in the more central CMZ express proneural genes, committing them to differentiation (Harris and Perron, 1998; Perron et al., 1998). In ND retinas, these progenitors are lost, indicating that their production ceases. But what happens to the progenitors that were already present at the start of ND? Do they complete their differentiation programme even in the absence of continued proliferation?
To answer this question, embryos were exposed to EdU for just 20 minutes, and then washed and nutrient deprived. The fate of these EdU-positive cells was examined 3 days later. As would be expected in normal retinas, we found EdU cells located in the differentiated layers of the retina (Fig. 3A). However, and perhaps surprisingly, ND retinas showed significantly more EdU-positive terminally differentiated cells outside of the CMZ than normal embryos, suggesting that many progenitors quickly exited the cell cycle and differentiated after ND (Fig. 3B). Some of these EdU-positive cells were labelled with Islet1, a marker of terminal differentiation of several retinal cell types in Xenopus [e.g. ON-bipolar, cholinergic amacrine and ganglion cells (Elshatory et al., 2007)] (Fig. 3C,D), suggesting that these cells not only had left the CMZ, but also had completed their differentiation programme.
Fig. 3.
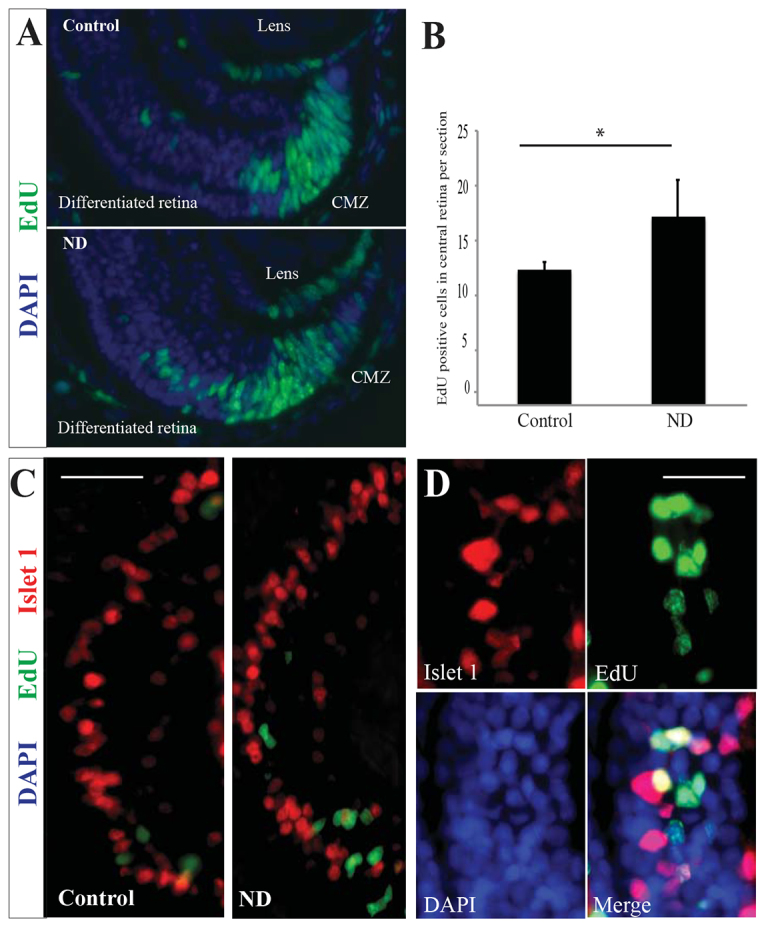
Committed progenitor cells continue to differentiate normally following ND. (A) Many cells labelled with EdU 20 minutes prior to ND were found in the differentiated central retina after a 3-day chase period. n=3, 12 embryos/condition. (B) These cells increased in number in the ND condition compared with control retinas. n=3, 12 embryos/condition. Error bars show 95% confidence intervals; *P≤0.05 (unpaired t-test). (C,D) High magnification of the central retina, which contains differentiated cells. Some EdU-labelled cells were also positive for the neuronal marker Islet1, which marks specific subsets of amacrine and bipolar cells in the inner nuclear layer of the differentiated retina. n=3, 12 embryos/condition. Scale bars: 40 μm (C); 10 μm (D).
This experiment, in combination with our previous results, suggests that the stem and progenitor cells of the CMZ respond differently to ND. Stem cells maintain their undifferentiated state, whereas many committed progenitors differentiate. The production of new progenitors is blocked because the expression of genes that direct the progenitor fate is also inhibited. This depletes the CMZ of progenitor cells while maintaining the stem cells at the periphery of the CMZ.
