Abstract
Our recent report demonstrated that a small subset of GABAergic interneurons in the cerebral cortex of rodents expresses Fos protein, a marker for neuronal activity, during slow wave sleep (Gerashchenko et al., 2008). The population of sleep-active neurons consists of strongly immunohistochemically-stained cells for the enzyme neuronal nitric oxide synthase. By virtue of their widespread localization within the cerebral cortex and their widespread projections to other cortical cell types, cortical neuronal nitric oxide synthase-positive neurons are positioned to play a central role in the local regulation of sleep waveforms within the cerebral cortex. Here, we review the possible functions of neuronal nitric oxide synthase and its diffusible gas product, nitric oxide, in regulating neuronal activity, synaptic plasticity and cerebral blood flow within the context of local sleep regulation in the cerebral cortex. We also summarize what is known, in addition to their expression of neuronal nitric oxide synthase, about the biochemical phenotype, synaptic connectivity and electrophysiological properties of this novel sleep-active population of cells. Finally, we raise some critical unanswered questions about the role of this population in local sleep regulation within the cerebral cortex and describe some experimental approaches that might be used to address those questions.
Keywords: Sleep, nitric oxide, interneurons, electroencephalographic slow waves, cerebral blood flow, neuropeptides, sleep homeostasis, synaptic plasticity
Identification of a Sleep-Active Population of Neurons in the Cerebral Cortex
The concept that there are discrete sleep-active neuronal populations - populations that exhibit high firing rates during sleep relative to wake - has been a common thread in sleep research for several decades. Sleep-active populations have historically been identified in subcortical areas through electrophysiological recordings (reviewed by Siegel, 2000; Szymusiak et al., 2001; Jacobs et al., 2002; Jones, 2004). In the past 15 years, subcortical sleep-active populations have also been identified immunohistochemically by the expression of immediate early genes, typically c-fos, in animals euthanized after sleep bouts (Sherin et al., 1996; Scammell et al., 2001; Gong et al., 2004).
Until recently, it was assumed that neurochemically-defined sleep-active populations exist only in a small number of subcortical sleep-regulatory nuclei and not in the cerebral cortex itself, where sleep electroencephalographic (EEG) waveforms are commonly recorded. Large-scale slow-wave activity (SWA) occurs in the cerebral cortex during slow-wave sleep (SWS; synonymous with non-rapid eye movement sleep in rodent behavioral state classification). The cortex has been generally conceptualized as having reduced cellular activity during SWS in comparison to wake; this may be true of excitatory cells, in particular. Regular spiking cells of the rat somatosensory cortex, most of which are excitatory glutamatergic cells, fire at lower rates during SWS (3.8 Hz on average) than quiet wake (4.7 Hz) and REMS (5.3 Hz). This rate of firing was slightly more than half of the firing rate during active wake (6.1 Hz; Vijayan et al., 2010). The firing rates of cortical neurons have been assessed across spontaneous sleep and wake states in cats (Steriade et al., 2001) and rats (Vyazovskiy et al., 2009). In a population of randomly sampled cells in cat cerebral cortex, the reduction of firing (from 15.7 Hz during wake to 11.4 Hz during SWS), was not statistically significant. However, when cells were classified into regular spiking (RS), fast-rhythmic bursting (FRB) and fast-spiking (FS) types based on their firing patterns, FS and FRB cells exhibited reduced activity during SWS relative to wake. The firing rates of FS cells (presumably, interneurons) during SWS was reduced to 61% of wake (14 Hz vs. 23 Hz); that of FRB cells (likely a mixed population of interneurons and pyramidal cells) was reduced to 50% of wake (7.5 Hz vs. 15 Hz; Steriade et al., 2001). Likewise, in the rat, the firing rate of cortical cells (the neurochemical and physiological properties of which were not defined) is higher by 40% in wake relative to SWS (Vyazovskiy et al., 2009).
However, reduced firing during SWS is not a universal property of cortical neurons. Nearly half (44%) of presumed excitatory RS neurons (corresponding to roughly 25% of all cortical neurons observed in a data set obtained in cats) were “wakesilent”, firing at higher rates during SWS and the SWS-to-wake transition than during active wake (Rudolph et al., 2007). Neurons that increase activity during SWS compared to wake have also been demonstrated in the guinea pig auditory cortex (Pena et al., 1999) and the monkey precentral gyrus (Evarts, 1964). Anatomical clustering of such neurons has been found only in the monkey subgenual cingulate cortex and may thus reflect a unique sleep-related role for that cortical region (Rolls et al., 2003).
