Abstract
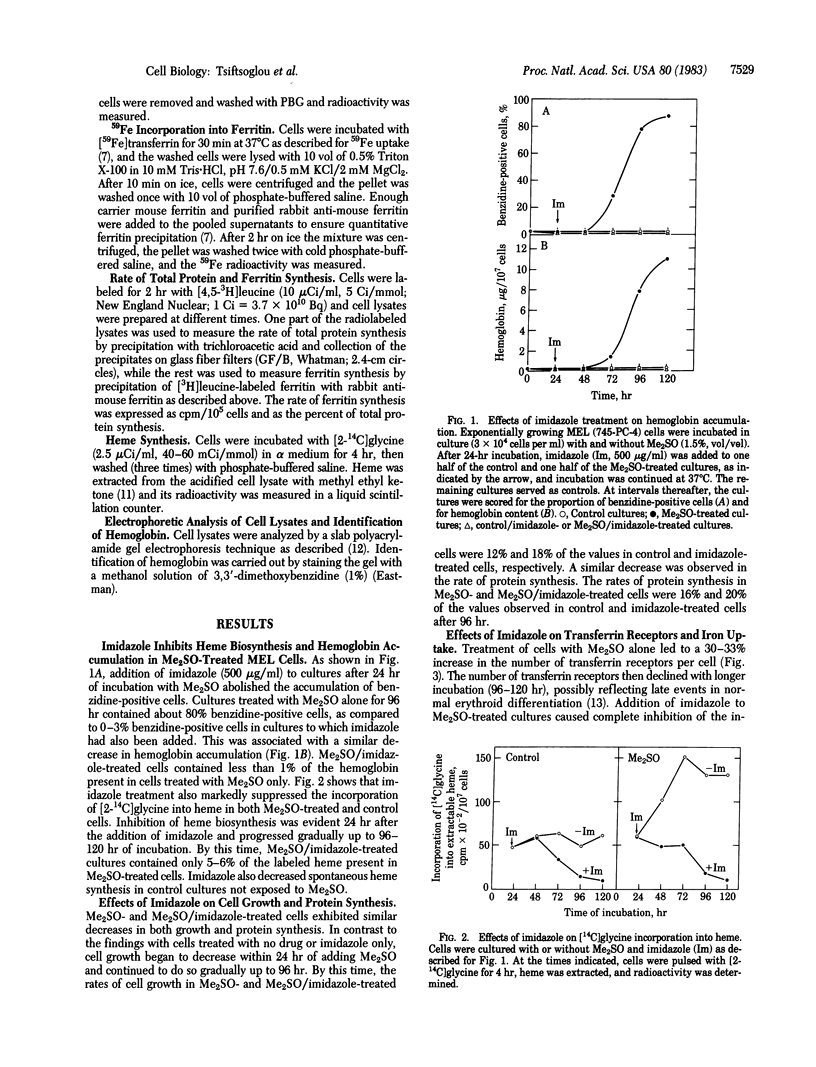
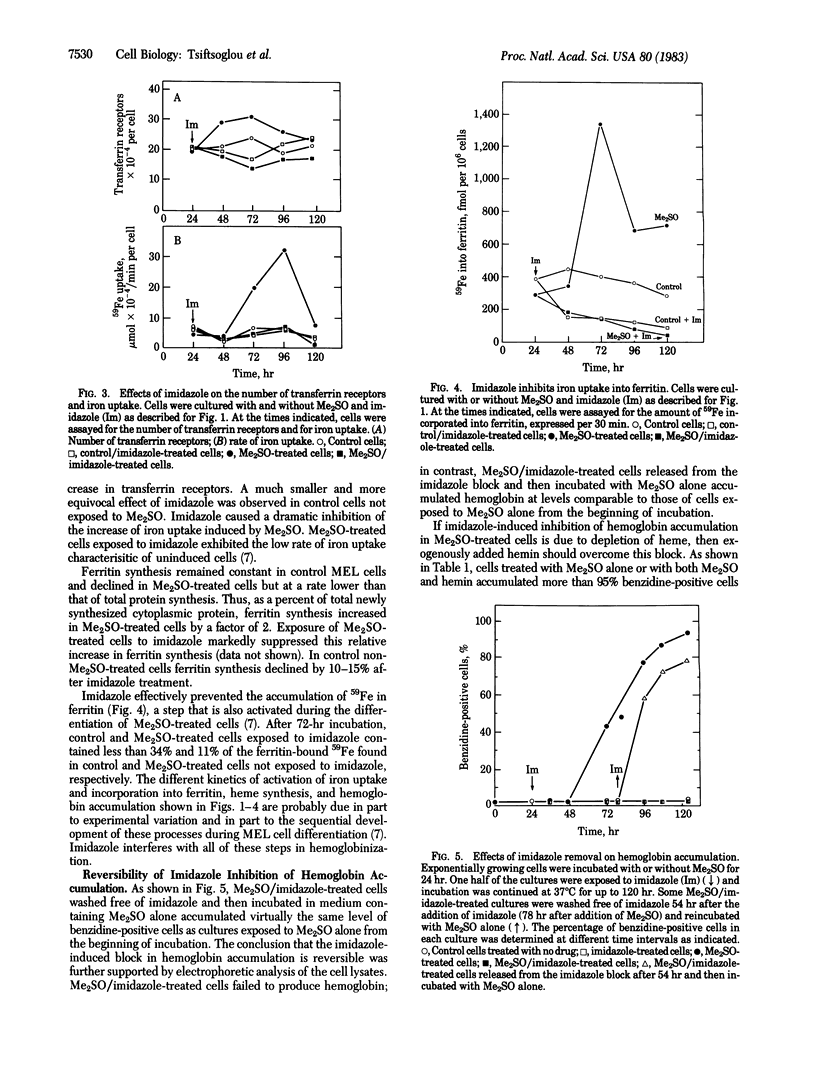
Treatment of murine erythroleukemia (MEL) cells with imidazole in the presence of dimethyl sulfoxide (Me2SO) has been shown to dissociate hemoglobin accumulation from commitment to terminal maturation. To explore the mechanism(s) of this effect, we studied iron transport and heme and hemoglobin synthesis in Me2SO-induced MEL cells that were then exposed to imidazole. Imidazole treatment (i) causes moderate inhibition of 125I-labeled transferrin binding to both control and Me2SO-treated MEL cells; (ii) markedly suppresses Me2SO-induced activation of iron uptake into MEL cells; (iii) markedly decreases the incorporation of iron into ferritin; and (iv) abolishes heme biosynthesis from [2-14C]glycine and hemoglobin accumulation in Me2SO-treated cells. Imidazole treatment does not inhibit other aspects of cellular maturation; cells treated with Me2SO in the presence or absence of imidazole exhibit similar changes in proliferative activity and protein synthesis and, as shown previously, in cell morphology. Inhibition of hemoglobin accumulation in MEL cells is reversible on withdrawal of imidazole but is not altered by exogenous hemin. These data indicate that commitment to terminal maturation is regulated independently from the systems for iron transport and heme biosynthesis during early phases of erythroid cell differentiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Goff S. C. Variable globin chain synthesis in mouse erythroleukemia cells. Blood. 1978 Nov;52(5):1047–1057. [PubMed] [Google Scholar]
- Cheung D. W., Daniel E. E. Imidazole inhibits a temperature-dependent component of mammalial skeletal muscle action potential. Nature. 1980 Jan 31;283(5746):485–486. doi: 10.1038/283485a0. [DOI] [PubMed] [Google Scholar]
- Cole R. J., Paul J. The effects of erythropoietin on haem synthesis in mouse yolk sac and cultured foetal liver cells. J Embryol Exp Morphol. 1966 Apr;15(2):245–260. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass J., Nunez M. T., Fischer S., Robinson S. H. Transferrin receptors, iron transport and ferritin metabolism in Friend erythroleukemia cells. Biochim Biophys Acta. 1978 Aug 3;542(1):154–162. doi: 10.1016/0304-4165(78)90241-6. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Tsiftsoglou A. S., Volloch V., Weil S. C., Neumann J., Housman D. E. Dissociation of hemoglobin accumulation and commitment during murine erythroleukemia cell differentiation by treatment with imidazole. J Cell Physiol. 1982 Oct;113(1):179–185. doi: 10.1002/jcp.1041130127. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Weil S. C., Tsiftsoglou A. S., Volloch V., Neumann J. R., Keys C., Housman D. E. Hemin does not cause commitment of murine erythroleukemia (MEL) cells to terminal differentiation. Blood. 1980 Sep;56(3):481–487. [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Housman D., Levenson R., Volloch V., Tsiftsoglou A., Gusella J., Parker D., Kernen J., Mitrani A., Weeks V., Witte O. Control of proliferation and differentiation in cells transformed by Friend virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1177–1185. doi: 10.1101/sqb.1980.044.01.127. [DOI] [PubMed] [Google Scholar]
- Hu H. Y., Gardner J., Aisen P. Inducibility of transferrin receptors on friend erythroleukemic cells. Science. 1977 Aug 5;197(4303):559–561. doi: 10.1126/science.267327. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel F., Allet B., Eisen H. Appearance of a chromatin protein during the erythroid differentiation of Friend virus-transformed cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):653–656. doi: 10.1073/pnas.74.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levenson R., Macara I. G., Smith R. L., Cantley L., Housman D. Role of mitochondrial membrane potential in the regulation of murine erythroleukemia cell differentiation. Cell. 1982 Apr;28(4):855–863. doi: 10.1016/0092-8674(82)90064-2. [DOI] [PubMed] [Google Scholar]
- Mager D. L., MacDonald M. E., Bernstein A. Growth in high-K+ medium induces Friend cell differentiation. Dev Biol. 1979 May;70(1):268–273. doi: 10.1016/0012-1606(79)90024-1. [DOI] [PubMed] [Google Scholar]
- Nunez M. T., Glass J., Fischer S., Lavidor L. M., Lenk E. M., Robinson S. H. Transferrin receptors in developing murine erythroid cells. Br J Haematol. 1977 Aug;36(4):519–526. doi: 10.1111/j.1365-2141.1977.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Ross J., Sautner D. Induction of globin mRNA accumulation by hemin in cultured erythroleukemic cells. Cell. 1976 Aug;8(4):513–520. doi: 10.1016/0092-8674(76)90219-1. [DOI] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Macara I. G., Levenson R., Housman D., Cantley L. Evidence that a Na+/Ca2+ antiport system regulates murine erythroleukemia cell differentiation. J Biol Chem. 1982 Jan 25;257(2):773–780. [PubMed] [Google Scholar]
- Tsiftsoglou A. S., Gusella J. F., Volloch V., Housman D. E. Inhibition by dexamethasone of commitment to erythroid differentiation in murine erythroleukemia cells. Cancer Res. 1979 Oct;39(10):3849–3855. [PubMed] [Google Scholar]
- Tsiftsoglou A. S., Mitrani A. A., Housman D. E. Procaine inhibits the erythroid differentiation of MEL cells by blocking commitment: possible involvement of calcium metabolism. J Cell Physiol. 1981 Sep;108(3):327–335. doi: 10.1002/jcp.1041080306. [DOI] [PubMed] [Google Scholar]