Re-feeding promotes reconstitution of the CMZ
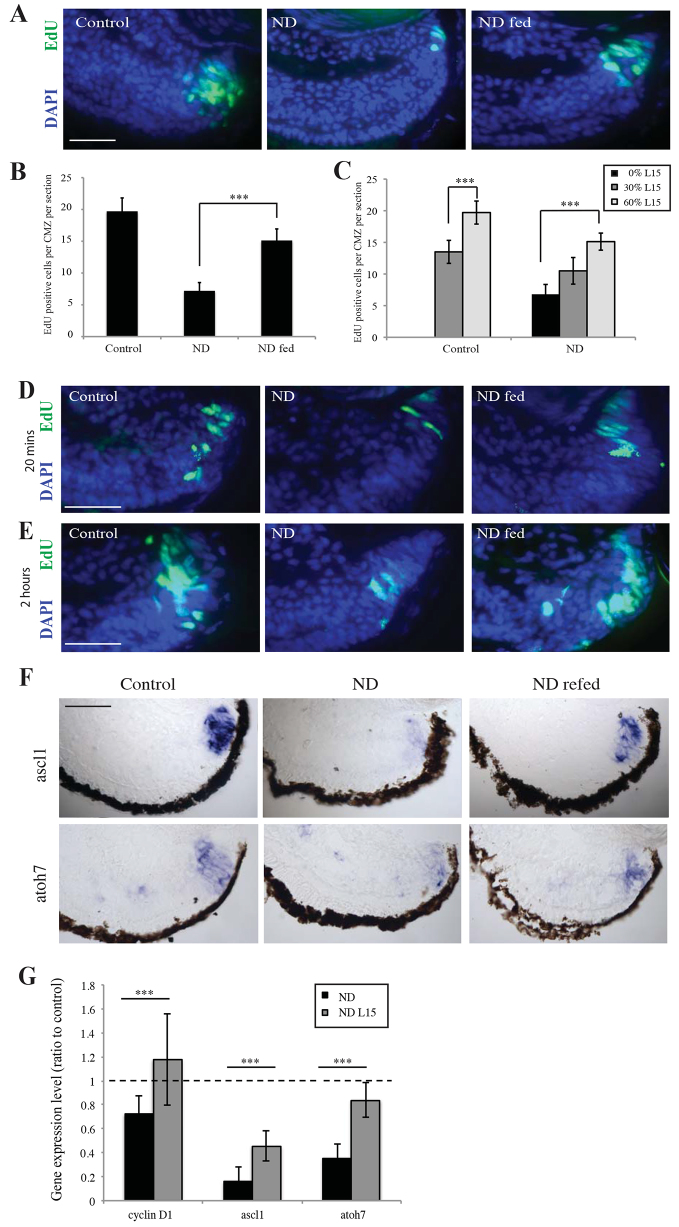
If retinal stem cells are maintained during ND, then feeding a starved embryo may repopulate the CMZ. Unfortunately, ND by dissection of yolk prevents feeding in vivo, as the experimental animals lack intestinal tracts. To circumvent this problem, we explanted whole eyes from ND animals into nutrient-rich media. We found that culturing retinas from ND embryos ex vivo in L15 medium, containing nutrients but no serum or growth factors, resulted in a significant increase in CMZ proliferation (Fig. 4A,B), demonstrating functionally that ND CMZ cells are not lost and are still responsive to growth-promoting conditions. Decreasing nutrient levels from 60% to 30% L15 for a 2-hour period decreased CMZ proliferation acutely in a dose-dependent manner (Fig. 4C), suggesting that proliferation is dynamically, and rapidly, attenuated by nutrient availability.
Fig. 4.
Re-feeding promotes reconstitution of the CMZ. (A,B) Explanted retinas from ND embryos grown in L15 medium containing sugars and amino acids for 24 hours showed an increase in CMZ proliferation after a 2-hour pulse with EdU. n=3, 18 retinas/condition. (C) This increase in proliferation varied with nutrient levels, suggesting that nutrients can regulate proliferation within a dynamic range. n=3, 18 retinas/condition. (D,E) Proliferation was initiated in the CMZ as early as 20 minutes after re-feeding (D), with robust proliferation being observed 2 hours after the addition of nutrients (E). (F,G) After culture for 24 hours in L15, a strong upregulation of Cyclin D1 and the proneural genes ascl1 and atoh7 was observed in re-fed retinas. n=3, 18 retinas/condition. (B,C,G) Error bars show 95% confidence intervals; ***P≤0.001 (unpaired t-test). Scale bars: 50 μm (A,D,E,F).
We next asked how quickly re-feeding initiates the repopulation of the CMZ. Remarkably, in as little as 20 minutes after explanting eyes into L15, a burst of EdU uptake could be observed in the peripheral edge of the CMZ (Fig. 4D), and by 2 hours there was a large increase in EdU uptake in the CMZ compared with ND retinas (Fig. 4E). After 24 hours, re-fed retinas showed increased expression of the cell cycle gene cyclin D1 and a significant upregulation of the proneural genes ascl1 and atoh7 (Fig. 4F,G; supplementary material Fig. S6A). These results demonstrate that the retinal stem cells at the CMZ periphery, and the progenitors whose proliferation had been blocked by ND, are still functional and, with a new supply of nutrients, are able to reinitiate proliferation and differentiation to reconstitute the entire CMZ.