Immunohistochemical data demonstrate considerably greater neuronal activity in the cerebral cortex during wake than sleep. Expression of the immediate early gene, c-fos (a marker of neuronal activity; Morgan and Curran, 1991), is much higher in the cerebral cortex when animals are euthanized after long wake bouts than after sleep bouts (O'Hara et al., 1993; Pompeiano et al., 1994; Cirelli et al., 1996). Thus, our recent report (Gerashchenko et al., 2008) of a neurochemically-defined population of cells that was active in the cerebral cortex during sleep was particularly noteworthy. Our report documented, in three rodent species, that a high percentage of the neurons in the cerebral cortex that are immunohistochemically positive for neuronal nitric oxide synthase (nNOS; also known as NOS-1, NOS1, or bNOS) express Fos protein when animals were euthanized after a period of sleep. Based on the suggestion of a reviewer of our 2008 paper, we will refer to this population as SANs (sleep-active neurons) hereafter. SANs expressed Fos in a time-of-day-dependent manner when animals underwent spontaneous sleep: the number of nNOS-positive cells expressing Fos is highest at the time of day when cerebral SWA is highest and is considerably lower at the time of day when SWA is lowest. To date, this is the only neurochemically-defined cortical neuron population that is activated in association with SWS. Other articles in this volume address the question of whether sleep serves a function, or is regulated, at the local level within the cerebral cortex. Here, we consider the possibility that SANs in the cerebral cortex serve an essential function within the cortical circuitry underlying SWS. The discussion is constrained by the limited information that is available on this population of cells at the present time. Therefore, we also raise some pressing questions about the SAN population and propose some methods that might be used to address them.
Neuronal NO as a Sleep-Inducing Factor: Local Regulation of Neuronal SWA
Since the expression of nNOS is one of few known features of SANs, one possibility is that NO produced by SANs is a regulator of SWA. In fact, NO meets some of the criteria for sleep regulatory substances (Gautier-Sauvigne et al., 2005). Systemic administration of the nNOS inhibitors 3-bromo-7-nitroindazole (Cavas and Navarro, 2006) and NG-nitro-L-arginine (L-NAME; Kapas et al., 1994b) during the light phase of the LD cycle reduced the amount of time spent in SWS, as did intracerebroventricular injection (Kapas et al., 1994a). However, when the same compound was administered at dark onset, only suppression of SWA occurred and time spent in SWS increased (Ribeiro and Kapas, 2005). This latter effect was hypothesized to result from the effect of the inhibitor on nNOS-immunoreactive cells present in the suprachiasmatic nucleus (Chen et al., 1997) whereas the elevation of SWS as a percentage of time, occurring early in the light phase when the drive for sleep is high, was presumably homeostatic in nature. Collectively, these results are compatible with a model in which SWS EEG SWA is facilitated by increased NO synthesis in sleep homeostatic regulatory circuits and suppressed by effects of NO on the circadian clock (Ribeiro and Kapas, 2005).
If NO is critical for the expression of sleep drive, then genetic inactivation of nNOS would be expected to attenuate the expression of sleep drive. Sleep has been studied in nNOS-deficient mice and this appears not to be the case. The time spent in each of the sleep states and in wake was not altered in nNOS-deficient animals relative to wild type mice. However, SWS delta power, a measure of SWA, was significantly greater in nNOS KO mice than in their wild type controls during all phases of the circadian cycle (Chen et al., 2003). The same authors also found that influenza-induced elevation of time spent in SWS was attenuated in nNOS-deficient mice (Chen et al., 2004). Thus, while nNOS may in fact suppress sleep SWA under baseline conditions, it appears to facilitate sleep in the context of viral infection. Whether the other isoforms of NOS (inducible and endothelial NOS) were upregulated in neurons or elsewhere as a compensatory reaction to nNOS knockout was not determined in the sleep studies on the nNOS knockout mice.
Biochemical and electrophysiological evidence is more supportive of an acute wake-promoting than a sleep-promoting role for nNOS. Upon its release by nNOS-positive cells, NO alters cell physiology by diffusing freely across cell membranes and activating a soluble guanylyl cyclase in target neurons (reviewed by Schuman and Madison, 1994). The guanylyl cyclase activated by NO synthesizes cyclic GMP, the consequences of which are manifestly excitatory in neurons involved in corticothalamic oscillations. NO inhibition by L-NAME decreases the firing of cortical and thalamic neurons (Cudeiro and Rivadulla, 1999). Application of the NO donor S-nitroso-N-acetyl-DL-penicillamine (SNAP), by contrast, increases neuronal firing (Cudeiro et al., 2000). In addition, firing elicited by sensory stimuli in visual cortex neurons is attenuated by a NOS inhibitor and potentiated by a NOS donor (Cudeiro et al., 2000). It is difficult to reconcile these data with the notion that NO promotes sleep. However, these pharmacological effects may be misleading with regard to the dynamics of NO activity. Whereas these pharmacological manipulations result in either a protracted decrease (L-NAME) or increase (SNAP) in NO levels, NO levels in vivo are highly labile, as the halflife of NO is less than one second (Barbosa et al., 2008). Rhythmic release of NO driven by rhythmic activation of nNOS-positive cells (see section below on Ca2+-dependence of NOS activity) may have quite different effects from tonic manipulations. So, the role of SANs in regulating sleep timing and sleep waveforms in the cerebral cortex remains unresolved by pharmacological, electrophysiological and genetic studies. The relevance of these studies is also limited by the fact that the manipulations were not cell type-specific. It is likely that individual effects of nNOS-deficiency in the suprachiasmatic nucleus, basal forebrain, SANs and other nNOS-positive sites have distinct effects, some of which are wake-promoting and others are sleep-promoting. These counterbalancing effects may, on the whole, prevent any significant sleep phenotype from being expressed. It will be necessary to specifically target interventions to the SAN population to resolve this matter.
