Abstract
Background & Aims
Tumor cells express vascular endothelial growth factor (VEGF), which induces angiogenesis. VEGF also activates VEGF receptors (VEGFRs) on or within tumor cells to promote their proliferation in an autocrine fashion. We studied the mechanisms of autocrine VEGF signaling in Barrett’s esophagus cells.
Methods
Using Barrett’s epithelial cell lines, we measured VEGF and VEGFR mRNA and protein, and studied the effects of VEGF signaling on cell proliferation and VEGF secretion. We studied the effects of inhibiting factors in this pathway on levels of phosphorylated phospholipase Cγ1 (PLCG1), protein kinase C (PKC), and extracellular signal-regulated kinases (ERK)1/2. We performed immunohistochemical analysis of phosphorylated VEGFR2 on esophageal adenocarcinoma tissues. We studied effects of sunitinib, a VEGFR2 inhibitor, on proliferation of neoplastic cells and growth of xenograft tumors in mice.
Results
Neoplastic and non-neoplastic Barrett’s cells expressed VEGF and VEGFR2 mRNA and protein, with higher levels in neoplastic cells. Incubation with recombinant human (rh)VEGF significantly increased secretion of VEGF protein and cell number; knockdown of PLCG1 markedly reduced the rhVEGF-stimulated increase in levels of phosphorylated PLCG1 and phosphorylated ERK1/2 in neoplastic cells. Esophageal adenocarcinoma tissues showed immunostaining for phosphorylated VEGFR2. Sunitinib inhibited VEGF signaling in neoplastic cells, and reduced weight and volume of xenograft tumors in mice.
Conclusions
Neoplastic and non-neoplastic Barrett’s epithelial cells have autocrine VEGF signaling. In neoplastic Barrett’s cells, VEGF activation of VEGFR2 initiates a PLCG1–PKC–ERK pathway that promotes proliferation and is self-sustaining (by causing more VEGF production). Strategies to reduce autocrine VEGF signaling (e.g. with sunitinib) might be used to prevent or treat cancer in patients with Barrett’s esophagus.
Keywords: vascular endothelial growth factor receptor 2, esophageal cancer, apoptosis, mouse model
Introduction
In the United States, there has been an alarming increase in the incidence of esophageal adenocarcinoma over the past few decades.1 Barrett’s esophagus, the condition in which esophageal squamous epithelium that has been damaged by gastroesophageal reflux is replaced by an intestinal-type columnar metaplasia, is the main precursor lesion for esophageal adenocarcinoma.2 Treatment for this cancer often involves a combination of chemotherapy, radiation therapy, and surgery.3;4 Despite recent advances in multimodality therapy, however, the overall 5-year survival rate for patients with esophageal adenocarcinoma remains below 15%.5 Clearly, better agents are needed to treat this lethal tumor.
Cancers develop in Barrett’s metaplasia through the accumulation of numerous genetic and epigenetic alterations that enable cells to acquire the physiologic hallmarks of cancer.6 Among these key physiologic traits is the ability to induce angiogenesis, the formation of new vascular supplies. Angiogenesis is regulated largely by signaling through vascular endothelial growth factor (VEGF) and its receptors, VEGFR1 (Flt-1) and VEGFR2 (Flk-1).7 Tumor cells produce VEGF, which interacts with VEGFRs on stromal and endothelial cells. This interaction results in the recruitment of endothelial progenitor cells to the region surrounding the tumor mass.7 The resultant neovascularization supplies the nutrients needed to sustain tumor growth.
In addition to the well known effects of VEGF on endothelial cells in angiogenesis, recent data suggest that autocrine VEGF signaling in tumor cells plays an important role in promoting their proliferation and inhibiting apoptosis. Many different human tumor cell types have been found to co-express VEGF and its receptors.8 Autocrine signaling occurs when VEGF secreted by the tumor cell binds VEGF receptors on the cell surface. Autocrine VEGF signaling has been linked with activation and nuclear translocation of VEGFR2 in primary endothelial cells.8 Autocrine effects of VEGF signaling appear to contribute importantly to tumorigenesis in K5-SOS transgenic mice, which develop epidermal skin tumors with high levels of both VEGF and VEGFR1.9 When VEGFR1 is genetically knocked out in the epidermal cells (but not in the endothelial cells) of these mice, they exhibit a decrease in cell proliferation and in the formation of skin tumors. The fact that the anti-tumor effects of VEGFR1 knockout occur in an angiogenesis-independent manner suggests that autocrine VEGF signaling is important in this animal model.9
A number of studies have documented cytoplasmic expression of VEGF in the epithelial cells of non-dysplastic Barrett’s metaplasia, and VEGF expression has been found to increase during the neoplastic progression of Barrett’s metaplasia through dysplasia to adenocarcinoma.10;11 Robust expression of VEGFR2 and weak expression of VEGFR1 has been found on endothelial cells lining new blood vessels in non-dysplastic Barrett’s mucosa. 12 However, few data are available on epithelial cell expression of VEGFRs in Barrett’s carcinogenesis. Moreover, the role of VEGF signaling in Barrett’s epithelial cells has not been investigated in Barrett’s carcinogenesis. Using neoplastic and non-neoplastic Barrett’s epithelial cell lines, we studied the molecular mechanisms of VEGF produced by Barrett’s cells on their growth, proliferation, and apoptosis, and we explored the contribution of VEGFR2 inhibition in mediating those effects in vitro using the cell lines and in vivo using tumor xenografts.
Materials and methods
Cell Lines
We used: 1) non-neoplastic, telomerase-immortalized Barrett’s epithelial (BAR-T) cell lines that were developed in our laboratory, 2) Barrett’s cell lines that we transformed by knockdown of p53 and expression of oncogenic H-Ras (P13R1 and P13R2), and 3) adenocarcinoma cell lines [OE33, JH-EsoAd1, FLO-1, (Supplemental methods)]. 13–16
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNAs were isolated from cell lines and subjected to real-time PCR for VEGF and VEGFR mRNA expression (Supplemental methods and Supplemental table 1).
Total Protein Extraction and Immunoblotting
Total protein was extracted from cultured cells and fresh human tissue specimens, analyzed using Western blot and quantified by densitometry (Supplemental methods and Supplemental table 2).
Promoter Reporter Gene Assays
The mouse VEGF (−807/+118) and VEGFR2 (−620/+304) promoter regions were inserted into the firefly luciferase reporter plasmid pGL3-Basic (Promega, Madison, WI). Data were expressed as fold luciferase activity calculated as a ratio to empty vector control (Supplemental methods).
Cell Growth, Proliferation, Apoptosis, and Enzyme-Linked Immunosorbent Assays (ELISA)
Cells numbers were determined using a Z1 particle counter (Beckman Coulter, Fullerton, CA). For proliferation assays, cells were labeled with bromodeoxyuridine (BrdU) for 6 hours using a Cell Proliferation Assay Kit per the manufacturer’s instructions (Calbiochem, Gibbstown, NJ). Apoptosis rates were measured by flow cytometry (FACScaliber, Becton Dickson, Franklin Lake, NJ) using Annexin-V. VEGF concentrations were determined using a commercially available ELISA (Invitrogen, Camarillo, CA) per manufacturer’s instructions (Supplemental methods).