The mTOR pathway is involved in the nutrient-sensitive restriction point
The serine/threonine kinase mTOR regulates cell growth, proliferation and survival by sensing and responding to nutrient availability (Sengupta et al., 2010; Wang and Proud, 2009) and is involved in the regulation of Drosophila eye development (Lanet et al., 2013; McNeill et al., 2008). To test whether mTOR regulates the effects of ND on the CMZ, we first immunostained for phospho-Ribosomal protein S6 (pS6), the cardinal marker of mTOR activity. pS6 was mostly absent from the differentiated cells in the retina but stained the CMZ intensely (Fig. 5A). Within the CMZ, it marked the progenitor cells, but not the most peripheral undifferentiated stem cells (Fig. 5B). pS6 staining was reduced by ND to levels similar to those of embryos treated with the mTOR inhibitor rapamycin (Fig. 5A,B). Thus, in CMZ progenitors, as in other systems, nutrients are necessary for mTOR activity.
Fig. 5.
The mTOR pathway is involved in the nutrient-sensitive restriction point. (A) The CMZ stained strongly for the mTOR target pS6 (red) in control embryos, whereas pS6 staining was lost in the CMZ of ND embryos or in embryos treated with the mTOR inhibitor rapamycin (n=40/40 embryos). (B) Within the CMZ, pS6 was present in committed progenitors but not in undifferentiated stem/progenitor cells (n=40/40 embryos). (C) Rapamycin or ND resulted in a significantly reduced eye size in stage 41 embryos. n=3, 12 embryos/condition. (D) Rapamycin treatment during stages 38-45 significantly reduced the number of proliferative cells in the CMZ. n=5-12 retinas. (E,F) ND or rapamycin reduced the fraction of G2/M cells that were EdU positive after a short EdU pulse (E), suggesting G2/M slowdown, and reduced the overall percentage of EdU-positive cells in the retina (F) from 13% in controls to 9% in rapamycin-treated and 7% in ND embryos. n=3 experiments. (G) Expression levels for the cell cycle genes cyclin D1, cyclin A2 and p27Xic1. ND and rapamycin expression levels are shown as ratio to control. n=6, 24 embryos/condition. (C-E,G) Error bars show 95% confidence intervals; ***P≤0.001, *P≤0.05 (unpaired t-test). Scale bars: 100 μm (A); 50 μm (B).
We next asked whether mTOR is necessary for CMZ proliferation. Incubating embryos with rapamycin, like ND, decreased eye growth (Fig. 5C) and inhibited proliferation in the CMZ (Fig. 5D). Both rapamycin treatment and ND reduced the fraction of the G2/M population that was EdU positive after a 1.5-hour pulse, suggesting a G2/M slowdown (Fig. 5E,F). Also similarly to ND, rapamycin inhibited expression of Cyclin D1 and Cyclin A2 (Fig. 5G), without significantly changing the level of p27Xic1 expression (Fig. 5G). These results suggest that mTOR signalling is sensitive to nutrient levels and is necessary for cell proliferation in the CMZ.
Rapamycin caused changes in gene expression similar to those observed with ND. The intensity of expression of the proneural genes ascl1, atoh7, ath3 and neurogenin2 in the central CMZ was significantly decreased both by ND and rapamycin treatment (Fig. 6A). Rapamycin also reduced the expression levels of the central progenitor markers sox2 and n-Myc, which lie upstream of proneural genes in the differentiation cascade (Van Raay et al., 2005; Xue and Harris, 2012) (Fig. 6B), but did not attenuate the expression of rx1 (Fig. 6C), c-Myc (Fig. 6D), hes1 or hes4 (Fig. 6E), as expressed by the most peripheral CMZ stem cells.
