Abstract
Tissue engineering and regenerative medicine (TERM) has caused a revolution in present and future trends of medicine and surgery. In different tissues, advanced TERM approaches bring new therapeutic possibilities in general population as well as in young patients and high-level athletes, improving restoration of biological functions and rehabilitation. The mainstream components required to obtain a functional regeneration of tissues may include biodegradable scaffolds, drugs or growth factors and different cell types (either autologous or heterologous) that can be cultured in bioreactor systems (in vitro) prior to implantation into the patient. Particularly in the ankle, which is subject to many different injuries (e.g. acute, chronic, traumatic and degenerative), there is still no definitive and feasible answer to ‘conventional’ methods. This review aims to provide current concepts of TERM applications to ankle injuries under preclinical and/or clinical research applied to skin, tendon, bone and cartilage problems. A particular attention has been given to biomaterial design and scaffold processing with potential use in osteochondral ankle lesions.
Keywords: ankle, biomaterials, osteochondral lesions, regenerative medicine, scaffold, tissue engineering
1. Introduction: fundamentals of tissue engineering and regenerative medicine
1.1. Tissue engineering and regenerative medicine surgical application potential in several ankle tissues
In the anatomical ankle region, several tissues develop injuries/pathologies with new emerging therapeutic possibilities arising from tissue engineering and regenerative medicine (TERM) strategies.
Tissue engineering (TE) and related therapeutic strategies, which mimic the mechanisms of tissue normal repair and regeneration, have been regarded as a revolution in medical sciences [1]. As stated by Langer & Vacanti [1], TE is the research field which combines the principles of engineering, and life and health sciences with the development of biological functional substitutes. The aim is to restore, defend (avoid disease progression) or improve the function of the damaged tissue and/or organ.
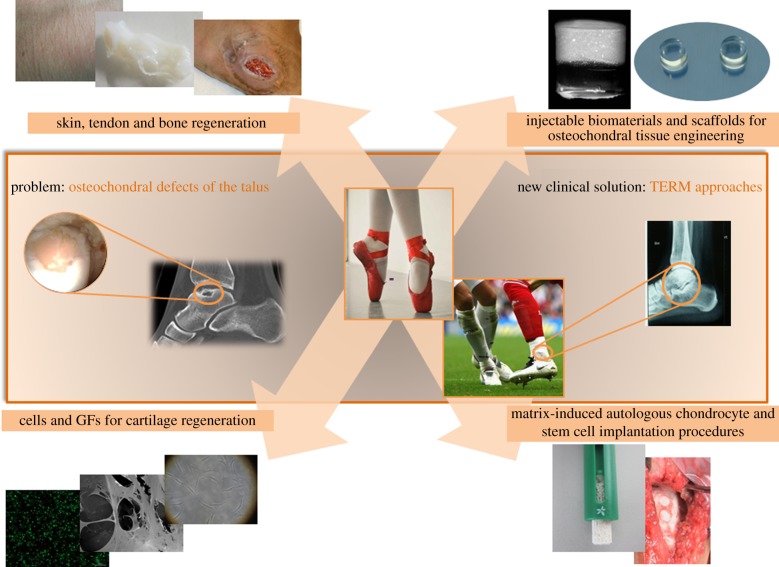
Application of ankle TE strategies [2,3] can consider, by definition, three main variables (figure 1): (i) tridimensional porous supports or scaffolds [4,5], (ii) cells (differentiated or undifferentiated), and (iii) bioactive agents, i.e. physical stimulus [6], and/or growth factors (GFs) [7,8]. Cells can be seeded and cultured onto a structure or scaffold capable of supporting three-dimensional tissue formation [9]. GFs can be used in the isolated form in injured tissue/organ, as a ‘pool’ of GFs or in association with scaffolds and/or cells [10,11]. The use of bioreactors (dynamic systems) as a way to improve the in vitro biological and mechanical properties of the TE constructs (cell-laden scaffolds) is also advantageous, as it can allow one to overcome the limitation of nutrients/metabolites diffusion observed in static cultures [12]. On the other hand, regenerative medicine (RM) is a broader concept which, besides that previously discussed for TE, also considers the use of bioactive soluble molecules [13,14], stem cell technologies [15,16], prolotherapy (i.e. injectable regenerative techniques) [17], genetic therapeutic strategies [18], nanotechnologies and several medical devices.
Figure 1.
TERM applications on the ankle joint.
The terms TE and RM can be used interchangeably, but both fields have been globally referred to in association as TERM [9,19].
In this review, an overview is given of the present applications in treatment of skin, tendon, bone and osteochondral lesions in the ankle joint.