Neuronal NOS as a Sleep-induced Factor: Regulation of Synaptic Plasticity and Cerebral Blood Flow
It is possible that rather than being a sleep-inducing factor, nNOS functions as a sleep-induced regulator of either synaptic function, sleep-related cerebral hemodynamics, or other functional concomitants of sleep. In addition to affecting neurotransmission acutely, NO has long-term effects on neuronal communication via its role in synaptic plasticity. NO-induced synthesis of the second messenger cyclic GMP can induce synaptic plasticity in the form of long-term potentiation (LTP) or long-term depression (LTD; reviewed by Schuman and Madison, 1994; Centonze et al., 1999). In the cerebral cortex during sleep, the latter scenario is more likely. nNOS is a calmodulin-sensitive enzyme (Knowles et al., 1989; Bredt and Snyder, 1990; Bredt et al., 1992) and its enzymatic activity is directly proportional to Ca2+ concentration in the cell (Tatsumi et al., 1998; Montgomery et al., 2000). The elevation of Fos in SANs during sleep is likely to be secondary to elevation of intracellular Ca2+, a major trigger for Fos expression (Morgan and Curran, 1991). Therefore, it is likely that there is a surge in Ca2+-dependent nNOS activity within these cells during SWS, paralleling Ca2+-dependent Fos expression. How does this increase relate to synaptic plasticity? The presumed elevation of NO due to Ca2+ influx in SANs occurs simultaneously with slow (approximately 1 Hz) oscillations between up and down states in nearby pyramidal cells, an electrophysiological hallmark of SWS (as described elsewhere in this issue by Timofeev). The coincidence of elevated NO concentration and 1 Hz cycles of synaptic input activation has been shown to induce long-term depression of both inhibitory (Le Roux et al., 2009) and excitatory (Huang and Hsu, 2010) inputs to and outputs from (Calabresi et al., 1999; Centonze et al., 1999) cortical pyramidal neurons, as well as in hippocampal circuits (Wu et al., 1998). By virtue of the coincident 1 Hz firing and elevation of NO during SWS, pyramidal cells are vulnerable to LTD.
Collectively, these data are compatible with a model in which SANs mediate synaptic LTD in a sleep-dependent manner (Figure 1). While this line of reasoning presents a set of tractable hypotheses, the specifics of the relationship between SWS and synaptic strength are not certain. While slow oscillations in the EEG may approximate a 1 Hz rhythm, their occurrence is irregular. Experimental stimulation paradigms that more accurately reflect the irregularity of in vivo activity patterns (i.e., Poisson-distributed stimuli with an average interstimulus interval of 1 sec) do not reliably cause LTD in pyramidal cells of the guinea pig cerebral cortex studied in vitro (Perrett et al., 2001). Furthermore, regular 1 Hz stimulation can induce LTP (i.e., an increase in synaptic strength), albeit subcortically in the amygdala or hippocampus, when delivered over protracted periods of time (reviewed by Habib and Dringenberg, 2010). Finally, whereas some of the above-cited studies on the relationship between NO and LTD involved brain tissues from immature animals less than 1 month old (Wu et al., 1998; Le Roux et al., 2009; Huang and Hsu, 2010), studies on the relationship between slow waves and synaptic strength have generally been made in more mature animals. Given that the EEG (Frank et al., 1998) and biochemical (Hairston et al., 2004) phenomena associated with sleep homeostasis in young animals are quite distinct from those in mature animals, caution is warranted in extrapolating from the in vitro work to the functional concomitants of SWS in vivo.
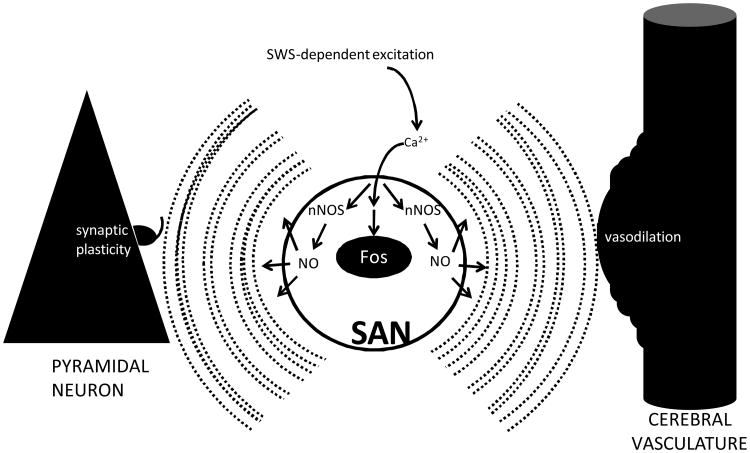
Figure 1.