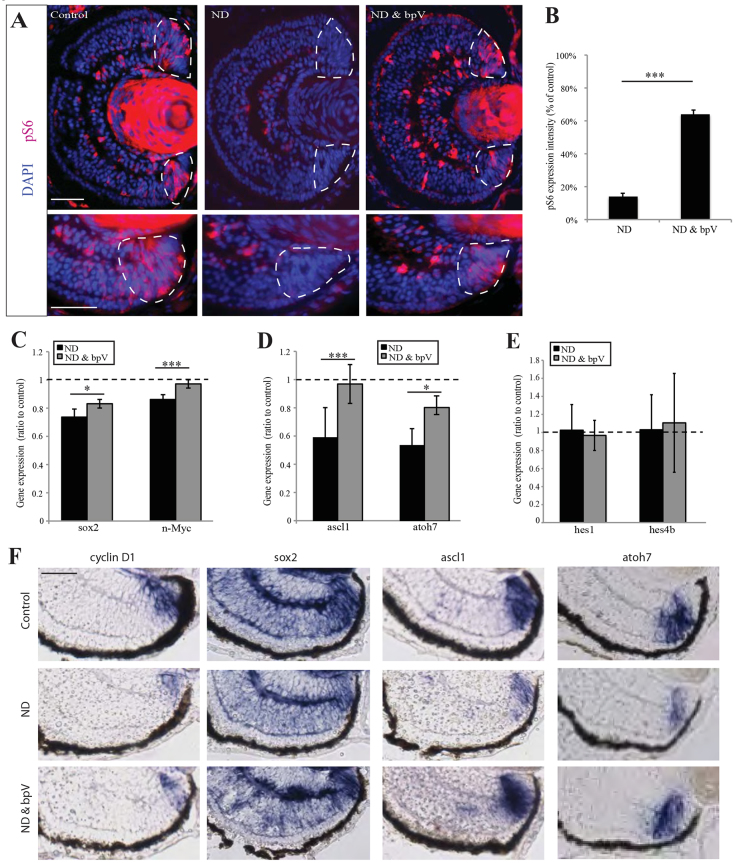
These results show that blocking mTOR activity mimics many of the effects of ND in the CMZ, raising the possibility that mTOR mediates the effects of ND. This hypothesis predicts that stimulation of the mTOR pathway in the absence of nutrients might rescue the ND phenotype. mTOR can be activated by the insulin mimetic compound and PTEN inhibitor bpV(phen) (hereafter bpV), which promotes PI3K signalling (Posner et al., 1994; Schmid et al., 2004). When we treated ND larvae with bpV, we found a robust rescue of pS6 specifically in the CMZ (Fig. 7A,B), similar to what was observed in explant cultures upon re-feeding (supplementary material Fig. S6B). Activation of mTOR by bpV in ND embryos was also sufficient to rescue n-Myc and sox2 expression in the central CMZ (Fig. 7C,F) and the expression of the proneural genes ascl1 and atoh7 (Fig. 7D,F). Interestingly, bpV did not cause an upregulation of pS6 in the CMZ stem cell zone at the far periphery, which also maintained expression of hes1 and hes4 (Fig. 7E), consistent with the restoration of mTOR signalling only in its physiological spatial domain. Treatment of control embryos with bpV did not result in an upregulation of pS6, nor did it have an influence on progenitor proliferation or differentiation (supplementary material Fig. S7A-E).
Fig. 7.
Activation of mTOR signalling is sufficient to maintain the progenitor pool. (A,B) Activation of mTOR with bpV rescues pS6 to a level 70% of that of controls in ND retinas (top row), specifically in CMZ progenitor cells (bottom row). Dashed lines encircle the CMZ. n=4, 16 embryos/condition. (C-F) bpV treatment rescued the downregulation of genes observed following ND, without affecting the expression of stem cell markers. n=4, 16 embryos/condition. (B-E) Error bars show 95% confidence intervals; ***P≤0.001, *P≤0.05 (unpaired t-test). Scale bars: 100 μm (A, top panel); 50 μm (A, bottom panel); 50 μm (F).
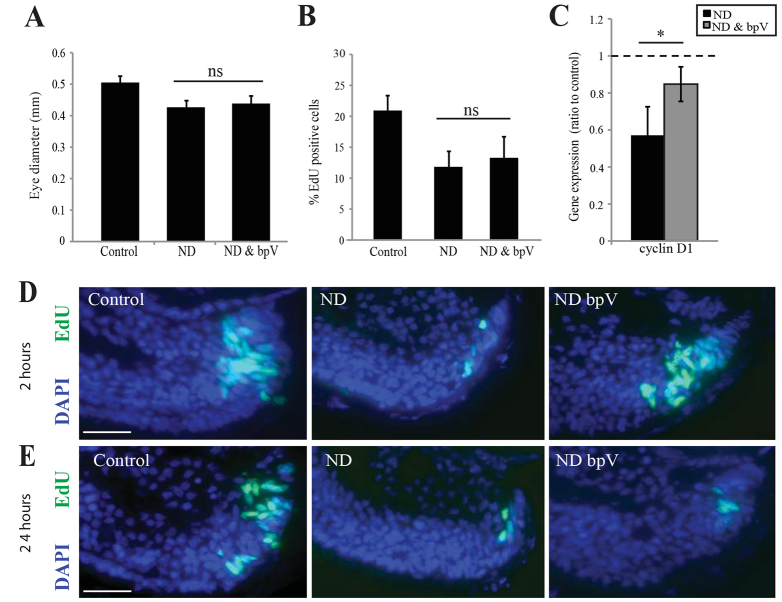
Although bpV rescued the differentiation block after ND, it failed to rescue the decrease in eye size (Fig. 8A) and proliferation (Fig. 8B) caused by ND. Consequently, the block by ND on progenitor differentiation can be uncoupled from, and is not simply downstream of, the impairment in progenitor proliferation. It is interesting to note in this regard that retinas from ND animals do show a transient increase in EdU incorporation 2 hours after the addition of bpV (Fig. 8D,E), and a partial rescue of Cyclin D1 expression (Fig. 8C). One possibility is that mTOR activation in the absence of nutrients does attempt to turn on the proliferation programme but, despite mTOR being necessary for progenitor proliferation (Fig. 5), it is insufficient to drive proliferation in the absence of actual nutrients. Indeed, at 24 hours of bpV treatment, proliferation receded to the levels observed in ND embryos (Fig. 8D,E). Therefore, whereas the effects of ND on differentiation are mediated by mTOR, its effects on proliferation are likely to be mediated by additional cellular mechanisms.