1.1.1. Applications of tissue engineering and regenerative medicine strategies to skin repair
Cutaneous ulcers around the ankle, secondary to trauma, vascular insufficiency or diabetes [20,21] are injuries that require special attention mainly owing to low vascular supply, a problem that is of great importance in poor subcutaneous tissue areas.
Simplicity of application and affordable price are the main reasons by which GFs have been widely applied for treatment of different injuries in orthopaedics but also in cardiovascular, plastic surgery and dentistry [22,23]. In a body injury, platelets participate in the natural healing process, being responsible for haemostasis and releasing of bioproteins or GFs that are crucial to the wound-healing process [22,24]. Platelet-rich plasma (PRP) can be harvested from patients’ own peripheral blood and after concentration it becomes ready to be administered at the injury site [25].
Biodegradable biomaterials [21,26] have also been processed as scaffolds and membranes as these systems can act as drug delivery carriers (figure 2), while serving as a three-dimensional template for supporting cell proliferation and repair at the damaged site.
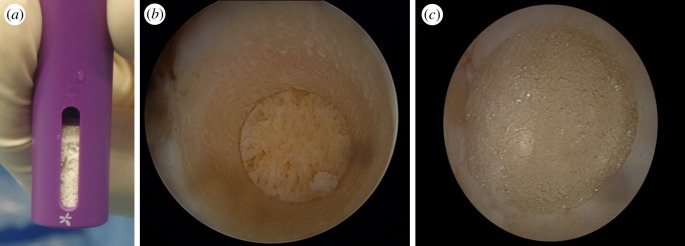
Figure 2.
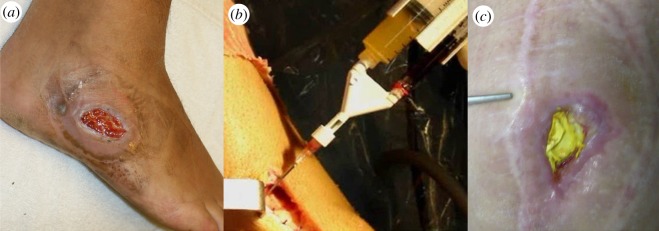
(a) Grade 3 ulcer, (b) PRP application in wound and (c) chronic infected wound protected by collagen membrane with gentamicin sulfate.
Bone morphogenetic proteins (BMPs) are members of the human transforming growth factor-β (TGF-β) superfamily and similarly to PRP have been demonstrated to have many therapeutic possibilities [27,28]. However, BMPs still present a considerably higher cost as compared with PRP. The biological mechanism of action for BMPs has been demonstrated by Urist [29]. BMP-2 and BMP-7 belong to TGF-β superfamily, and BMP-1 is considered a metalloprotease. It is undeniable the importance of these GFs in the field of tissue engineering, owing to their effect in regeneration of body tissues, specially bone and cartilage. More than 15 BMPs have been described, and their specific characteristics and mechanism of action are under investigation [28].
Tissue-engineered skin with allogeneic dermal fibroblasts and epidermal keratinocytes [21] has been successfully used in chronic wounds that fail to heal with standard wound care. Allogeneic dermal products seem to have the necessary cytokines for wound healing, presenting not only superior effective rate, but also reduced time of treatment. Yamada et al. [30] proposed the use of a bilayered hyaluronan/atelocollagen sponge seeded with fibroblasts for wound-healing (e.g. leg, ankle or foot ulcers) applications. That work has shown the beneficial effect of using cell-seeded scaffolds when treating ulcers as it improved wound healing.
TERM approach using acellular dermal graft has also been described [31]. This technique allies tissue-engineered matrices to the cells and GFs present in the human recipient following transplantation. Brigido [32] reported a clinical trial which demonstrated that Graftjacket tissue matrix showed a statistically significant higher percentage of wound healing with respect to wound, and it is more effective than sharp debridement in this small case-control trial. The disadvantage of allogeneic dermal products as compared to the acellular graft is that they require multiple applications and can only be applied to the treatment of superficial full thickness ulcers.
Another relevant issue is related to the treatment of infection in this area. Using TERM technologies such as nanotechnology [17,20,33] (e.g. micro- and nanoparticles or nanospheres developed as systems to deliver drugs in a controlled manner), it is possible to increase simultaneously the delivery of antibiotics at the damaged site and promote tissue repair [13].
