Abstract
Objective
Compare the initial phases of virologic decay when acute/early and advanced HIV-infected adults are administered the same treatment regimen.
Design
Mathematical modeling of a previously completed prospective treatment pilot study involving treatment-naive patients with early and advanced immunosuppression.
Methods
We analyzed data from a treatment protocol in which 18 individuals with acute or recent HIV-1 seroconversion and six patients with advanced AIDS were administered the same four-drug antiretroviral regimen. Initial treatment responses were compared by fitting a mathematical model to frequent viral load measurements in order to calculate the first and second phase kinetics of viral clearance, and also by comparing viral load suppression over 24 weeks. Patients were also comprehensively compared in terms of protease inhibitor drug levels, HIV-specific immune responses at baseline, and the presence of drug resistance-conferring mutations.
Results
There was no statistically meaningful difference in first phase clearance of comparable high-level viremia in the two groups, whether protease inhibitor levels were inserted into the model or 100% antiviral drug effectiveness was assumed. In contrast, acute/early patients had inferior sustained responses than advanced patients, reflecting erratic adherence.
Conclusions
Despite many years of intervening immune destruction, the initial virologic decay on therapy appears to be the same at the extremes of the HIV disease spectrum.
Keywords: acute HIV infection, mathematical modeling, viral clearance, viral dynamics
Acute HIV-1 infection is associated with high-level viremia (≥1 × 106 HIV-1 RNA copies/ml) [1]. Even without medical interventions, viremia declines over 2–6 months, concurrent with development of HIV-specific immune responses [2–5] to approach a steady state in which viral production and clearance are relatively matched [6]. The natural history of untreated infection then involves years of clinical stability, followed by inexorable CD4+ T-cell count decline (reviewed in [7]). In the advanced immunosuppression setting, antiretroviral therapy (ART) results in rapid plasma viremia decline, CD4+ T-cell count improvement, and decreased disease progression and mortality [8]. Treatment during acute seroconversion also hastens the viremia decline beyond the natural equilibration seen in untreated patients [9,10], but there is no consensus regarding the long-term risks and benefits of early treatment [7,8,11].
Very limited data directly compare treatment responses among individuals with early and late stage disease. It remains unclear whether comparably high viremia levels observed at the opposite ends of the disease spectrum would decline at the same rate in response to identical treatment interventions. Previously, we completed a pathogenesis-driven study involving both acute/early and highly advanced HIV-infected patients [12]. We revisited this unique dataset to compare and contrast treatment responses at the disease spectrum extremes. We hypothesized that virologic suppression would be more rapid and complete during acute than advanced disease, perhaps because of rescue of immune clearance mechanisms [13] or incomplete population of viral reservoirs [14].
Methods
All studies were approved by the Institutional Review Board. Eligibility required no prior ART, Karnofsky performance of at least 80, and safety laboratory results were within acceptable ranges. Acute/early patients met Acute Infection Early Disease Research Program (AIEDRP) diagnostic criteria. Acute patients had negative or indeterminant HIV serologies [enzyme-linked immunoassay (ELISA) and western blot] concurrent with plasma viral loads greater than 5000 HIV RNA copies/ml. Early patients were seropositive but had documented negative ELISA results less than 12 months prior or a current nonreactive ‘detuned’ ELISA (Blood Centers of the Pacific, San Francisco, California, USA) and a clinical history compatible with recent infection. Advanced disease patientswere seropositive with CD4+ T-cell counts less than 50.
All patients received open-label indinavir (1200 mg), nelfinavir (1250 mg), lamivudine (150 mg), and stavudine (30–40 mg) (depending on body weight), all twice daily. The patients returned each weekday the first week, then three times weekly for 3 weeks, twice weekly for 4 weeks, weekly for 4 weeks, twice monthly for 12 weeks, and monthly thereafter. Treatment modifications based on standard of care were allowed; alternative drugs were not provided by the protocol.