Fig. 8.
Activation of mTOR signalling is sufficient to initiate, but not maintain, progenitor proliferation. (A-C) Eye size at stage 41 was not rescued by bpV (A), nor was proliferation (B), despite expression of Cyclin D1 being significantly higher than in ND embryos (C). n=4, 16 embryos/condition. Error bars show 95% confidence intervals; *P≤0.05 (unpaired t-test). (D,E) In explanted cultures a short-term incubation (2 hours) with bpV was sufficient to initiate proliferation in ND retinas; however, bpV treatment was not sufficient to maintain proliferation for longer periods. n=4, 20 retinas/condition. Scale bars: 50 μm (E,F).
These results support the idea that mTOR signalling, which is lost as a result of ND, is essential for both the differentiation and proliferation of retinal progenitor cells. Even in the absence of nutrients, mTOR is sufficient to drive the progenitor differentiation programme. Retinal stem cells, by contrast, are not dependent on mTOR or abundant nutrients for their maintenance and proliferation.
DISCUSSION
We introduce a new ND paradigm for frog and fish embryos and use it to uncover a nutrient-sensitive restriction point in the proliferation-differentiation programme in the CMZ, a well-organised retinal stem cell niche. When embryos are deprived of nutrients, proliferation in the CMZ is shut down and committed progenitors quickly differentiate. However, the slowly dividing stem cells at the extreme periphery of the CMZ are maintained throughout ND. Our results indicate that nutrient abundance controls the expression of multiple genes required for a specific step in the proliferation-differentiation programme, defining a restriction point downstream of retinal stem cells. This means that the CMZ can be repopulated and growth can re-initiate as soon as nutrients become available again. Previous work in adult flies and mice showed that some intestinal and germline stem cells are maintained during caloric restriction or amino acid deprivation, whereas differentiated progenitor populations may be more reduced (Drummond-Barbosa and Spradling, 2001; McLeod et al., 2010; Yilmaz et al., 2012). Although differences are expected between the physiology of adult caloric restriction and embryonic ND, the nutrient-sensitive restriction point that we describe here could play a role in other tissues to enable stem cells to preferentially withstand ND compared with restricted progenitors.
Key to the response of progenitor cells to nutrient availability is the cellular nutrient-sensing pathway mediated by mTOR. Several growth factors, including the mTOR activator insulin/IGF, promote retinal progenitor proliferation (Agathocleous and Harris, 2009). Activation of mTOR by deletion of PTEN, a negative regulator of the PI3K/mTOR pathway, leads to premature neurogenesis in the developing central retina (Jo et al., 2012), suggesting that the PI3K/mTOR pathway regulates differentiation. Similarly, TOR signalling promotes differentiation in the Drosophila eye (Bateman and McNeill, 2004) and the chick neural tube (Fishwick et al., 2010). The mTOR pathway has been implicated in antagonising stem cell self-renewal in several systems. mTOR activation can deplete stem cells in mouse haematopoietic stem cells (Yilmaz et al., 2006; Chen et al., 2008), Drosophila germ stem cells (Sun et al., 2010) and mouse hair follicle stem cells (Castilho et al., 2009). In the subventricular zone (SVZ), mTOR is required for the proliferation of transit-amplifying progenitors, but is absent from SVZ populations that include neural stem cells (Paliouras et al., 2012). Our results suggest that this general function of mTOR in promoting committed progenitor fates in a variety of tissues might reflect a strategy used by tissues to limit stem cell differentiation when nutrients are scarce and to renew growth by stimulating proliferation of rapidly dividing progenitors when nutrients become available. How is it that stem cells, unlike progenitors, are relatively immune to ND? It is possible that they express negative mTOR regulators such as TSC2, which limits mTOR signalling in Drosophila intestinal stem cells but not progenitors (Kapuria et al., 2012), or that stem cells are more adept than progenitors at using low levels of nutrients for proliferation, perhaps because of their slower cycling (Xue and Harris, 2012).
In summary, our results support a model in which abundant nutrients activate mTOR causing both proliferation and production of committed retinal progenitor cells. When mTOR is inhibited under conditions of ND, these cells differentiate quickly but are not replenished. The remaining, presumably earlier stage, progenitors stop dividing and their differentiation programme is blocked upstream of the proneural genes. Thus, ND leads to the preferential loss of committed progenitors, but it does not block retinal stem cell fate or proliferation. Our results thus define a nutrient-sensitive restriction point in the proliferation-differentiation programme within retinal progenitor cells in the CMZ, and provide a mechanism whereby growth can be restricted, when nutrients are unavailable, without losing the capacity to reinitiate growth if conditions of plenty return.