Inhibition of VEGF/VEFGR2 Signaling
Cells were treated with (a) the VEGFR2 inhibitor SU1498 at doses ranging from 5 to 20 μM, (b) a VEGF neutralization antibody at 6 μg/ml, (c) sunitinib at doses of 5 and 10μM, or (d) specific VEGFR2 siRNA or shRNA (Supplemental methods).
Inhibition of the PLC Signaling Pathway
Cells were treated with the PLC-γ inhibitor U73122 (Tocris Bioscience, Ellisville, MO) at 2.5 or 5 μM doses or specific PLC-γ1 siRNA (Thermo Fisher Scientific) (Supplemental methods).
Activation of VEGF Pathway Signaling
Cells were treated with 30 ng/ml of recombinant human VEGF (rhVEGF) for times ranging from 0–6 hours (Supplemental methods).
Immunofluorescence
Cells were seeded at a density of 1 × 105 cells per well onto glass cover slips and incubated with primary antibodies against total and phospho-VEGFR2 (Supplemental methods and Supplemental table 2).
Immunohistochemistry
Human esophageal adenocarcinoma tissue arrays purchased from US Biomax (BC0211; Rockville, MD) containing 20 esophageal adenocarcinoma tissues with 20 adjacent, histologically-normal esophageal epithelial tissues were stained with phospho-VEGFR2. Tumor masses from xenograft experiments were processed using standard histological procedures, and viable areas of the tumor were evaluated for CD31 and Ki67 staining (Supplemental methods and Supplemental table 2).
Tumor Xenografts
All procedures were approved by the Institutional Animal Care and Use Committee at the Dallas VA Medical Center. P13R1 cells or P13R1 cells (population or clone B3) that were infected with VEGFR2 shRNA were suspended in 50% Matrigel and 50% culture medium and implanted subcutaneously into the dorsal skin of female NOD/SCID mice (6 weeks old; Taconic, Hudson, NY). For sunitinib studies, when mice developed a palpable mass (≥.5 cm in greatest diameter), they were administered a daily oral gavage with either 4 mg/kg sunitinib (Sutent®, Pfizer) or vehicle-only solution. Tumor size was measured twice a week with calipers, and volumes for total tumor burden and index tumors were calculated based on a published method (Supplemental methods). 17
Data Analyses
All the experiments were repeated at least twice and each experiment was performed in duplicate or triplicate. Data analyses were performed as described in Supplemental methods. P values ≤ 0.05 were considered significant for all analyses.
Results
Increased VEGF and VEGFR2 mRNA and protein expression in transformed Barrett’s and OE33 adenocarcinoma cells
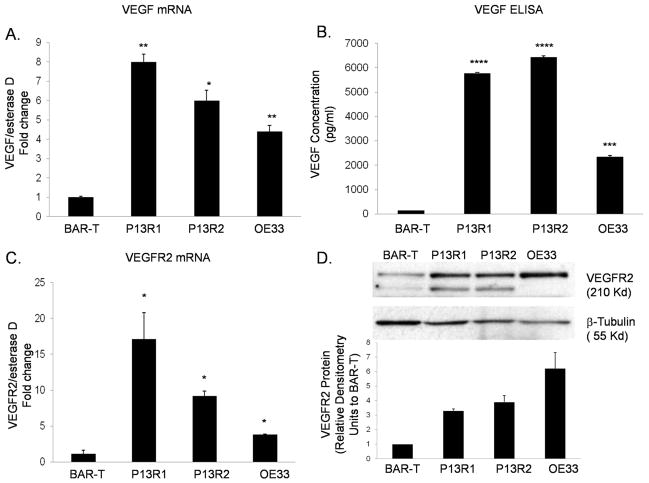
Earlier studies using immunohistochemical staining of biopsy specimens showed that Barrett’s epithelial cells express more VEGF than esophageal squamous cells, and that esophageal adenocarcinoma cells express even greater VEGF levels than benign Barrett’s cells. 11 To determine if these differences in VEGF expression levels were recapitulated in our benign and neoplastic Barrett’s cell lines, we measured their VEGF promoter activity, and their mRNA and protein expression levels. We found that VEGF mRNA levels and promoter activity were significantly higher in transformed Barrett’s and OE33 cancer cells than in non-neoplastic BAR-T cells (Figure 1A and Supplemental Figure 1A), suggesting that the increased mRNA expression in the neoplastic cells was due to increased transcriptional activity. Since secretion of VEGF out of the cell is required for autocrine signaling, we measured the amount of VEGF protein secreted in the media using an ELISA assay. Secretion levels of VEGF protein were minimal in BAR-T cells, and significantly greater in the transformed Barrett’s and OE33 cells (Figure 1B). We found minimal expression of VEGFR1 mRNA and protein in all the cell lines, with no significant difference between the BAR-T and neoplastic cells (data not shown). In contrast, VEGFR2 mRNA expression levels and transcriptional activities were significantly higher in transformed Barrett’s and OE33 cells than in BAR-T cells (Figure 1C and Supplemental Figure 1B). VEGFR2 protein expression levels were 3 to 6 fold higher in transformed Barrett’s and OE33 cells than in BAR-T cells (Figure 1D). Staining by immunofluorescence demonstrated membranous and cytoplasmic expression of total VEGFR2 in all cell lines (Supplemental Figure 1C).
Figure 1.
Upregulation of VEGF (A&B) and VEGFR2 (C&D) mRNA and protein in transformed Barrett’s and OE33 adenocarcinoma cells. Data are the means ± SEM of at least 2 separate experiments. *, p≤ 0.05; **, p≤ 0.01; ***, p≤ 0.001; ****, p≤ 0.0001 compared to BAR-T cells.