Hypothetical model for the functions of SAN activation during SWS. At SWS onset, SANs are released from the tonic inhibition that they receive via monoaminergic and cholinergic inputs during wake. Excitation of SANs results in increased intracellular Ca2+ levels, which induces expression of Fos and activates nNOS. nNOS activation increases the concentration of NO within the cell. Being membrane permeant, NO disperses to nearby pyramidal cells. This increase in NO concentration is simultaneous with sleep-associated slow (approximately 1 Hz) oscillations in the electrical potential of pyramidal cells. The coincidence of 1 Hz oscillations and increased NO concentration induces long-term depression of synaptic inputs to the pyramidal cells (left). At the same time, NO triggers activity-dependent vasodilation of the cerebral microvasculature (right).
As described elsewhere (Benington and Frank, 2003) and in this issue (Ferrara and DeGennaro, 2010; Hanlon et al., 2010), regulation of synaptic plasticity, including possibly LTD, is a likely functional concomitant of sleep. By releasing NO during SWS, SANs may play an essential role in sleep-dependent synaptic plasticity. Given the evidence that sleep regulates synaptic plasticity within the cerebral cortex, a definitive assessment of this model is needed. Specifically, it would be of value to determine whether disruption of nNOS activity in the SAN population, independently of other nNOS-positive populations, disrupts the sleep history-dependent changes in synaptic strength, as measured by EEG dynamics or local field potentials (as in Vyazovskiy et al., 2007). It is interesting to note that EEG delta power is elevated in nNOS-deficient mice relative to wild type mice (Chen et al., 2003). Inasmuch as delta power serves as an indirect measure of synaptic strength (Esser et al., 2007), these data are compatible with the notion that nNOS deficiency confers a sleep-related deficit in synaptic downscaling.
Vasodilation is another well-documented response to NO in the cerebral cortex and elsewhere (Estrada and DeFelipe, 1998; Cauli et al., 2004). Although SWS is characterized by reduced cerebral blood flow relative to wakefulness (reviewed by (Madsen and Vorstrup, 1991), the vasodilatory response to neuronal activation is more robust during SWS than during wake or REM sleep (Schei and Rector, this issue). Since SWS is also the time that SANs are active, it is possible that NO produced by these cells mediates this state-specific response. NO release by GABAergic interneurons underlies transient increases in cerebral blood flow in response to shortterm activation of brain circuitry (reviewed in Cauli and Hamel, 2010). By contrast, other vasoactive agents (likely to be produced by astrocytes) are likely to underlie the protracted neurovascular response that occurs when neuronal activation is sustained (Cauli and Hamel, 2010), as it is in wake. Activation of cortical nNOS-positive neurons located in the vicinity of cerebral microvessels results in vasodilation of cerebral microvessels over an interval of several minutes (Cauli et al., 2004). (More rapid dynamics might be expected in vivo as these in vitro studies were done at 20-25°C). This effect was mimicked by an NO donor (Cauli et al., 2004). Triggering vasodilation during SWS may prove to be a function of the SAN population. The possible role of nNOS in regulating SWS-specific changes in cortical blood flow thus requires a closer look.
What Do We Know About SANs Other Than That They are nNOS-positive?
It is also possible that other signaling mechanisms utilized by SANs are important to cortical sleep waveforms and functions. This section describes what is known about the neurochemistry, morphology and electrophysiology of these cells in addition to their expression of nNOS. There are two types of nNOS-immunoreactive neurons in the cortex (reviewed by Kilduff et al., 2011): Type I neurons have a large soma and exhibit intense NADPH-d activity and nNOS immunoreactivity. While there are some species differences, Type I cells in the cat cerebral cortex are located infragranularly in the deeper layers and even in the white matter. In contrast, Type II neurons have a small soma, weak NADPH-d activity and modest nNOS immunoreactivity. These cell bodies are located in the more superficial layers of the cerebral cortex. The SANs are Type I nNOS-positive cells (Gerashchenko et al., 2010). Although both Type I and Type II cells are GABAergic (Yan et al., 1996), they have morphological differences and appear at different times during development (Yan and Ribak, 1997; Ohyu and Takashima, 1998).
Type I nNOS-positive cells are quite rare in the cerebral cortex. The vast majority of neurons in the cerebral cortex are either glutamatergic pyramidal cells (70-80%) or GABAergic interneurons (20-30%; DeFelipe and Farinas, 1992). nNOS-positive cells represent a small subset of the GABAergic interneuron population (Druga, 2009). About 0.5% of the GABAergic neurons are nNOS-positive (Estrada and DeFelipe, 1998). Early immunohistochemical characterizations demonstrated a 100% overlap between brain neurons expressing nNOS and NADPH diaphorase immunoreactivity and, in fact, nNOS engages in both NO synthesis and NADPH diaphorase activity (Hope et al., 1991). Although limited by the lack of reagents to unequivocally distinguish between Type I and Type II nNOS cortical neurons, our estimate is that the Type II nNOS neurons outnumber the Type I cells by a factor of 10. Thus, the SAN population is a very rare cell type.