MATERIALS AND METHODS
Animal maintenance and pharmacological treatment
Xenopus laevis embryos, obtained by fertilisation, were raised in 0.1× modified Barth’s solution (MBS) and staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). Larvae were nutrient deprived at stage 34/35 and analysed at stage 41 unless otherwise indicated. For drug treatments, 1-5 μM rapamycin (Calbiochem) or 300 nM bpV(phen) (Calbiochem) were bath applied in embryo medium at stages 28 and 34, respectively, or at stages otherwise indicated. Zebrafish embryos were raised at 25-32°C and staged as hpf (Kimmel et al., 1995). All animal work was approved by Local Ethical Review Committee at the University of Cambridge and performed in accordance with the UK Home Office license PPL 80/2606.
Dissection of yolk from Xenopus and zebrafish
Xenopus and zebrafish embryos, like other externally developing organisms, rely on maternally derived yolk platelets for nutrition during the first few days of life (Jorgensen et al., 2009). The yolk sacs of stage 34 Xenopus embryos were removed by microdissection using fine dissecting pins as illustrated (Fig. 1A). Removal of yolk from larval zebrafish was carried out at 56 hpf in methylcellulose by first making a fine lesion with a dissection pin, and then carefully removing the contents of the yolk sac by suction through a glass pipette. In both Xenopus and zebrafish care was taken to avoid damaging the blood vessels and maintain blood circulation.
Immunostaining
Immunostaining was performed on 12 μm sections using rabbit anti-active Caspase 3 (1:500; BD Biosciences; 59565), rabbit anti-phospho-Ribosomal protein S6 (Ser235; 1:500; Upstate; 07-433), mouse anti-Islet1 (1:100; Developmental Studies Hybridoma Bank; 39.3f7), Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 594 goat anti-rabbit (both 1:1000; Invitrogen). Antigen retrieval was performed by steaming with 0.01 M sodium citrate (pH 6) prior to staining for pS6. Nuclei were labelled with 0.1 μg/ml DAPI (Invitrogen). Immunofluorescent sections were imaged with an Olympus Fluoview FV1000 laser-scanning confocal microscope or with a Nikon Eclipse 80i microscope and Hamamatsu Orca-ER camera. All images were analysed using ImageJ (NIH) or Openlab (Perkin Elmer).
BrdU/EdU labelling
To mark cycling cells, 5 mM EdU was bath applied to Xenopus embryos, or embryos were injected with BrdU or EdU 2 hours prior to fixation. For pulse chase experiments, 5 mM EdU was bath applied with 1% DMSO for 20 minutes before being washed out. BrdU incorporation was detected as previously described (Agathocleous et al., 2009), and EdU detection was by Click-iT chemistry performed in accordance with the manufacturer’s instructions (Invitrogen). Flow cytometry was performed using a CyAN flow cytometer (Beckman Coulter) and analysed using FlowJo software (Tree Star). Statistical analysis was determined by Student’s t-test or one-way ANOVA.
In situ hybridisation
In situ hybridisation was performed on 20 μm sections as described previously (Xue and Harris, 2012) using digoxigenin-labelled antisense mRNA probes against rx1 (Mathers et al., 1997), atoh7 (Kanekar et al., 1997), ascl1, neurogenin2 (a gift from Dr Anna Philpott, University of Cambridge, UK), ath3 (Takebayashi et al., 1997), c-Myc and n-Myc (Bellmeyer et al., 2003), sox2 (Mizuseki et al., 1998), p27Xic1, cyclin A2 (Howe et al., 1995), cyclin D1 (a gift from Dr Tim Hunt, CRUK, UK) and hes1 and hes4 (a gift from Dr Muriel Perron, Universite Paris-Sud, France). Images were acquired using a Zeiss Axioskop microscope and Q-imaging camera and staining intensity was analysed using ImageJ. Intensity measurements were normalised to background intensity, then the intensity ratio of experimental conditions relative to controls was determined. Statistical significance was tested using a Student’s t-test.
Explant cultures
Stage 41 Xenopus embryos were deprived of their yolk for 24 hours prior to retinal dissection. For ex vivo re-feeding conditions, explanted retinas from control and nutrient-deprived embryos were cultured in a 60% solution of Leibovitz L-15 medium (Sigma) containing galactose and amino acids as nutrient sources. For ex vivo ND, retinas were cultured in 1× MBS, a salt buffer without nutrients. In proliferation experiments, explants were cultured for 22 hours at 20°C then incubated with EdU for 2 hours prior to fixation. Explants for in situ hybridisation were cultured in either 60% L15 or 1× MBS for 24 hours and then fixed. The insulin mimetic bpV was used to activate mTOR in explanted retinas from embryos deprived of nutrients for 24 hours. Eyes were cultured in 300 nM bpV for 2 or 24 hours. To label proliferating cells, explants were either cultured at 20°C with EdU for 2 hours, or cultured for 22 hours and EdU added for 2 hours prior to fixation.
Supplementary Material
Acknowledgments
We thank N. Coutts for technical assistance and N. Miller for help with flow cytometry. We are also grateful to A. McNabb, K. Scott, T. Dyl and S.Dudczig for frog and fish maintainance.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
N.K.L performed most of the experiments and co-wrote the paper with M.A. M.A. initiated the project and performed experiments in Fig. 1A-H and Fig. 5A-F. N.K. generated the data in supplementary material Fig. S4 and R.L. generated data in supplementary material Fig. S3. W.A.H guided the research and helped write the manuscript.