Transformed Barrett’s and adenocarcinoma cells are more sensitive to the growth suppressive effects of VEGF/VEGFR2 inhibition than non-neoplastic Barrett’s cells
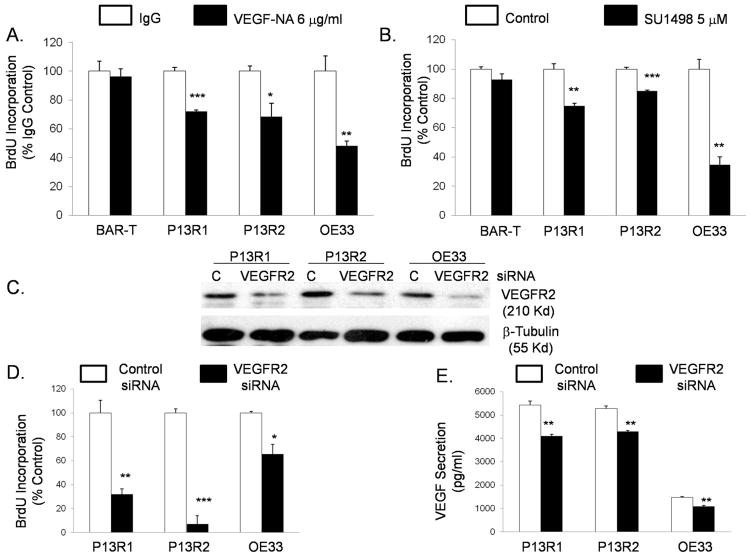
To determine whether VEGF signaling affects Barrett’s cell growth, we treated our cells with a VEGF-neutralization antibody (NA). VEGF-NA had no effect on BAR-T cell number, but significantly decreased transformed Barrett’s (P13R1 and P13R2) and OE33 cell numbers (Supplemental Figure 2A). We next treated BAR-T and P13R1 cells with the VEGFR2 inhibitor SU1498 at concentrations ranging from 5 to 20 μM. Concentrations of SU1498 ≥10 μM caused a significant, dose-dependent decrease in BAR-T cell number, whereas significant decreases in P13R1 cell number were observed with SU1498 in concentrations as low as 5 μM (Supplemental Figure 2B). SU1498 in a concentration of 5 μM also caused significant reductions in P13R2 and OE33 cell numbers (Supplemental Figure 2C). These findings show that neoplastic Barrett’s cells are more sensitive to the growth suppressive effects of VEGF/VEGFR2 inhibition than benign Barrett’s cells, and suggest that inhibition of VEGF/VEGFR2 signaling might be an effective therapeutic target for Barrett’s-associated neoplasms.
Epithelial cells of human esophageal adenocarcinomas and their matching non-neoplastic esophageal squamous tissues demonstrate VEGF/VEGFR2 signaling
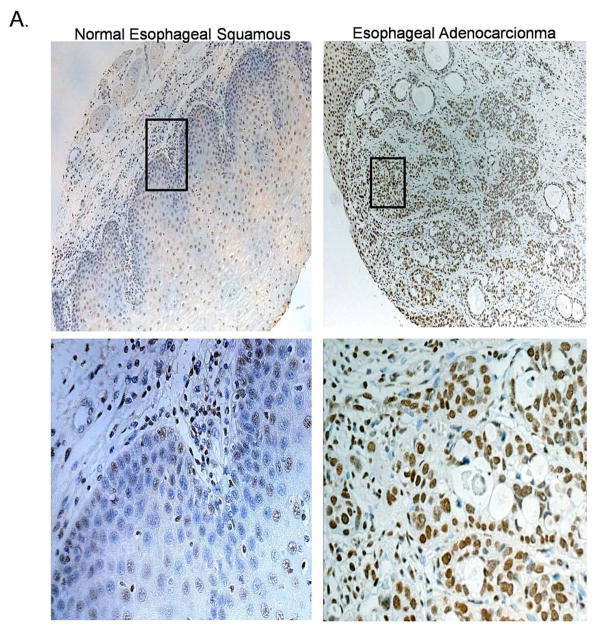
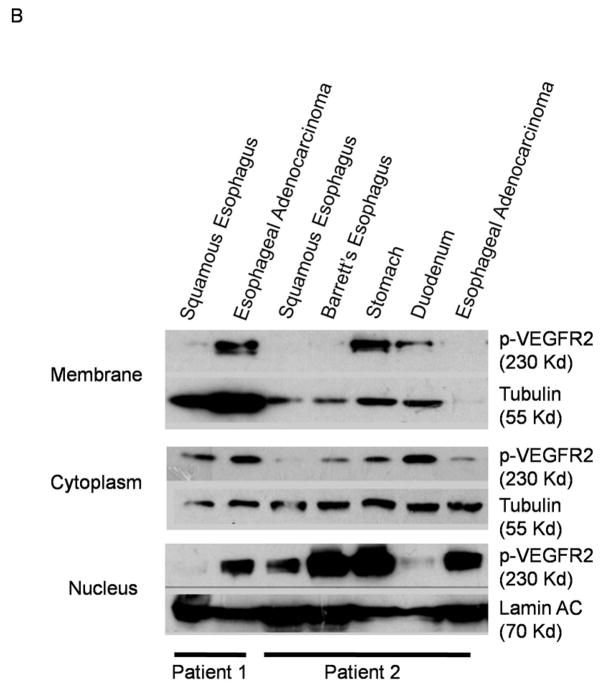
To determine whether VEGF/VEGFR2 signaling occurs in primary esophageal adenocarcinomas as well as in neoplastic Barrett’s cell lines, we performed immunohistochemistry for phosphorylated VEGFR2 on 20 esophageal adenocarcinoma tissues, and on their 20 matching (from the same patients) and histologically normal-appearing esophageal squamous tissues. When activated by VEGF, VEGFR2 becomes phosphorylated and moves from the surface membrane into the cytoplasm and nucleus.8 We found phospho-VEGFR2 predominantly in the nucleus, with lower levels in the cytoplasm of epithelial cells in both the non-neoplastic squamous epithelium (Figure 2A left panels) and in the esophageal adenocarcinomas (Figure 2A right panels). This finding was confirmed using fresh human esophageal adenocarcinoma tissues (Figure 2B). Approximately 40% of adenocarcinoma and squamous tissues were negative for phospho-VEGFR2 expression. In the esophageal adenocarcinoma tissues, staining intensity for phospho-VEGFR2 was strong in 12.5% whereas none of the histologically normal tissues had strong staining for this phospho-protein.
Figure 2.
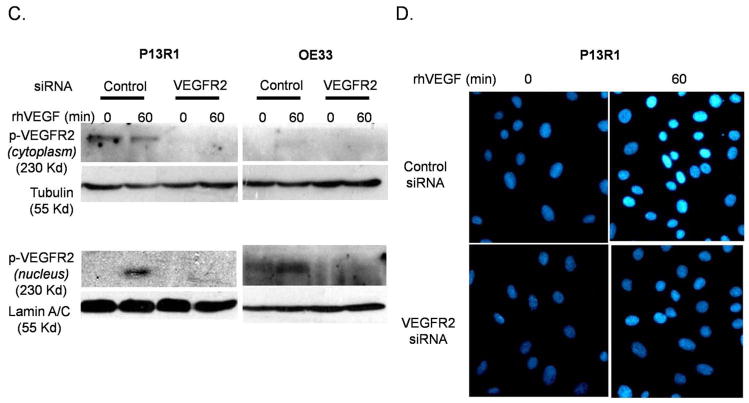
(A) Representative photomicrographs showing expression of phospho-VEGFR2 in an esophageal adenocarcinoma (right panels) and in histologically-normal esophageal squamous epithelium (left panels) from the same patient. Lower panels show high magnification (400X) of the boxed area in the upper panels (100X). (B) Phospho-VEGFR2 expression in fresh tissue biopsies from two patients. (C&D) By 60 minutes, treatment with rhVEGF causes nuclear translocation of phospho-VEGFR2 in transformed Barrett’s (P13R1) and OE33 cells containing control siRNA, but not VEGFR2 siRNA as detected by (B) Western blot and (C) immunofluorescence. VEGFR2 was stained with anti-human phospho-VEGFR2 antibody (turquoise) and nuclei were counterstained with DAPI (blue).
