Abstract
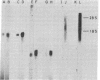
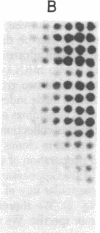
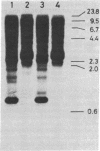
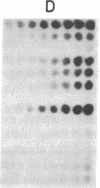
The sequences specifically transcribed in tumor cells are believed to be closely related to transformed phenotypes. For the isolation of such sequences, a cDNA clone library was constructed by using poly(A)+ RNAs from azo-dye-induced rat ascites hepatomas. Thirty-one tumor RNA-responsive clones were isolated by screening 4,000 clones of this library with conventional techniques, differential colony hybridization, and RNA blot hybridization. These clones were categorized into two groups with respect to their size distribution of mRNAs from which clones were derived. The first group was complementary to a single distinct species, either about 1.5 or 0.6 kilobases in length, of poly(A)+ RNA, and the second showed no distinct bands but a smear on a RNA blot. Semiquantitative RNA dot blot assays revealed that the sequences of these clones were expressed very little, if at all, in normal and regenerating livers, while generally high in ascites hepatomas. This specificity was also true for other solid lines of tumors, such as Morris hepatoma 5123D of Buffalo rat and Walker 256 carcinosarcoma of Wistar rat. The smear class sequences were transcribed from middle-repetitive sequences of DNA, indicating that a class of middle-repetitive sequences is specifically transcribed in tumor cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augenlicht L. H., Kobrin D. Cloning and screening of sequences expressed in a mouse colon tumor. Cancer Res. 1982 Mar;42(3):1088–1093. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B., Droller M. J., Baylin S. B., Nelkin B. D. Mutation affecting the 12th amino acid of the c-Ha-ras oncogene product occurs infrequently in human cancer. Science. 1983 Jun 10;220(4602):1175–1177. doi: 10.1126/science.6304875. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Activation of cellular genes by avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5351–5354. doi: 10.1073/pnas.77.9.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Interspersion of repetitive and single-copy sequences in nuclear ribonucleic acid of high molecular weight. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1108–1112. doi: 10.1073/pnas.71.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Birnie G. D. Post-transcriptional regulation of messenger abundance in rat liver and hepatoma. Nucleic Acids Res. 1980 Jul 25;8(14):3087–3103. doi: 10.1093/nar/8.14.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöchel W., Patel N. T., Holoubek V. Polysomal poly(A)+ RNA in liver of rats fed 3'-methyl-4-dimethylaminoazobenzene and in hepatoma induced by the same carcinogen. Biochim Biophys Acta. 1980;606(1):67–75. doi: 10.1016/0005-2787(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyzis R. K., Grady D. L., Li D. W., Mirvis S. E., Ts'o P. O. Extensive homology of nuclear ribonucleic acid and polysomal poly(adenylic acid) messenger ribonucleic acid between normal and neoplastically transformed cells. Biochemistry. 1980 Mar 4;19(5):821–832. doi: 10.1021/bi00546a001. [DOI] [PubMed] [Google Scholar]
- ODASHIMA S. ESTABLISHMENT OF ASCITES HEPATOMAS IN THE RAT, 1951-1962. Natl Cancer Inst Monogr. 1964 Dec;16:51–93. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Shiosaka T., Saunders G. F. Differential expression of selected genes in human leukemia leukocytes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4668–4671. doi: 10.1073/pnas.79.15.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Supowit S. C., Rosen J. M. Gene expression in normal and neoplastic mammary tissue. Biochemistry. 1980 Jul 22;19(15):3452–3460. doi: 10.1021/bi00556a008. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Bloom F. E., Lerner R. A. Common 82-nucleotide sequence unique to brain RNA. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4942–4946. doi: 10.1073/pnas.79.16.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Hoffman R., Penman S. The extensive homology between mRNA sequences of normal and SV40-transformed human fibroblasts. Cell. 1977 Aug;11(4):901–907. doi: 10.1016/0092-8674(77)90301-4. [DOI] [PubMed] [Google Scholar]
- Zuker C., Lodish H. F. Repetitive DNA sequences cotranscribed with developmentally regulated Dictyostelium discoideum mRNAs. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5386–5390. doi: 10.1073/pnas.78.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]