Funding
N.K.L. is a member of the Wellcome Trust 4-Year Programme in Stem Cell Biology. W.A.H. is funded by a Senior Investigator Award (SIA) grant from the Wellcome Trust. M.A. was funded by a Gonville and Caius College Research Fellowship and an 1851 Royal Commission Research Fellowship. Deposited in PMC for immediate release.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.103978/-/DC1
References
- Agathocleous M., Harris W. A. (2009). From progenitors to differentiated cells in the vertebrate retina. Annu. Rev. Cell Dev. Biol. 25, 45–69 [DOI] [PubMed] [Google Scholar]
- Agathocleous M., Iordanova I., Willardsen M. I., Xue X. Y., Vetter M. L., Harris W. A., Moore K. B. (2009). A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development 136, 3289–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. M., McNeill H. (2004). Temporal control of differentiation by the insulin receptor/tor pathway in Drosophila. Cell 119, 87–96 [DOI] [PubMed] [Google Scholar]
- Bellmeyer A., Krase J., Lindgren J., LaBonne C. (2003). The protooncogene c-myc is an essential regulator of neural crest formation in Xenopus. Dev. Cell 4, 827–839 [DOI] [PubMed] [Google Scholar]
- Benmimoun B., Polesello C., Waltzer L., Haenlin M. (2012). Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development 139, 1713–1717 [DOI] [PubMed] [Google Scholar]
- Borday C., Cabochette P., Parain K., Mazurier N., Janssens S., Tran H. T., Sekkali B., Bronchain O., Vleminckx K., Locker M., et al. (2012). Antagonistic cross-regulation between Wnt and Hedgehog signalling pathways controls post-embryonic retinal proliferation. Development 139, 3499–3509 [DOI] [PubMed] [Google Scholar]
- Castilho R. M., Squarize C. H., Chodosh L. A., Williams B. O., Gutkind J. S. (2009). mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 5, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveny K. L., Cavodeassi F., Turner K. J., de Jong-Curtain T. A., Heath J. K., Wilson S. W. (2010). The zebrafish flotte lotte mutant reveals that the local retinal environment promotes the differentiation of proliferating precursors emerging from their stem cell niche. Development 137, 2107–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell J. M., Brand A. H. (2010). Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell 143, 1161–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Liu Y., Liu R., Ikenoue T., Guan K.-L., Liu Y., Zheng P. (2008). TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 205, 2397–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denayer T., Locker M., Borday C., Deroo T., Janssens S., Hecht A., van Roy F., Perron M., Vleminckx K. (2008). Canonical Wnt signaling controls proliferation of retinal stem/progenitor cells in postembryonic Xenopus eyes. Stem Cells 26, 2063–2074 [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D., Spradling A. C. (2001). Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 231, 265–278 [DOI] [PubMed] [Google Scholar]
- El Yakoubi W., Borday C., Hamdache J., Parain K., Tran H. T., Vleminckx K., Perron M., Locker M. (2012). Hes4 controls proliferative properties of neural stem cells during retinal ontogenesis. Stem Cells 30, 2784–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshatory Y., Everhart D., Deng M., Xie X., Barlow R. B., Gan L. (2007). Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J. Neurosci. 27, 12707–12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishwick K. J., Li R. A., Halley P., Deng P., Storey K. G. (2010). Initiation of neuronal differentiation requires PI3-kinase/TOR signalling in the vertebrate neural tube. Dev. Biol. 338, 215–225 [DOI] [PubMed] [Google Scholar]
- Harris W. A., Perron M. (1998). Molecular recapitulation: the growth of the vertebrate retina. Int. J. Dev. Biol. 42, 299–304 [PubMed] [Google Scholar]
- Hietakangas V., Cohen S. M. (2009). Regulation of tissue growth through nutrient sensing. Annu. Rev. Genet. 43, 389–410 [DOI] [PubMed] [Google Scholar]
- Holt C. E., Bertsch T. W., Ellis H. M., Harris W. A. (1988). Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron 1, 15–26 [DOI] [PubMed] [Google Scholar]
- Howe J. A., Howell M., Hunt T., Newport J. W. (1995). Identification of a developmental timer regulating the stability of embryonic cyclin A and a new somatic A-type cyclin at gastrulation. Genes Dev. 9, 1164–1176 [DOI] [PubMed] [Google Scholar]
- Hsu H. J., Drummond-Barbosa D. (2009). Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc. Natl. Acad. Sci. USA. 106, 1117–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. A. (2002). Nutrients, Growth, and the Development of Programmed Metabolic Function. pp. 41–55 Boston, MA: Kluwer Academic Publishers; [DOI] [PubMed] [Google Scholar]
- Jasper H., Jones D. L. (2010). Metabolic regulation of stem cell behavior and implications for aging. Cell Metab. 12, 561–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H. S., Kang K. H., Joe C. O., Kim J. W. (2012). Pten coordinates retinal neurogenesis by regulating Notch signalling. EMBO J. 31, 817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P. (2008). Yolk. Curr. Biol. 18, R103–R104 [DOI] [PubMed] [Google Scholar]