Other non-ubiquitous proteins that exhibit partial overlap with nNOS in the hippocampus (Price et al., 2005) or cerebral cortex (Estrada and DeFelipe, 1998; Druga, 2009) at the immunohistochemical level include two neuropeptides (somatostatin and NPY), a microfilament α-actinin (Fuentealba et al., 2008) and the GABAA receptor-δ subunit (Olah et al., 2009). The overlap with nNOS among these markers is far from complete. Of NPY-positive interneurons in the cerebral cortex identified using single cell polymerase chain reaction assays, about one third were nNOS-positive (see Figure 1A in Karagiannis et al., 2009). This relatively high percentage of nNOS-positive NPY cells is in contrast to somatostatin (SOM) and parvalbumin-positive neurons, of which less than 10 percent were nNOS-positive, and VIP-positive cells, of which 2% were nNOS-positive (Karagiannis et al., 2009). Of the nNOS-positive cells identified by Karagiannis et al., the majority (80%) were NPY-positive. Other studies concur that the neurochemical marker that most strongly overlaps with nNOS in the brain (including cerebral cortex and hippocampus) is NPY (Cauli et al., 2004; Fuentealba et al., 2008). NPY-positive cells may therefore be informative with regard to the physiological properties of SANs in the cerebral cortex with two caveats. First, as mentioned above, only one third of NPY-positive cortical cells are nNOS-positive at the mRNA level. Second, the NPY-positive cells studied electrophysiologically were mostly present in layers 1-3, where nNOS cells are more likely to be Type II cells (i.e., not SANs) than in deeper layers (Karagiannis et al., 2009). It is the SOM/NPY/NOS cells that are the Type I cells and likely to be SANs (Kilduff et al., in press). The NPY/NOS-positive SOM-negative cells are much more common and are likely Type II (Karagiannis et al., 2009).
The temporal and spatial lability of NO concentration allows for fine-tuning of electrophysiological events, including oscillations. There is evidence that rhythmic electrophysiological events in the hippocampus are modulated by nNOS-dependent changes in NO concentration. Fuentealba and colleagues (Fuentealba et al., 2008) studied the electrophysiological properties of nNOS/NPY/GABA-positive “ivy cells” (so named for their widespread arborization) in the hippocampus. These cells synapse onto the pyramidal cells that undergo theta oscillations during wake and rapid eye movement sleep. Ivy cells and cortical nNOS-positive cells are both derived from median ganglionic eminence precursor cells and are dependent on the transcriptional regulator Nkx2-1 for their development (Pleasure et al., 2000; Tricoire et al., 2010). A related population of nNOS- and NPY-positive cells in the hippocampus, termed neurogliaform cells, are also derived from the same precursor population and have electrophysiological properties similar to those of ivy cells (Tricoire et al., 2010). Electrophysiological properties of ivy cells and nNOS-positive neurogliaform cells may therefore provide data relevant to the function of nNOS-positive cells in the cortex. Since the firing of ivy cells was phase locked with the trough of hippocampal theta and gamma oscillations, it appears that these cells function in the generation of slow theta oscillations. More generally speaking, the function of these NOS-positive GABAergic cells may be to sustain the cellular down state that is produced by rhythm-generating circuitry (Fuentealba et al., 2008). If the same effect was involved in the cerebral cortex, it would provide a mechanism whereby nNOS could reinforce slow oscillations. Another characteristic of nNOS-positive cortical interneurons that makes them a possible player in slow-wave generation is their morphology. nNOS is commonly detected immunohistochemically in cortical interneurons that send corticocortical and other long-range projections (Tomioka et al., 2005; Higo et al., 2007; Tomioka and Rockland, 2007). It is likely that these cells are Type I nNOS cells (i.e. SANs), since long-range projections are typically found on cells with large cell bodies. Cells with long-range projections are ideally situated to enforce the synchrony across large ensembles of cells that typifies SWS.
What Makes SANs Active During Sleep?
Wake-active subcortical projections have profound influence on the electrophysiological activity of the cerebral cortex (Siegel, 2000; Jones, 2004). Projections from these wake-active populations are potential sources of afferent input to the SANs. Cortical nNOS cells are contacted by choline acetyltransferase- (ChAT) positive neuronal terminals (Vaucher et al., 1997; Cauli et al., 2004) and the number of such terminals is significantly reduced when the substantia innominata is lesioned (Vaucher et al., 1997). Cortical nNOS cells are also innervated by serotonergic terminals (Cauli et al., 2004), however, the type of nNOS cells (Type I SANs, Type II non-SANs, or both) innervated by cholinergic and serotonergic terminals is unknown. Both cholinergic tone and serotonergic tone are high during wake and low during SWS (for review see Siegel, 2000; Jones, 2004). We hypothesize that SANs are inhibited by acetylcholine and serotonin, and that the withdrawal of cholinergic and serotonergic tone disinhibits these cells during SWS (see Figure 1). At least in the case of serotonin, there is evidence for receptor expression (5-HT3A receptors, specifically) on NPY-positive neurogliaform cells in the cerebral cortex (Vucurovic et al., 2010).