Plasma for viral load assessments and HIV drug resistance genotyping was separated and frozen at −85°C. Larger blood draws for banking of cryopreserved peripheral blood mononuclear cells (PBMC) and CD4+ T-cell quantitation were obtained twice at baseline, weekly for 4 weeks, twice monthly for 8 weeks, and then monthly. Plasma HIV RNA assays were performed using Amplicor HIV-1 Monitor (Version 1.0; Roche Molecular Systems, Alameda, California USA, and Branchburg, New Jersey; limit of detection <50 copies/ml). Virologic responses were defined as at least two consecutive results less than 50 copies/ml. Time to virologic response was calculated as weeks required to achieve the first of consecutive results less than 50 copies/ml. Virologic relapses were defined as at least two consecutive results greater than 1000 copies/ml.
Statistical significance for group comparisons was calculated using χ2 test, Fisher's exact test, Mann–Whitney test, or t-test.
Plasma drug level assays
Plasma samples for determination of protease inhibitor concentrations were collected within 2–4 h of morning doses at days 7, 10, 14, 17, 21, 28, 42, 63, and 77. Assays for indinavir, nelfinavir, and the nelfinavir M8 metabolite were performed using validated high-performance liquid chromatography (HPLC)-UV methodology [15].
HIV drug resistance assay
HIV drug-resistance genotyping testing was performed for all at baseline, and for those who experienced virologic relapses, as previously described [16], and results were interpreted in accordance with International AIDS Society–USA Drug Resistance Mutations Group recommendations (October 2003 version; www.iasusa.org).
HIV-specific immune response assays
Interferon (IFN)-γ ELISpot assays for HIV-specific T-cell responses was performed at baseline by stimulating 105 PBMCs overnight with HIV-1 peptides in culture as described previously [17].
Mathematical modeling and statistical analysis
A previously published and validated viral kinetic model predicts that viral load will decline on 100% effective combination therapy as [18]
in which V0 is the baseline viral load, δ is the death rate of short-lived productively infected cells, μ is the death rate of long-lived infected cells, τ is the delay before viral load decline is observed, c is the virion clearance rate, k is the infection rate constant, T0 is the baseline CD4 cell count, and N is the viral burst size.
Results
Acute/early (n = 18) and advanced (n = 6) patients had distinctly different CD4 cell count ranges (T0 in Table 1). Using nonlinear least squares regression, we fitted patient data to the model given by Eq. (1) and estimated V0, the baseline viral load; δ, the death rate of short-lived productively infected cells; μ, the death rate of long-lived infected cells; τ, the delay; and the composite parameter NkT0. Virion clearance occurred too rapidly to estimate c. Thus, c was held constant at the previously determined mean value c = 23/day [19].
Table 1.
Parameter estimates determined by fitting Eq. (1) to the viral load data for 16 patients in the early group and six patients in the advanced group.
| T0 (cells/ml) | log(V0) (per ml) | δ (per day) | μ (per day) | τ (day) | |
|---|---|---|---|---|---|
| P1 | 361 | 5.7 | 1.08 | 0.040 | 2.39 |
| P2 | 140 | 5.6 | 0.89 | 0.033 | 6.78 |
| P3 | 249 | 6.0 | 0.99 | 0.030 | 1.10 |
| P4 | 408 | 5.4 | 0.50 | 0.022 | 0.99 |
| P5 | 774 | 5.0 | 0.53 | 0.043 | 0 |
| P7 | 870 | 5.0 | 1.31 | 0.058 | 0.98 |
| P8 | 518 | 5.3 | 0.41 | 0.076 | 0 |
| P9 | 446 | 5.4 | 0.80 | 0.044 | 0.95 |
| P10 | 244 | 5.3 | 1.28 | 0.11 | 3.43 |
| P11 | 250 | 5.8 | 0.39 | 0.050 | 2 |
| P12 | 238 | 5.9 | 0.50 | 0.025 | 0 |
| P13 | 514 | 4.5 | 0.57 | 0.027 | 1 |
| P14 | 502 | 5.6 | 0.31 | 0.051 | 0 |
| P16 | 304 | 6.5 | 1.04 | 0.056 | 0.80 |
| P17 | 408 | 6.7 | 0.70 | 0.027 | 0.17 |
| P18 | 832 | 4.9 | 0.67 | 0.057 | 0.84 |
| Early/mean | 441 | 5.54 | 0.75 | 0.047 | 1.35 |
| Early/SD | 221 | 0.57 | 0.32 | 0.023 | 1.71 |
| Early/median | 408 | 5.50 | 0.69 | 0.044 | 0.97 |
| P1 | 25 | 5.3 | 0.83 | 0.039 | 0.98 |
| P2 | 5 | 5.4 | 0.95 | 0.089 | 2.00 |
| P3 | 6 | 5.0 | 0.56 | 0.103 | 2.99 |
| P4 | 19 | 5.5 | 1.38 | 0.052 | 2.67 |
| P5 | 15 | 6.0 | 0.72 | 0.043 | 0.08 |
| P6 | 8 | 5.9 | 0.50 | 0.052 | 1.99 |
| Advanced/mean | 13 | 5.52 | 0.82 | 0.063 | 1.79 |
| Advanced/SD | 8.02 | 0.38 | 0.32 | 0.026 | 1.08 |
| Advanced/median | 11.5 | 5.45 | 0.78 | 0.052 | 2.00 |
The baseline viral load, V0, and first and second phase decay rates, which are determined by δ and μ, respectively, are not significantly different between the groups. T0, baseline CD4 cell count; τ, delay before viral load decline.