- Jorgensen P., Steen J. A. J., Steen H., Kirschner M. W. (2009). The mechanism and pattern of yolk consumption provide insight into embryonic nutrition in Xenopus. Development 136, 1539–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekar S., Perron M., Dorsky R., Harris W. A., Jan L. Y., Jan Y. N., Vetter M. L. (1997). Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron 19, 981–994 [DOI] [PubMed] [Google Scholar]
- Kapuria S., Karpac J., Biteau B., Hwangbo D., Jasper H. (2012). Notch-mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage. PLoS Genet. 8, e1003045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- Lanet E., Gould A. P., Maurange C. (2013). Protection of neuronal diversity at the expense of neuronal numbers during nutrient restriction in the Drosophila visual system. Cell Rep. 3, 587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers P. H., Grinberg A., Mahon K. A., Jamrich M. (1997). The Rx homeobox gene is essential for vertebrate eye development. Nature 387, 603–607 [DOI] [PubMed] [Google Scholar]
- McLeod C. J., Wang L., Wong C., Jones D. L. (2010). Stem cell dynamics in response to nutrient availability. Curr. Biol. 20, 2100–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H., Craig G. M., Bateman J. M. (2008). Regulation of neurogenesis and epidermal growth factor receptor signaling by the insulin receptor/target of rapamycin pathway in Drosophila. Genetics 179, 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K., Kishi M., Matsui M., Nakanishi S., Sasai Y. (1998). Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development 125, 579–587 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1967). Normal table of Xenopus Laevis. North Holland Publishing Company: Amsterdam, The Netherlands: [Google Scholar]
- Paliouras G. N., Hamilton L. K., Aumont A., Joppé S. E., Barnabé-Heider F., Fernandes K. J. L. (2012). Mammalian target of rapamycin signaling is a key regulator of the transit-amplifying progenitor pool in the adult and aging forebrain. J. Neurosci. 32, 15012–15026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parain K., Mazurier N., Bronchain O., Borday C., Cabochette P., Chesneau A., Colozza G., El Yakoubi W., Hamdache J., Locker M., et al. (2012). A large scale screen for neural stem cell markers in Xenopus retina. Dev. Neurobiol. 72, 491–506 [DOI] [PubMed] [Google Scholar]
- Perron M., Kanekar S., Vetter M. L., Harris W. A. (1998). The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev. Biol. 199, 185–200 [DOI] [PubMed] [Google Scholar]
- Posner B. I., Faure R., Burgess J. W., Bevan A. P., Lachance D., Zhang-Sun G., Fantus I. G., Ng J. B., Hall D. A., Lum B. S., et al. (1994). Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J. Biol. Chem. 269, 4596–4604 [PubMed] [Google Scholar]
- Schmid A. C., Byrne R. D., Vilar R., Woscholski R. (2004). Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 566, 35–38 [DOI] [PubMed] [Google Scholar]
- Sengupta S., Peterson T. R., Sabatini D. M. (2010). Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40, 310–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J., Mukherjee T., Banerjee U. (2012). Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat. Cell Biol. 14, 394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Nunes R., Yee L. L., Gould A. P. (2011). Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature 471, 508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Quan Z., Zhang B., Wu T., Xi R. (2010). TSC1/2 tumour suppressor complex maintains Drosophila germline stem cells by preventing differentiation. Development 137, 2461–2469 [DOI] [PubMed] [Google Scholar]
- Takebayashi K., Takahashi S., Yokota C., Tsuda H., Nakanishi S., Asashima M., Kageyama R. (1997). Conversion of ectoderm into a neural fate by ATH-3, a vertebrate basic helix-loop-helix gene homologous to Drosophila proneural gene atonal. EMBO J. 16, 384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raay T. J., Moore K. B., Iordanova I., Steele M., Jamrich M., Harris W. A., Vetter M. L. (2005). Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron 46, 23–36 [DOI] [PubMed] [Google Scholar]
- Wang X., Proud C. G. (2009). Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol. 19, 260–267 [DOI] [PubMed] [Google Scholar]
- Wehman A. M., Staub W., Meyers J. R., Raymond P. A., Baier H. (2005). Genetic dissection of the zebrafish retinal stem-cell compartment. Dev. Biol. 281, 53–65 [DOI] [PubMed] [Google Scholar]
- Xue X. Y., Harris W. A. (2012). Using myc genes to search for stem cells in the ciliary margin of the Xenopus retina. Dev. Neurobiol. 72, 475–490 [DOI] [PubMed] [Google Scholar]
- Yilmaz Ö. H., Valdez R., Theisen B. K., Guo W., Ferguson D. O., Wu H., Morrison S. J. (2006). Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 [DOI] [PubMed] [Google Scholar]
- Yilmaz O. H., Katajisto P., Lamming D. W., Gültekin Y., Bauer-Rowe K. E., Sengupta S., Birsoy K., Dursun A., Yilmaz V. O., Selig M., et al. (2012). mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486, 490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.