Future Directions
Progress in understanding the role of the SAN population in sleep is hindered by a lack of both knowledge of their neurochemical makeup and suitable experimental tools for their characterization. Even the designation of the cells as sleep-active is based on a biochemical marker (Fos expression) rather than observations of their electrophysiological properties in vivo during EEG-defined sleep. Whether SANs are rendered active due to disinhibition by the cessation of neurotransmitter release by terminals of subcortical origin, changes in local circuit inputs, or by direct effects of sleep-inducing substances via receptors expressed on the SANs is unknown. Transcriptome characterization of the receptor repertoire of SANs (isolated by laser capture microdissection, for instance) might address this question. Transcriptome characterization might also identify a promoter suitable for targeting these cells for ablation or optogenetic activation, two approaches that have proved fruitful in characterizing the roles of other neurochemically-defined populations in regulating sleep (Hara et al., 2001; Adamantidis et al., 2007). Unfortunately, the currently known nNOS promoter targets reporter gene expression to the Type II cortical nNOS neurons rather than the Type I nNOS cells, which includes the SAN population (www.gensat.org). Therefore, another protein that is co-expressed in SANs must be identified. The transcription factors Nkx2-1, Dlx1, Dlx2, Dlx5 and Dlx6 regulate development of GABAergic forebrain neurons and, as such, might be useful for genetic targeting to GABAergic cells. The promoters of these genes are sufficient to drive transgene expression in GABAergic interneurons of the cerebral cortex, but are by no means selective to nNOS-positive cells (Potter et al., 2009). More specific developmental regulators of the SAN population remain to be identified. Efforts to learn more about these cells would be well placed. It is clear that cortical interneurons play a unique role in maintaining the up state/down state oscillations that characterize SWS (Vyazovskiy et al., 2002; Watson et al., 2008) and Timofeev, this issue). The SAN population provides a novel inroad to begin addressing this role in more detail.
Acknowledgments
Research supported by Defense Advanced Research Projects Agency (N66001-09-1-2117, JPW) and NIH (R01 HL059658, TSK). We thank Bruno Cauli for his comments on this manuscript.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa RM, Lourenco CF, Santos RM, Pomerleau F, Huettl P, Gerhardt GA, Laranjinha J. In vivo real-time measurement of nitric oxide in anesthetized rat brain. Methods Enzymol. 2008;441:351–367. doi: 10.1016/S0076-6879(08)01220-2. [DOI] [PubMed] [Google Scholar]
- Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol. 2003;69:71–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Ferris CD, Snyder SH. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992;267:10976–10981. [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Sancesario G, Morello M, Giorgi M, Pisani A, Bernardi G. A critical role of the nitric oxide/cGMP pathway in corticostriatal long-term depression. J Neurosci. 1999;19:2489–2499. doi: 10.1523/JNEUROSCI.19-07-02489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavas M, Navarro JF. Effects of selective neuronal nitric oxide synthase inhibition on sleep and wakefulness in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:56–67. doi: 10.1016/j.pnpbp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Bernardi G, Calabresi P. Permissive role of interneurons in corticostriatal synaptic plasticity. Brain Res Brain Res Rev. 1999;31:1–5. doi: 10.1016/s0165-0173(99)00018-1. [DOI] [PubMed] [Google Scholar]
- Chen D, Hurst WJ, Ding JM, Faiman LE, Mayer B, Gillette MU. Localization and characterization of nitric oxide synthase in the rat suprachiasmatic nucleus: evidence for a nitrergic plexus in the biological clock. J Neurochem. 1997;68:855–861. doi: 10.1046/j.1471-4159.1997.68020855.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Majde JA, Krueger JM. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 2003;973:214–222. doi: 10.1016/s0006-8993(03)02484-3. [DOI] [PubMed] [Google Scholar]
- Chen L, Duricka D, Nelson S, Mukherjee S, Bohnet SG, Taishi P, Majde JA, Krueger JM. Influenza virus-induced sleep responses in mice with targeted disruptions in neuronal or inducible nitric oxide synthases. J Appl Physiol. 2004;97:17–28. doi: 10.1152/japplphysiol.01355.2003. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- Cudeiro J, Rivadulla C. Sight and insight--on the physiological role of nitric oxide in the visual system. Trends Neurosci. 1999;22:109–116. doi: 10.1016/s0166-2236(98)01299-5. [DOI] [PubMed] [Google Scholar]
- Cudeiro J, Rivadulla C, Grieve KL. A possible role for nitric oxide at the sleep/wake interface. Sleep. 2000;23:829–835. [PubMed] [Google Scholar]
- DeFelipe J, Farinas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- Druga R. Neocortical inhibitory system. Folia Biol (Praha) 2009;55:201–217. [PubMed] [Google Scholar]
- Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30:1617–1630. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada C, DeFelipe J. Nitric oxide-producing neurons in the neocortex: morphological and functional relationship with intraparenchymal microvasculature. Cereb Cortex. 1998;8:193–203. doi: 10.1093/cercor/8.3.193. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Temporal Patterns of Discharge of Pyramidal Tract Neurons during Sleep and Waking in the Monkey. J Neurophysiol. 1964;27:152–171. doi: 10.1152/jn.1964.27.2.152. [DOI] [PubMed] [Google Scholar]
- Ferrara M, DeGennaro L. Going Local: Insights from EEG and Stereo-EEG Studies of the Human Sleep-Wake Cycle. Curr Topics Med Chem. 2010 doi: 10.2174/156802611797470268. in press. [DOI] [PubMed] [Google Scholar]
- Frank MG, Morrissette R, Heller HC. Effects of sleep deprivation in neonatal rats. Am J Physiol. 1998;275:R148–157. doi: 10.1152/ajpregu.1998.275.1.R148. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Begum R, Capogna M, Jinno S, Marton LF, Csicsvari J, Thomson A, Somogyi P, Klausberger T. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier-Sauvigne S, Colas D, Parmantier P, Clement P, Gharib A, Sarda N, Cespuglio R. Nitric oxide and sleep. Sleep Med Rev. 2005;9:101–113. doi: 10.1016/j.smrv.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Wisor JP, Kilduff TS. Sleep-active cells in the cerebral cortex and their role in slow-wave activity. Sleep Biol Rhythms. 2010 doi: 10.1111/j.1479-8425.2010.00461.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Wisor JP, Burns D, Reh RK, Shiromani PJ, Sakurai T, de la Iglesia HO, Kilduff TS. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci U S A. 2008;105:10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib D, Dringenberg HC. Low-frequency-induced synaptic potentiation: a paradigm shift in the field of memory-related plasticity mechanisms? Hippocampus. 2010;20:29–35. doi: 10.1002/hipo.20611. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Peyron C, Denning DP, Ruby NF, Flores J, Sapolsky RM, Heller HC, O'Hara BF. Sleep deprivation effects on growth factor expression in neonatal rats: a potential role for BDNF in the mediation of delta power. J Neurophysiol. 2004;91:1586–1595. doi: 10.1152/jn.00894.2003. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Vyazovskiy VV, Ugo F, Tononi G, Cirelli C. Synaptic potentiation and sleep need: clues from molecular and electrophysiological studies. Curr Topics Med Chem. 2010 doi: 10.2174/156802611797470312. in press. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Higo S, Udaka N, Tamamaki N. Long-range GABAergic projection neurons in the cat neocortex. J Comp Neurol. 2007;503:421–431. doi: 10.1002/cne.21395. [DOI] [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Activation of muscarinic acetylcholine receptors induces a nitric oxide-dependent long-term depression in rat medial prefrontal cortex. Cereb Cortex. 2010;20:982–996. doi: 10.1093/cercor/bhp161. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Kapas L, Fang J, Krueger JM. Inhibition of nitric oxide synthesis inhibits rat sleep. Brain Res. 1994a;664:189–196. doi: 10.1016/0006-8993(94)91969-0. [DOI] [PubMed] [Google Scholar]
- Kapas L, Shibata M, Kimura M, Krueger JM. Inhibition of nitric oxide synthesis suppresses sleep in rabbits. Am J Physiol. 1994b;266:R151–157. doi: 10.1152/ajpregu.1994.266.1.R151. [DOI] [PubMed] [Google Scholar]
- Karagiannis A, Gallopin T, David C, Battaglia D, Geoffroy H, Rossier J, Hillman EM, Staiger JF, Cauli B. Classification of NPY-expressing neocortical interneurons. J Neurosci. 2009;29:3642–3659. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilduff TS, Cauli B, Gerashchenko D. Activation of cortical interneurons during sleep: an anatomical link to sleep homeostasis? Trends Neurosci. 2011 doi: 10.1016/j.tins.2010.09.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux N, Amar M, Moreau AW, Fossier P. Roles of nitric oxide in the homeostatic control of the excitation-inhibition balance in rat visual cortical networks. Neuroscience. 2009;163:942–951. doi: 10.1016/j.neuroscience.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Vorstrup S. Cerebral blood flow and metabolism during sleep. Cerebrovasc Brain Metab Rev. 1991;3:281–296. [PubMed] [Google Scholar]
- Montgomery HJ, Romanov V, Guillemette JG. Removal of a putative inhibitory element reduces the calcium-dependent calmodulin activation of neuronal nitric-oxide synthase. J Biol Chem. 2000;275:5052–5058. doi: 10.1074/jbc.275.7.5052. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- O'Hara BF, Young KA, Watson FL, Heller HC, Kilduff TS. Immediate early gene expression in brain during sleep deprivation: preliminary observations. Sleep. 1993;16:1–7. doi: 10.1093/sleep/16.1.1. [DOI] [PubMed] [Google Scholar]