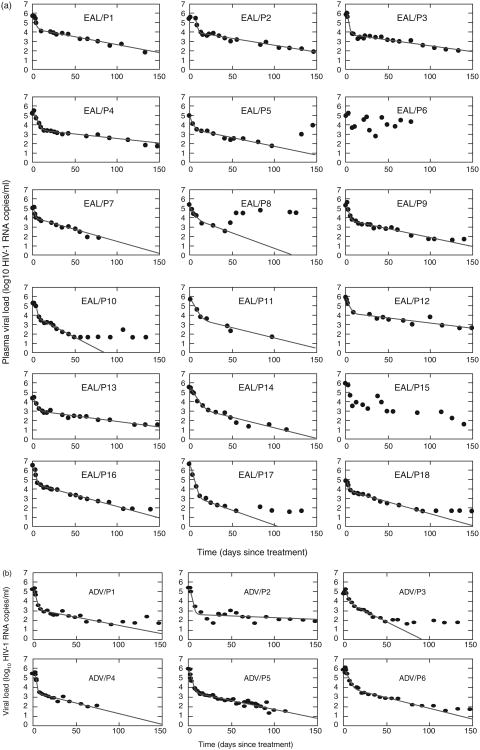
For the first phase decay characterized by δ, δ = 0.75 ± 0.32/day for early/acute versus δ = 0.82 ± 0.32 /day for advanced patients. For the second phase of decay, μ = 0.047 ± 0.023/day for the early and μ = 0.063 ± 0.026/day for the advanced group. There were no significant differences in first and second viral decay characteristics for recently infected versus advanced patients (P = 0.32 for δ and P = 0.11 for μ, t-test) (Fig. 1).
Fig. 1. Best fit of Eq. (1) (solid line) to the HIV-1 RNA data (black dots) obtained from patients.
(a) Patients with early/acute infection. (b) Patients with advanced AIDS. In (a) the data from patients 6 and 15 did not follow the expected monotonic decline and were not fit; these were the only participants who had interruptions in therapy during the first 40 days (both experienced major psychosocial events during the early treatment period). Patient 8 reported excellent initial adherence but attempted suicide and stopped therapy on day 42; thus, whereas his treatment response is not prolonged there were sufficient data points prior to day 40 to establish good fit with the model. The best fit values of δ (per day), μ (per day), and τ (days) are summarized in Tables 1 and 2.
Although CD4+ cell count changes initially were more dynamic in acute/early (fluctuating +/− 200–600 cells) than advanced patients, a substantial median increase occurred in both groups over 24 weeks [+168 (+37%) versus +75 (+577%) cells/μl in acute/early and advanced, respectively].