To determine if VEGF signaling causes nuclear translocation of phospho-VEGFR2 in neoplastic Barrett’s cells, P13R1 and OE33 cells were treated with rhVEGF, and subcellular fractionation and immunofluorescence for VEGFR2 were performed. In addition, cells were transfected with either control or VEGFR2 siRNA. P13R1 cells containing control siRNA demonstrated baseline phospho-VEGFR2 in the cytoplasm, but not in the nucleus, whereas OE33 adenocarcinoma cells demonstrated minimal nuclear and no cytoplasmic expression of phospho-VEGFR2 (Figure 2C). Following treatment with rhVEGF, there was a marked increase in nuclear phospho-VEGFR2 expression levels in both P13R1 and OE33 cells. In contrast, we observed no cytoplasmic or nuclear expression of phospho-VEGFR2 either at baseline or following treatment with rhVEGF in the P13R1 and OE33 cells that contained the VEGFR2 siRNA (Figure 2D). Following treatment with rhVEGF, there was intense nuclear immunofluorescence staining of phospho-VEGFR2 in cells expressing the control siRNA, but not in cells containing the specific VEGFR2 siRNA (Figure 2C).
Inhibition of VEGF/VEGFR2 signaling decreases proliferation and VEGF secretion in transformed Barrett’s and OE33 adenocarcinoma cells
To explore the mechanism underlying the growth suppressive effects of VEGF/VEGFR2 inhibition, we studied cell proliferation and apoptosis. Both the VEGF-NA and 5 μM SU1498 had no significant effect on BrdU incorporation in BAR-T cells, but significantly decreased BrdU incorporation in transformed Barrett’s and OE33 cells (Figure 3 A&B). Compared to controls, we found no significant changes in rates of apoptosis after treating cells with VEGF-NA or with 5 μM SU1498 (data not shown). Since pharmacologic inhibitors can have non-specific effects, we used a specific VEGFR2 siRNA to confirm our findings. Knock-down of VEGFR2 protein in transformed and OE33 cells was confirmed by western blotting (Figure 3C). VEGFR2 siRNA caused a significant decrease in BrdU incorporation in all the cell lines (Figure 3D), confirming that inhibition of VEGFR2 decreases proliferation in transformed Barrett’s and OE33 cells. VEGFR2 siRNA also caused a significant decrease in VEGF protein secretion in all the cell lines (Figure 3E), demonstrating that VEGFR2 signaling regulates secretion of its own ligand. These findings suggest that VEGF secretion by neoplastic Barrett’s cells may be contributing to cell proliferation by binding to VEGFR2 in an autocrine manner.
Figure 3.
Treatment with (A) VEGF neutralization antibody (VEGF-NA), (B) SU1498, and (D) VEGFR2 siRNA causes a reduction in BrdU incorporation in transformed Barrett’s and OE33 cells. (E) VEGFR2 siRNA decreases VEGF secretion by the neoplastic cells. Data are means ± SEM of 3 separate experiments. *, p≤ 0.05; **, p≤ 0.01; ***, p≤ 0.001 compared to corresponding controls. (C) Representative Western blot demonstrating VEGFR2 knockdown by siRNA.
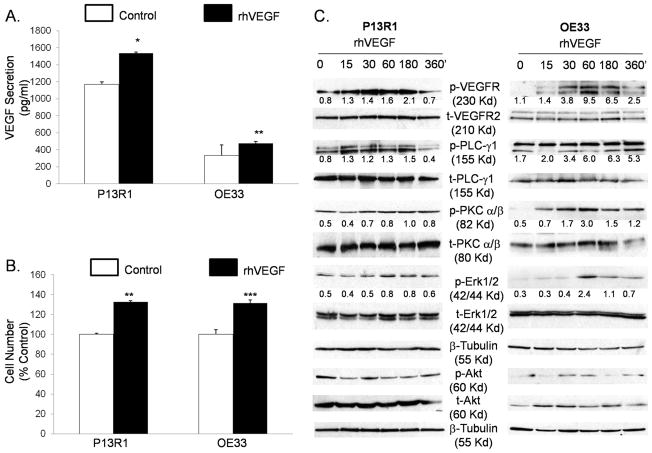
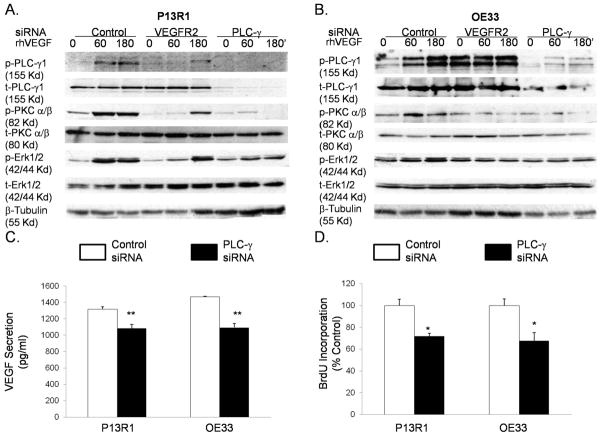
Treatment with VEGF stimulates it own production, increases proliferation, and signals through a PLC-PKC-ERK1/2 pathway in transformed Barrett’s and OE33 adenocarcinoma cells
To confirm that VEGF can stimulate its own production and increase cell growth by activating VEGFR2, we treated P13R1 and OE33 cells with rhVEGF and measured VEGF secretion and cell number. Treatment with rhVEGF caused a significant increase in VEGF protein secretion (Figure 4A) and cell number (Figure 4B) in both neoplastic cell lines. To explore the VEGFR2 signaling pathways whereby VEGF exerts its effects, we performed Western blotting for phospho- and total PLC-γ1, PKC-α/β, ERK1/2, and Akt proteins. Phosphorylation of VEGFR2 increased after 15 minutes of rhVEGF treatment, and peaked between 30 and 180 minutes in both cell lines (Figure 4C). Increased levels of phospho-proteins PLC-γ1, PKC-α/β, and ERK1/2 were detected after VEGF treatment in both cell lines (Figure 4C). In contrast, phospho-Akt expression levels were either unchanged, slightly decreased (P13R1) or only slightly increased [OE33, (Figures 4C)]. To confirm these findings, we knocked down VEGFR2 with siRNA before stimulating with rhVEGF. As expected, cells with control siRNAs showed an increase in phospho-PLC-γ1, PKC-α/β, and ERK1/2 expression (Figure 5A & B). In contrast, P13R1 cells containing VEGFR2 siRNA demonstrated a delayed and substantially reduced increase in these phospho-proteins, whereas OE33 cells with VEGFR2 siRNA showed no increase in any of the phospho-proteins (Figure 5A&B). Taken together, these results suggest that activated VEGFR2 signals primarily through the PLC, PKC, and ERK1/2 pathways in transformed Barrett’s and OE33 adenocarcinoma cells.
Figure 4.
Treatment with rhVEGF causes an increase in (A) VEGF secretion and (B) cell number in transformed Barrett’s and OE33 cells. Data are the means ± SEM of 3 separate experiments. *, p≤ 0.05; **, p≤ 0.01; ***, p≤ 0.001 compared to corresponding controls. (C) Representative Western blots demonstrating phosphorylation of VEGFR2, PLC-γ1, PKC-α/β, and ERK1/2, and Akt by rhVEGF in the neoplastic cells. Numbers represent the relative quantity of protein with respect to the loading control.