- Ohyu J, Takashima S. Developmental characteristics of neuronal nitric oxide synthase (nNOS) immunoreactive neurons in fetal to adolescent human brains. Brain Res Dev Brain Res. 1998;110:193–202. doi: 10.1016/s0165-3806(98)00107-2. [DOI] [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JL, Perez-Perera L, Bouvier M, Velluti RA. Sleep and wakefulness modulation of the neuronal firing in the auditory cortex of the guinea pig. Brain Res. 1999;816:463–470. doi: 10.1016/s0006-8993(98)01194-9. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Dudek SM, Eagleman D, Montague PR, Friedlander MJ. LTD induction in adult visual cortex: role of stimulus timing and inhibition. J Neurosci. 2001;21:2308–2319. doi: 10.1523/JNEUROSCI.21-07-02308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleasure SJ, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein DH, Rubenstein JL. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Cirelli C, Tononi G. Immediate-early genes in spontaneous wakefulness and sleep: expression of c-fos and NGFI-A mRNA and protein. J Sleep Res. 1994;3:80–96. doi: 10.1111/j.1365-2869.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, Rubenstein JL. Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons. Mol Cell Neurosci. 2009;40:167–186. doi: 10.1016/j.mcn.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci. 2005;25:6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AC, Kapas L. Day- and nighttime injection of a nitric oxide synthase inhibitor elicits opposite sleep responses in rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R521–R531. doi: 10.1152/ajpregu.00605.2004. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Inoue K, Browning A. Activity of primate subgenual cingulate cortex neurons is related to sleep. J Neurophysiol. 2003;90:134–142. doi: 10.1152/jn.00770.2002. [DOI] [PubMed] [Google Scholar]
- Rudolph M, Pospischil M, Timofeev I, Destexhe A. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci. 2007;27:5280–5290. doi: 10.1523/JNEUROSCI.4652-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. Nitric oxide and synaptic function. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Brainstem mechanisms generating REM sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 3. Philadelphia: W.B. Saunders; 2000. pp. 112–133. [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, Steininger T, Alam N, McGinty D. Preoptic area sleep-regulating mechanisms. Arch Ital Biol. 2001;139:77–92. [PubMed] [Google Scholar]
- Tatsumi S, Itoh Y, Ma FH, Higashira H, Ukai Y, Yoshikuni Y, Kimura K. Inhibition of depolarization-induced nitric oxide synthase activation by NS-7, a phenylpyrimidine derivative, in primary neuronal culture. J Neurochem. 1998;70:59–65. doi: 10.1046/j.1471-4159.1998.70010059.x. [DOI] [PubMed] [Google Scholar]
- Tomioka R, Rockland KS. Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J Comp Neurol. 2007;505:526–538. doi: 10.1002/cne.21504. [DOI] [PubMed] [Google Scholar]
- Tomioka R, Okamoto K, Furuta T, Fujiyama F, Iwasato T, Yanagawa Y, Obata K, Kaneko T, Tamamaki N. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21:1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Daw MI, Sousa VH, Miyoshi G, Jeffries B, Cauli B, Fishell G, McBain CJ. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci. 2010;30:2165–2176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucher E, Linville D, Hamel E. Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neuroscience. 1997;79:827–836. doi: 10.1016/s0306-4522(97)00033-x. [DOI] [PubMed] [Google Scholar]
- Vijayan S, Hale GJ, Moore CI, Brown EN, Wilson MA. Activity in the barrel cortex during active behavior and sleep. J Neurophysiol. 2010 doi: 10.1152/jn.00474.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, Monyer H, Rossier J, Vitalis Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30:1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Deboer T, Rudy B, Lau D, Borbely AA, Tobler I. Sleep EEG in mice that are deficient in the potassium channel subunit K.v.3.2. Brain Res. 2002;947:204–211. doi: 10.1016/s0006-8993(02)02925-6. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson BO, MacLean JN, Yuste R. Up states protect ongoing cortical activity from thalamic inputs. PLoS One. 2008;3:e3971. doi: 10.1371/journal.pone.0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang Y, Rowan MJ, Anwyl R. Evidence for involvement of the cGMPprotein kinase G signaling system in the induction of long-term depression, but not long-term potentiation, in the dentate gyrus in vitro. J Neurosci. 1998;18:3589–3596. doi: 10.1523/JNEUROSCI.18-10-03589.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Ribak CE. Prenatal development of nicotinamide adenine dinucleotide phosphate-diaphorase activity in the human hippocampal formation. Hippocampus. 1997;7:215–231. doi: 10.1002/(SICI)1098-1063(1997)7:2<215::AID-HIPO8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Yan XX, Jen LS, Garey LJ. NADPH-diaphorase-positive neurons in primate cerebral cortex colocalize with GABA and calcium-binding proteins. Cereb Cortex. 1996;6:524–529. doi: 10.1093/cercor/6.3.524. [DOI] [PubMed] [Google Scholar]