Mean indinavir levels were higher among advanced (4928 ng/ml) than early (3194 ng/ml) patients. More early patients had substantial indinavir level variability (>100 fold) (6/17 early versus 1/6 advanced patients) and more experienced undetectable indinavir levels (5/17 early versus 0/6 advanced patients). We examined whether there was a correlation between average drug levels during the first 10 days and the δ estimated assuming 100% drug efficacy. Prior work has shown that if drug efficacy ε is included, then the first phase decay slope is ∼εδ, in which ε = 1 means 100% efficacy [20,21]. Thus, as shown in [21], one would expect a correlation between drug efficacy and first phase viral decay slope. Here we found no significant difference in first phase clearance between groups, although there was a trend in advanced patients for higher mean protease inhibitor levels to correlate with more rapid virologic suppression [for indinavir (r = 0.82) and nelfinavir (r = 0.42) but not the nelfinavir metabolite (r = −0.24)]. Overall, patients with cases of undetectable levels of either protease inhibitor had longer mean time to treatment response (19.6 weeks) [8–24] compared with patients with always-detectable levels (15.6 weeks) [7–24].
HIV-specific immune responses
Acute/early patients had greater HIV-specific T-cell responses at every target site tested, with trends toward more responses and greater magnitudes of spot-forming units (SFU) per response within each target region. No responses were detected within Pol or Env among advanced patients , and for Env this result was significantly different from the early group [by Mann–Whitney for response magnitude (140 versus 0 SFU, P = 0.04) and by Fisher's exact test for response number (11/17 versus 0/6, P = 0.02)]. Overall, immune response intensity did not correlate with time to virologic response (data not shown).
Antiviral drug resistance
Four acute/early patients and four advanced patients had 1 –3 protease gene mutations associated with drug resistance (combinations of M36I, L63P, A71V, and L10I). Three acute/early (versus zero of advanced) patients had reverse transcriptase mutations known to confer resistance (V118I; T215S; M41L and T215S). No individual had concurrent protease inhibitor-resistance and reverse transcriptase-resistance conferring mutations. There was no evidence that these ‘minor’ resistance-conferring mutations adversely impacted initial virologic response. On serial testing of the acute/early patients with incomplete or delayed virologic responses, in no case were newly acquired or evolved resistance-conferring mutations seen. No patient had evidence of treatment failure triggering repeat resistance testing.
Extended follow-up
Although initial viral load decay rates were not significantly different for acute/early versus advanced disease patients, four patients in the acute/early group did not achieve less than 50 copies (all achieved <1000) within 24 weeks. Time to virologic response was 17.8 weeks [7–24] for acute/ early versus 14.3 weeks [11–21] for advanced patients (P = 0.22). Mean time to less than 500 (<2.70 log10) and less than 5000 (<3.70 log10) copies per milliliter was 11.2 weeks [5–18] versus 5.7 [3–11] weeks (P = 0.05) and 3.1 weeks [1–7] versus 1.7 [1–3] weeks (P = 0.07), respectively. Time-to-virologic response correlated with neither peak viral load nor baseline CD4 count (data not shown). These longer term differences were not explained by more effective pathogen-specific cellular immune responses or antiviral drug resistance. Instead, trends suggest erratic adherence over time in the acute/early group (including in the context of suicide and drug overdose attempts, homelessness, and relationship discord).
Discussion
Mathematical modeling of HIV RNA declines in acute/early patients and patients with advanced disease treated with the same therapy demonstrated that the first and second phases of treatment-enhanced viral clearance are not different at the extremes of the HIV disease spectrum, despite years of intervening viral replication and T-cell depletion. Further, both groups had favorable responses in terms of absolute CD4+ T-cell counts, during the study and over longer-term follow-up.
Putter et al. [10] previously reported a slower relative decline on therapy (involving various drug combinations) during primary versus chronic infection. These authors speculated that differences were due to an immature immune response in primary infection, enabling longer persistence of productively infected cells. In contrast, our analyses suggest a greater magnitude of HIV-specific immune responses, as measured by IFN-γ ELISpot assays, in acute/early cases. These findings support Little et al. who showed that the first viral decay phase in patients treated during acute HIV infection was comparable with that reported for chronically infected patients [22]. This prior study, however, did not involve chronically infected patients treated in parallel for direct comparisons of treatment responses to the same intervention. Data from representative subsets of acute/ early and chronic patients treated with the same drug regimens from the study by Louie et al. [21] also demonstrate no difference in first-phase decay rates (Table 2) [25–28]. Table 2 compares viral kinetic results found in a variety of studies in which drug was given to patients presumably at or near set-point viral loads so that Eq. (1) and/or variants could be used to analyze the data. In general, the more potent the therapy, the faster the viral decline [21,23].