Figure 5.
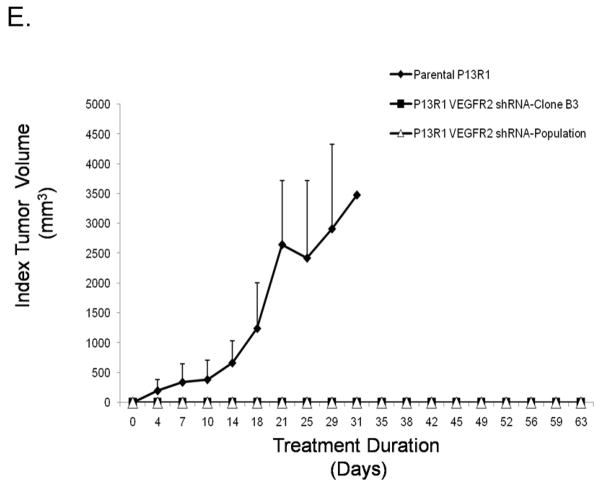
Representative Western blots demonstrating that siRNAs against VEGFR2 and PLC-γ decrease rhVEGF-induced phospho-PLC-γ1, PKC-α/β, and ERK1/2 in (A) transformed Barrett’s and (B) OE33 cells. PLC-γ siRNA decreases (C) VEGF secretion and (D) BrdU incorporation in transformed Barrett’s and OE33 cells. Data are the means ± SEM of 3 separate experiments. *, p≤ 0.05; **, p≤ 0.01 compared to corresponding controls. (E) Mouse xenografts of transformed, parental P13R1 cells (without VEGFR2 shRNA) exhibited robust tumor growth, whereas both the population and selected clone (B3) of P13R1 cells with VEGFR2 knockdown exhibited no tumor growth whatsoever.
VEGFR2 can activate ERK1/2 directly through Ras or indirectly via the PLC/PKC pathway.18 To explore the mechanism of ERK1/2 phosphorylation, we knocked down PLC-γ1 using siRNA, and then stimulated the cells with rhVEGF. Compared to control cells, PLC-γ1 knockdown cells demonstrated marked reductions in rhVEGF-stimulated phospho-PKC-α/β and -ERK1/2 expression levels (Figure 5 A&B). These findings suggest that VEGFR2 activates ERK1/2 indirectly through the PLC/PKC pathway in transformed Barrett’s and OE33 adenocarcinoma cells.
We next confirmed that the PLC/PKC signaling pathway was responsible for the VEGF-induced increase in proliferation and VEGF production that we had previously observed. Treatment with U73122, a pharmacologic inhibitor of PLC-γ, caused a significant decrease in VEGF secretion and cell number in both P13R1 and OE33 cells (Supplementary Figure 3A&B). Similarly, reductions in VEGF secretion and BrdU incorporation were observed in both cell lines with PLC-γ1siRNA (Figure 5C&D). These data show that VEGF binding to VEGFR2 activates signaling via a PLC-PKC-ERK pathway that stimulates production of its ligand (VEGF) and promotes cell proliferation in neoplastic Barrett’s cells.
To explore the effects of VEGFR2 knockdown on tumor growth in vivo, we produced a stable line of P13R1 cells infected with VEGFR2 shRNA, and confirmed knockdown of VEGFR2 by Western blot both in the population and in a selected clone (B3, data not shown). We injected these VEGFR2 shRNA cell lines and the parental P13R1 cells (without VEGFR2 shRNA) subcutaneously into NOD/SCID mice. Unlike the parental cells, xenografts of the population and of the B3 clone of P13R1 cells with VEGFR2 knockdown exhibited no tumor growth whatsoever (Figure 5E). These findings suggest that VEGFR2 signaling is required for tumor growth of neoplastic Barrett’s cells in vivo.
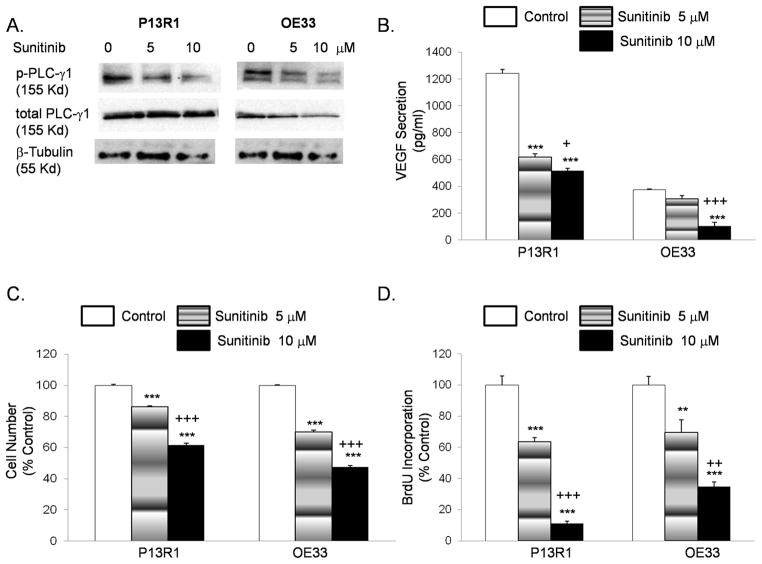
Sunitinib decreases phospho-PLC-γ1 protein expression and inhibits cell proliferation and VEGF protein secretion in transformed Barrett’s and OE33 adenocarcinoma cells
Sunitinib is a small-molecule, multi-target receptor tyrosine kinase inhibitor that includes VEGFR2 among its targets. Treatment with sunitinib decreased phospho-PLC-γ1 protein in P13R1 and OE33 cells in a dose-dependent fashion (Figure 6A). These same doses of sunitinib significantly decreased VEGF protein secretion, cell number, and BrdU incorporation in a dose-dependent fashion in transformed Barrett’s and OE33 adenocarcinoma cells (Figure 6B–D). Sunitinib also significantly reduced VEGF protein secretion and cell number in two additional adenocarcinoma cell lines, JH-EsoAd1 and FLO-1 (Supplemental Figure 4).
Figure 6.
Sunitinib decreases (A) phospho-PLC-γ1 protein expression, (B) VEGF secretion, (C) cell number and (D) BrdU incorporation in a dose dependent manner in transformed Barrett’s and OE33 cells. Data are the means ± SEM of at least 3 separate experiments. **, p≤ 0.01; ***, p≤ 0.001 compared to corresponding controls; +, p≤ 0.05; ++, p≤ 0.01; +++, p≤ 0.001 compared to corresponding 5 μM treated cells.