Table 2.
Viral decay rates reported from a variety of studies.
| Study | Disease stage | Therapy | δ (per day) | μ (per day) |
|---|---|---|---|---|
| This study | Acute/early | IDV, NFV, LMV, d4T | 0.75 ± 0.32 | 0.047 ± 0.023 |
| This study | Advanced | IDV, NFV, LMV, d4T | 0.82 ± 0.32 | 0.063 ± 0.026 |
| Perelson et al. [18] | Chronic | NFV, ZDV, LMV | 0.70 ± 0.25 | 0.066 ± 0.038 |
| Perelson et al. [24] | Chronic | RTV | 0.49 ± 0.13 | |
| Louie et al. [21] | Acute/early | LPV, RTV, EFV, TDF, LMV | 0.52 ± 0.03 | |
| Louie et al. [21] | Chronic | LPV, RTV, EFV, TDF, LMV | 0.54 ± 0.07 | |
| Louie et al. [25] | Chronic | TDF | 0.48 ± 0.15 | |
| Markowitz et al. [26] | Chronic | LPV, RTV, EFV, LMV, TDF | 1.0 ± 0.3 | |
| Notermans et al. [23] | Chronic | RTV/ZDV/LMV | 0.61 ± 0.19 | |
| Kuritzkes et al. [27] | Chronic | EFV, ZDV, LMV | 0.67 | 0.055 |
| Murray et al. [28] | Chronic | raltegravir | 0.58 | 0.035 |
ABC, abacavir; d4T, stavudine; EFV, efavirenz; TDF, tenofovir disoproxil fumarate; IDV, indinavir; LMV, lamivudine; LPV, lopinavir; NFV, nelfinavir; RTV, ritonavir; ZDV, zidovudine.
The study design employed here allowed us to explore detailed, clinically relevant comparisons despite the limited number of patients . The regimen utilized in this protocol was difficult to tolerate; indinavir and nelfinavir are now rarely combined because of overlapping toxicities. The poor regimen tolerability compromised our ability to define sustained optimal treatment responses, but does not call into question the first and second phase kinetics analyses, because all patients initiated the same regimen and the kinetics are largely determined by early viral responses [18].
Although sustained viral load suppression on therapy may be differentially affected by diverse factors (host immune responses, antiviral drug resistance, latent cellular reservoirs, and perhaps most importantly, psychosocial and behavioral determinants of adherence), our findings do not suggest a difference in initial plasma viral load decay rates when therapy is initiated early versus late in infection.
Acknowledgments
This study was supported by the NIH Acute Infection and Early Disease Research Program (AI49126 and AI41530) and UAB General Clinical Research Center (NCRR MO1 RR00032). Bristol–Myers Squibb and Agouron Pharmaceuticals provided drug and partial funding support for the clinical trial. Glaxo Smith Kline and Merck Pharmaceuticals provided drugs for the clinical trial. The AACTG virology (V.A.J.) and pharmacology (E.P.A.) laboratories are supported by the NIH/NIAID Adult AIDS Clinical Trials Group (U01AI38858). V.A.J. acknowledges the following grant support from NIH grants AI32775 and U01AI38858. V.A.J. also acknowledges Birmingham Veterans Affairs Medical Center and CFAR core laboratory research facilities, and infrastructure and resources provided by the NIH CFAR Core Grant P30 AI27767. A.S.P. acknowledges support from NIH grants AI28433 and RR06555.