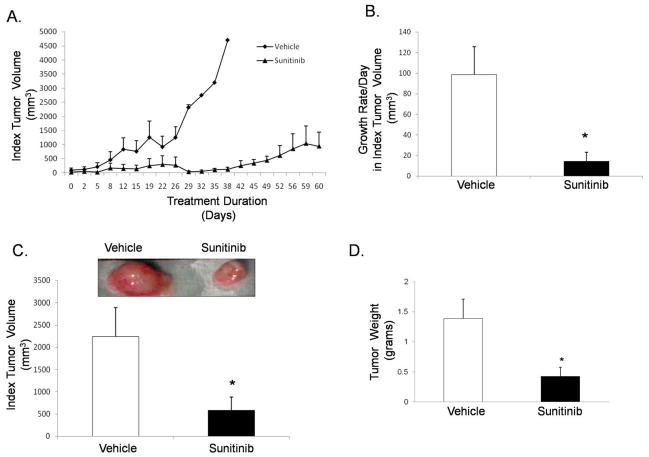
Sunitinib delays tumor growth and decreases tumor volume in mouse xenografts
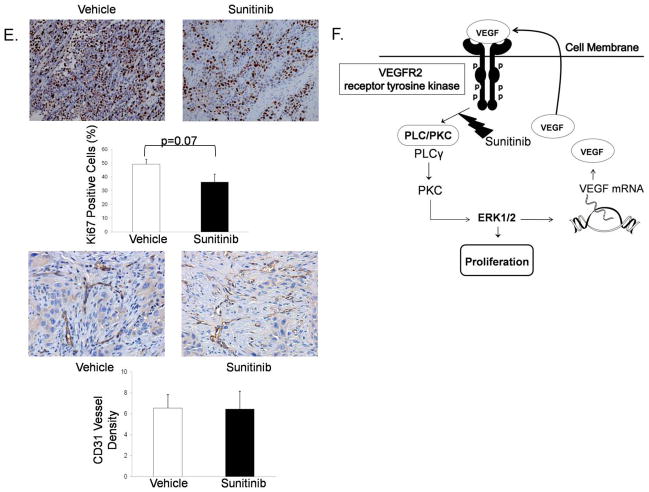
We next injected P13R1 cells subcutaneously into NOD/SCID mice. We selected P13R1 because it forms tumors within 10 weeks of inoculation, allowing for a long time course over which to assess the effects of sunitinib. When mice first developed a palpable (≥.5cm) mass (the index tumor), they were treated with either sunitinib (4 mg/kg/day) or vehicle solution daily until sacrifice. Compared to vehicle-treated controls, sunitinib-treated mice demonstrated a delay in growth of the index tumor (Figure 7A). The growth rate per day in index tumor volume (Figure 7B) and the index tumor volume at the time of sacrifice (Figure 7C) also were significantly lower in the sunitinib-treated mice. At sacrifice, the average total tumor weight (i.e. the weight of the index tumor plus any additional tumors) for sunitinib-treated mice was significantly lower than that of vehicle-treated control mice (Figure 7D). Sunitinib-treated tumors had lower numbers of Ki-67 staining cells and exhibited a similar density of microvessels compared to the vehicle-treated controls (Figure 7E). Finally, sunitinib-treated tumors had decreased phospho-PLC-γ1 protein and exhibited no apparent differences in expression of VEGF protein compared to the vehicle-treated controls (Supplemental Figure 5A&B). A schematic model summarizing our findings is provided in Figure 7F.
Figure 7.
In mouse xenografts of transformed Barrett’s cells, sunitinib (A) delays index tumor growth, (B) decreases growth rate per day in index tumor volume, and (C) decreases index tumor volume at sacrifice. Sunitinib-treated tumors have (D) lower total tumor weights, (E) lower numbers of Ki-67 positive staining cells and no difference in microvessel density compared to vehicle-treated tumors. *, p≤ 0.05 compared to vehicle. (F) Schematic model demonstrating the pro-proliferative effect of autocrine VEGF/VEGFR2 signaling and sunitinib’s inhibition of this pathway in neoplastic Barrett’s cells.
Discussion
We have shown that both neoplastic and non-neoplastic Barrett’s epithelial cells produce VEGF that contributes to their growth by activating VEGFR2. However, the neoplastic Barrett’s cells express more VEGF and VEGFR2, and are more sensitive to the growth-suppressive effects of VEGF/VEGFR2 inhibition than their non-neoplastic counterparts. We have found robust nuclear and cytoplasmic staining for active, phosphorylated VEGFR2 in the epithelial cells of human esophageal adenocarcinoma tissues, further supporting a role for VEGF signaling in the growth of those tumors. In neoplastic Barrett’s cells, we have demonstrated that VEGF activation of VEGFR2 initiates a PLC-PKC-ERK pathway that is both pro-proliferative and self-sustaining in that it causes the production of more VEGF. We have shown that sunitinib, a multi-target tyrosine kinase inhibitor, inhibits PLC signaling in neoplastic Barrett’s cells, which reduces their proliferation and decreases their VEGF production. Finally, in mice with tumor xenografts, we have shown that knockdown of VEGFR2 abolishes tumor growth, and that treatment with sunitinib delays tumor growth and decreases tumor weight and volume.
The role of VEGF in inducing angiogenesis has been appreciated for decades. Hypoxia is a potent stimulus for VEGF production, and it is conceivable that local tissue hypoxia induced by reflux esophagitis underlies the increased VEGF protein expression and accompanying neovascularization that have been described in non-dysplastic Barrett’s metaplasia.12 Both benign and neoplastic Barrett’s epithelial cells have been found to express high levels of VEGF that correlate with local vessel density.19 Recent studies suggest that VEGF produced by tumor cells also can activate receptors on or within the tumor cells themselves to sustain growth in an angiogenesis-independent manner.9 Our study provides evidence for angiogenesis-independent, pro-proliferative VEGF effects in both neoplastic and non-neoplastic Barrett’s epithelial cells. We have shown that these cells demonstrate transcriptional activation of VEGF mRNA and protein secretion, with the highest levels in the neoplastic cells. The finding that our cell lines recapitulate the differences in epithelial VEGF expression that have been observed in biopsy specimens of benign and neoplastic Barrett’s tissues suggests that they are appropriate models for the study of VEGF signaling in Barrett’s esophagus.11
When activated by VEGF, VEGFR2 becomes phosphorylated and then moves from the surface membrane into the cell cytoplasm and nucleus.8 VEGF signaling through VEGFR2 is not restricted to endothelial cells, and appears to occur frequently in both benign and malignant cells of many different types. Stewart et al. used immunohistochemistry to study phospho-VEGFR2 localization in a panel of normal human tissues from 18 different organs (not including esophagus), and in tumor tissues from 5 different organs (also not including esophagus).20 They found phosphorylated VEGFR2 in the cytoplasm and nucleus of disparate cell types in both the normal and tumor tissues from all organs evaluated, with more intense staining in the tumors. In our study, we found cytoplasmic and nuclear immunostaining for phospho-VEGFR2 in both esophageal adenocarcinoma tissues and in the accompanying esophageal squamous epithelium from the same patients, generally with stronger staining in the tumors. Although the squamous epithelium from these patients appears normal histologically, it has been reported that such squamous tissues can exhibit a carcinogenic “field effect”. 21 Compared to the squamous esophagus of healthy controls, for example, the squamous esophagus of patients with esophageal adenocarcinoma can show increased expression of VEGF mRNA.21 Thus, it is possible that the VEGF/VEGFR2 signaling that we observed in the histologically-normal squamous tissues of the adenocarcinoma patients might be exaggerated due to a carcinogenic field effect. Further studies on VEGF/VEGFR2 signaling in esophageal squamous tissue from healthy controls are warranted.