References
- 1.Clark SJ, Saag MS, Decker WD, Campbell-Hill S, Roberson JL, Veldkamp PJ, et al. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991;324:954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 5.Richmann DD, Wrin T, Little SJ, Petopoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann GR, Cunningham P, Kelleher AD, Zaunders J, Carr A, Vizzard J, et al. Patterns of viral dynamics during primary human immunodeficiency virus type 1 infection. The Sydney Primary HIV Infection Study Group. J Infect Dis. 1998;178:1812–1815. doi: 10.1086/314480. [DOI] [PubMed] [Google Scholar]
- 7.Pilcher CD, Eron JJ, Jr, Galvin S, Gay C, Cohen MS. Acute HIV revisited: new opportunities for treatment and prevention. J Clin Invest. 2004;113:937–945. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz M, Vesanen M, Tenner-Racz K, Cao Y, Binley JM, Talal A, et al. The effect of commencing combination antire-troviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:527–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 10.Putter H, Prins JM, Jurriaans S, Roos M, Ferguson NM, van Praag R, et al. Slower decline of plasma HIV-1 RNA following highly suppressive antiretroviral therapy in primary compared with chronic infection. AIDS. 2000;14:2831–2839. doi: 10.1097/00002030-200012220-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kinloch-de Loes S. Treatment of acute HIV-1 infection: is it coming of age? J Infect Dis. 2006;194:721–724. doi: 10.1086/506625. [DOI] [PubMed] [Google Scholar]
- 12.Hockett RD, Kilby JM, Derdeyn CA, Saag MS, Sillers M, Squires K, et al. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J Exp Med. 1999;189:1545–1554. doi: 10.1084/jem.189.10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 15.Turner ML, Reed-Walker K, King JR, Acosta EP. Simultaneous determination of nine antiretroviral compounds in human plasma using liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:331–341. doi: 10.1016/s1570-0232(02)00822-x. [DOI] [PubMed] [Google Scholar]
- 16.Kuritzkes DR, Grant RM, Feorino P, Griswold M, Hoover M, Young R, et al. Performance characteristics of the TRUGENE HIV-1 Genotyping Kit and the Opengene DNA Sequencing System. J Clin Microbiol. 2003;41:1594–1599. doi: 10.1128/JCM.41.4.1594-1599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 19.Ramratnam B, Bonhoeffer S, Binley J, Hurley A, Zhang L, Mittler JE, et al. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet. 1999;354:1782–1785. doi: 10.1016/S0140-6736(99)02035-8. [DOI] [PubMed] [Google Scholar]
- 20.Perelson AS, Nelson P. Mathematical analysis of HIV-1 dynamics in vivo. SIAM Rev. 1999;41:3–44. [Google Scholar]
- 21.Louie M, Hogan C, Di Mascio M, Hurley A, Simon V, Rooney J, et al. Determining the relative efficacy of highly active anti-retroviral therapy. J Infect Dis. 2003;187:896–900. doi: 10.1086/368164. [DOI] [PubMed] [Google Scholar]
- 22.Little SJ, McLean AR, Spina CA, Richman DD, Havlir DV. Viral dynamics of acute HIV-1 infection. J Exp Med. 1999;190:841–850. doi: 10.1084/jem.190.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Notermans DW, Goudsmit J, Danner SA, de Wolf F, Perelson AS, Mittler J. Rate of HIV-1 decline following antiretroviral therapy is related to viral load at baseline and drug regimen. AIDS. 1998;12:1483–1490. doi: 10.1097/00002030-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 25.Louie M, Hogan C, Hurley A, Simon V, Chung C, Padte N, et al. Determining the antiviral activity of tenofovir disoproxil fumarate in treatment-naïve chronically HIV-1-infected individuals. AIDS. 2003;17:1151–1156. doi: 10.1097/00002030-200305230-00006. [DOI] [PubMed] [Google Scholar]
- 26.Markowitz M, Louie M, Hurley A, Sun E, Di Mascio M, Perelson AS, Ho DD. A novel antiviral intervention results in more accurate assessment of human immunodeficiency virus type 1 replication dynamics and T cell decay in vivo. J Virol. 2003;77:5037–5038. doi: 10.1128/JVI.77.8.5037-5038.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuritzkes DR, Ribaudo HJ, Squires KE, Koletar SL, Santana J, Riddler SA, et al. Plasma HIV-1 RNA dynamics in antiretroviral-naïve subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166s. J Infect Dis. 2007;195:1169–1176. doi: 10.1086/512619. [DOI] [PubMed] [Google Scholar]
- 28.Murray JM, Emery S, Kelleher AD, Law M, Chen J, Hazuda DJ, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS. 2007;21:2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]