When we treated our neoplastic Barrett’s cells with VEGF, we found prominent nuclear translocation of phospho-VEGFR2. In primary endothelial cells, VEGF binding of VEGFR2 has been shown to induce its phosphorylation and translocation to the nucleus, where the VEGFR2 binds its promoter to regulate its own transcription.8 Thus, nuclear translocation of VEGFR2 might serve to amplify the angiogenesis and cell proliferation effects induced by VEGF activation.8 Further studies are needed to establish the role of nuclear VEGFR2 in Barrett’s-associated neoplasia.
In both neoplastic and non-neoplastic Barrett’s cell lines, we found that 48 hours of treatment with the VEGFR2 inhibitor SU1498 decreased cell numbers, suggesting that autocrine VEGF/VEGFR2 signaling contributes to epithelial cell growth in both benign and neoplastic Barrett’s metaplasia. This autocrine signaling appears to be especially important for the growth of neoplastic Barrett’s cells, however. Compared to non-neoplastic Barrett’s cells, growth suppression was achieved with lower concentrations of SU1498 in neoplastic Barrett’s cells, and inhibition of their VEGF signaling through multiple methods (VEGF-neutralizing antibody, pharmacologic VEGFR2 inhibition, VEGFR2 siRNA) decreased VEGF production and cell proliferation without significant effects on apoptosis. Recently, Chatterjee et al. reported that autocrine VEGF/VEGFR2 signaling is required for the initiation of tumor growth in vivo. 22 This observation is supported by our finding that VEGFR2 knockdown in transformed Barrett’s cells abolished their ability to form tumors in immunodeficient mice. Our study suggests that agents that target VEGFR2 might be used to prevent or treat cancer in Barrett’s esophagus.
To delineate the downstream molecular events in VEGF signaling, we stimulated our neoplastic Barrett’s cells with rhVEGF and observed the effects of a series of pharmacologic inhibitors and siRNAs. In support of our observation that VEGF signaling affects cell proliferation but not apoptosis, we found that VEGF induced the pro-proliferative PLC/PKC and ERK1/2 pathways, but not the pro-survival Akt pathway. VEGFR2 is known to activate ERK1/2 directly through Ras, or indirectly via the PLC/PKC pathway.18 Our observation that siRNA against PLC-γ1 markedly reduced expression of phospho-ERK1/2 suggests that VEGFR2 activates ERK1/2 indirectly (via PLC) in neoplastic Barrett’s cells. In primary endothelial cells, Takahashi et al. showed that VEGF-induced PLC-γ activation was required both for ERK activation and for cell proliferation. 23 In agreement with those data, we found that inhibition of PLC-γ1 decreased phosphorylated ERK expression and cell number in our neoplastic Barrett’s cells.
Although drugs that target VEGF signaling traditionally have been called “anti-angiogenesis agents”, our study suggests that these agents might have substantial angiogenesis-independent effects on cancer in Barrett’s esophagus. Some so-called anti-angiogenesis agents have established efficacy in the treatment of metastatic colorectal cancer, metastatic renal cell carcinoma, and hepatocellular carcinoma, but these agents have not prolonged survival for patients with advanced esophagogastric cancers in Phase II and III clinical trials.24 The anti-angiogenesis agent used in most of those clinical trials was bevacizumab, a monoclonal antibody directed against VEGF (Reviewed in 24).
In malignancies that exhibit autocrine VEGF signaling, agents that target primarily extracellular VEGF (e.g. bevacizumab) are not as effective as those that can target intracellular VEGF signaling (e.g. sunitinib, a small-molecule inhibitor of VEGFR kinase). 9 In our neoplastic Barrett’s cells (which exhibit autocrine VEGF signaling), we found that sunitinib decreased levels of phospho-PLC-γ1, VEGF secretion, and cell proliferation in a dose-dependent manner. Sunitinib treatment of mice bearing xenografts of transformed Barrett’s cells resulted in a significant delay in tumor growth and a significant reduction in tumor volume and weight. Moreover, xenografts from sunitinib-treated mice demonstrated decreased protein levels of phospho-PLC-γ1, but not VEGF. The failure of sunitinib to reduce VEGF levels is not surprising because, in tumor cells in vivo, VEGF production can be stimulated by a number of factors in addition to autocrine VEGF signaling including cell stress and inflammatory cytokines. 25 Thus, our study suggest that intracellularly-targeted inhibitors of VEGF signaling such as sunitinib have a potential role as anti-tumor agents for patients with Barrett’s-associated neoplasms.
Sunitinib currently is used clinically for the initial treatment of advanced renal cell carcinoma, for second-line treatment of gastrointestinal stromal tumors, and as one of the molecularly-targeted therapies available for progressive pancreatic neuroendocrine tumors. In a study of patients with advanced esophageal adenocarcinomas that had not responded to conventional chemotherapy, sunitinib plus paclitaxel did not appear to prolong progression-free survival. 26 It is conceivable that the prior chemotherapy might have altered the tumors’ responsiveness to sunitinib, and the results of this small, phase II trial cannot be considered definitive. Our study suggests that investigations still are warranted on the role of sunitinib and other inhibitors of VEGF signaling for patients with cancer in Barrett’s esophagus.
In conclusion, our study provides evidence for angiogenesis-independent, pro-proliferative VEGF effects in both neoplastic and non-neoplastic Barrett’s epithelial cells. We have demonstrated that VEGF produced by neoplastic Barrett’s cells activates their VEGFR2, thereby initiating a PLC-PKC-ERK pathway that causes cell proliferation and the production of more VEGF. Our finding that neoplastic Barrett’s cells are highly sensitive to the effects of VEGF/VEGFR2 inhibition with drugs like sunitinib suggests that agents targeting molecules involved in autocrine VEGF signaling might be used to prevent or treat cancer in Barrett’s esophagus.
Supplementary Material
Acknowledgments
This work was supported by the Office of Medical Research, Departments of Veterans Affairs (R.F.S., S.J.S., D.H.W.), VISN 17 Start-Up Award (Q.Z.), the National Institutes of Health (R01-DK63621, R01-CA134571 to R.F.S. and S.J.S., K12 HD-068369-01 to E.C.), and the American Gastroenterological Association Institute (Fellow to Faculty Transition Award to E.C.)
Abbreviations
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- PLC
protein lipase C
- PKC
protein kinase C
- ERK
extracellular signal-regulated kinases
- PI3-kinase
phosphoinositol 3 kinase
- MAPK
mitogen activated protein kinase
- PCR
polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
- VEGF-NA
vascular endothelial growth factor neutralization antibody
- rhVEGF
recombinant human vascular endothelial growth factor
- SEM
standard error of the mean
Footnotes
Financial Disclosures: No conflicts of interest exist for any of the authors.
Authors names in bold designate shared co-first authorship.
Author Contributions:
Q. Zhang: study design; technical and material support; analysis and interpretation of data; critical revision of manuscript; important intellectual content; drafting of manuscript
Yu: study design; technical and material support; analysis and interpretation of data; important intellectual content
Peng: technical and material support; important intellectual content
Xu: technical and material support; important intellectual content
Wright: technical and material support; important intellectual content
X Zhang: technical and material support; important intellectual content
Huo: technical and material support; important intellectual content
Cheng: technical and material support; important intellectual content
Pham: technical and material support; important intellectual content
Asanuma: technical and material support; important intellectual content
Hatanpaa: technical and material support; important intellectual content
Rezai: technical and material support; important intellectual content
Wang: technical and material support; important intellectual content
Sarode: technical and material support; analysis and interpretation of data; important intellectual content
Melton: technical and material support; analysis and interpretation of data; important intellectual content
Genta: technical and material support; analysis and interpretation of data; important intellectual content
Spechler: study concept; analysis and interpretation of data; critical revision of manuscript; important intellectual content
Souza: study concept/design; analysis and interpretation of data; critical revision of manuscript; important intellectual content; drafting of manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468–1470. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 2.Souza RF, Spechler SJ. Concepts in the prevention of adenocarcinoma of the distal esophagus and proximal stomach. CA Cancer J Clin. 2005;55:334–351. doi: 10.3322/canjclin.55.6.334. [DOI] [PubMed] [Google Scholar]
- 3.Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 4.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 5.Varghese TK, Jr, Hofstetter WL, Rizk NP, Low DE, Darling GE, Watson TJ, Mitchell JD, Krasna MJ. The society of thoracic surgeons guidelines on the diagnosis and staging of patients with esophageal cancer. Ann Thorac Surg. 2013;96:346–356. doi: 10.1016/j.athoracsur.2013.02.069. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 8.Domingues I, Rino J, Demmers JA, de Lanerolle P, Santos SC. VEGFR2 translocates to the nucleus to regulate its own transcription. PLoS One. 2011;6:e25668. doi: 10.1371/journal.pone.0025668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Islam A, Banerjee S, Kambhampati S, Baranda J, Banerjee S, Weston AP, Saxena NK, Banerjee SK. Angiogenic switch in Barrett’s adenocarcinoma: the role of vascular endothelial growth factor. Front Biosci. 2006;11:2336–2348. doi: 10.2741/1973. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths EA, Pritchard SA, McGrath SM, Valentine HR, Price PM, Welch IM, West CM. Increasing expression of hypoxia-inducible proteins in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Br J Cancer. 2007;96:1377–1383. doi: 10.1038/sj.bjc.6603744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auvinen MI, Sihvo EI, Ruohtula T, Salminen JT, Koivistoinen A, Siivola P, Ronnholm R, Ramo JO, Bergman M, Salo JA. Incipient angiogenesis in Barrett’s epithelium and lymphangiogenesis in Barrett’s adenocarcinoma. J Clin Oncol. 2002;20:2971–2979. doi: 10.1200/JCO.2002.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Jaiswal KR, Morales CP, Feagins LA, Gandia KG, Zhang X, Zhang HY, Hormi-Carver K, Shen Y, Elder F, Ramirez RD, Sarosi GA, Jr, Spechler SJ, Souza RF. Characterization of telomerase-immortalized, non-neoplastic, human Barrett’s cell line (BAR-T) Dis Esophagus. 2007;20:256–264. doi: 10.1111/j.1442-2050.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Yu C, Wilson K, Zhang H, Melton SD, Huo X, Wang DH, Genta RM, Spechler SJ, Souza RF. Malignant transformation of non-neoplastic Barrett’s epithelial cells through well-defined genetic manipulations. PLoS One. 2010;5:e13093. doi: 10.1371/journal.pone.0013093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soldes OS, Kuick RD, Thompson IA, Hughes SJ, Orringer MB, Iannettoni MD, Hanash SM, Beer DG. Differential expression of Hsp27 in normal oesophagus, Barrett’s metaplasia and oesophageal adenocarcinomas. Br J Cancer. 1999;79:595–603. doi: 10.1038/sj.bjc.6690094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez H, Koorstra JB, Hong SM, Boonstra JJ, Dinjens WN, Foratiere AA, Wu TT, Montgomery E, Eshleman JR, Maitra A. Establishment and characterization of a bona fide Barrett esophagus-associated adenocarcinoma cell line. Cancer Biol Ther. 2008;7:1753–1755. doi: 10.4161/cbt.7.11.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holloway SE, Beck AW, Shivakumar L, Shih J, Fleming JB, Brekken RA. Selective blockade of vascular endothelial growth factor receptor 2 with an antibody against tumor-derived vascular endothelial growth factor controls the growth of human pancreatic adenocarcinoma xenografts. Ann Surg Oncol. 2006;13:1145–1155. doi: 10.1245/ASO.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 19.Kleespies A, Guba M, Jauch KW, Bruns CJ. Vascular endothelial growth factor in esophageal cancer. J Surg Oncol. 2004;87:95–104. doi: 10.1002/jso.20070. [DOI] [PubMed] [Google Scholar]
- 20.Stewart M, Turley H, Cook N, Pezzella F, Pillai G, Ogilvie D, Cartlidge S, Paterson D, Copley C, Kendrew J, Barnes C, Harris AL, Gatter KC. The angiogenic receptor KDR is widely distributed in human tissues and tumours and relocates intracellularly on phosphorylation. An immunohistochemical study. Histopathology. 2003;43:33–39. doi: 10.1046/j.1365-2559.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- 21.Brabender J, Marjoram P, Lord RV, Metzger R, Salonga D, Vallbohmer D, Schafer H, Danenberg KD, Danenberg PV, Selaru FM, Baldus SE, Holscher AH, Meltzer SJ, Schneider PM. The molecular signature of normal squamous esophageal epithelium identifies the presence of a field effect and can discriminate between patients with Barrett’s esophagus and patients with Barrett’s-associated adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14:2113–2117. doi: 10.1158/1055-9965.EPI-05-0014. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee S, Heukamp LC, Siobal M, Schottle J, Wieczorek C, Peifer M, Frasca D, Koker M, Konig K, Meder L, Rauh D, Buettner R, Wolf J, Brekken RA, Neumaier B, Christofori G, Thomas RK, Ullrich RT. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest. 2013;123:3183. doi: 10.1172/JCI65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 24.Okines AF, Reynolds AR, Cunningham D. Targeting angiogenesis in esophagogastric adenocarcinoma. Oncologist. 2011;16:844–858. doi: 10.1634/theoncologist.2010-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt JM, Sommers SR, Fisher W, Ansari R, Robin E, Koneru K, McClean J, Liu Z, Tong Y, Hanna N. Sunitinib plus paclitaxel in patients with advanced esophageal cancer: a phase II study from the Hoosier Oncology Group. J Thorac Oncol. 2012;7:760–763. doi: 10.1097/JTO.0b013e31824abc7c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.