1.1.2. Applications of tissue engineering and regenerative medicine strategies to tendon repair
Another relevant group of injuries located in the ankle region is the tendon lesions. Most tendons have the ability to heal after injury, but the newly formed tissue is functionally different from normal tendon. Achilles tendon pathologies (in their several classifications) [34] have high impact in both high-level athletes and the general population. Figure 3 shows a magnetic resonance image (MRI) of a typical Achilles tendon partial rupture. Tendon acute tears treatments are managed by direct suturing techniques [35,36], and the most common form of healing is scar formation. Poor tissue vascularization explains the slow healing rate and the observed scar tissue in the repaired tendon. The latter can affect tissue functioning as scar tissue results in adhesion formation, which disrupts tendons. Therefore, it represents a higher risk of further damage [37–39]. All these facts contribute to distorted motion and consequently reduced life quality [40]. In the last few years, several TERM approaches have been investigated with the promise of a more successful outcome for patients, where acute tendon pathology and chronic tendon ruptures have been diagnosed [41–43]. This can be achieved by means of both inhibiting degeneration process [44–47] and helping to relieve pain [48].
Figure 3.
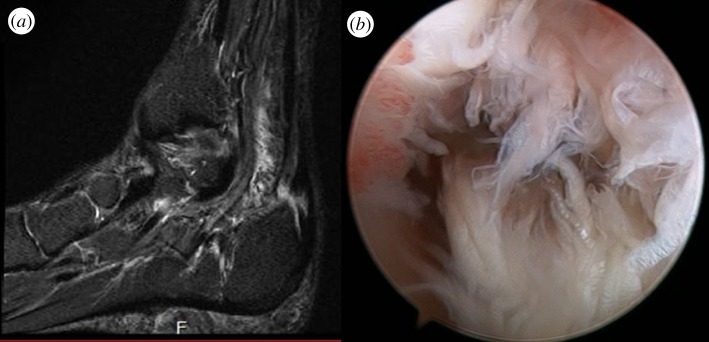
(a) Achilles tendon defect partial rupture identified in T2 MRI (arrow) and (b) endoscopic view of the defect.
Several GFs have been found to be useful in tendon wound healing [40], like TGF-β [44], BMP, fibroblast growth factor (FGF) [49] and insulin-like growth factor (IGF) [50]. All aforementioned approaches using GFs proved to accelerate the wound-healing process and strength of the repair. However, depending on the concentration, half-time and applied technique, it can also promote undesired fibrosis, with excessive disordered collagen deposition, i.e. the structural properties are improved, but not the tissue functioning [44].
Several studies have reported that PRP has a positive effect on proliferation and metabolism of human tenocytes, and thus enhances tendon repair [22,51]. Meanwhile, the main problem might be the standardization of the methods used in the clinical setting, and concentration of platelets and GFs to be used. One of the most challenging goals is related to the need for establishing the optimal concentration, half-life and local of injection and avoiding clearance of the PRP from lesion sites [48].
Tenocytes present low mitotic rate, which obviously influences any therapeutic approach. Particularly, in an attempt to reverse/decelerate the degenerative process, controlled drug delivery systems, such as micro- or nanoparticle proteins or polymer-based systems [52,53], have been tried. Figure 4 illustrates gellan gum microparticles obtained by precipitation in a phosphate buffer saline solution. Nanotechnology-based approaches are promising when it is envisioned to stabilize and to achieve a controlled release of a given therapeutic agent at the defect site.
Figure 4.
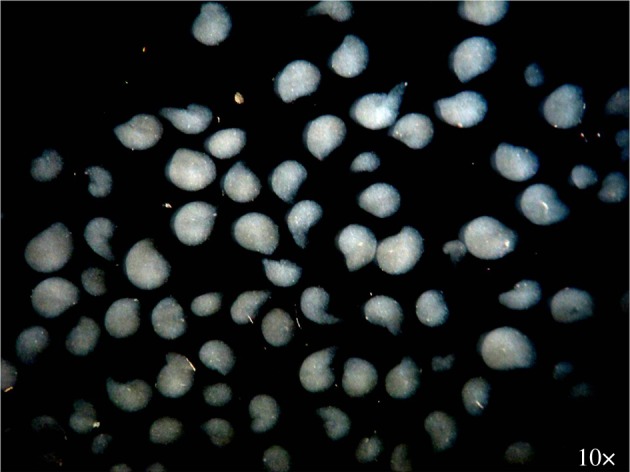
Photograph of the gellan gum microparticles obtained by precipitation in a phosphate buffered saline (pH 7.4) solution and possessing a size between 500 and 2000 µm.
Several authors have proposed both acellular and cellular silk fibroin-based scaffolds for ligament/tendon tissue engineering with promising results, in vitro and in vivo [54,55]. TERM approaches using a ligament/tendon with similar mechanical and functional characteristics as the native tissue can prevent several complications associated with the traditional methods. Scaffolds can be combined with stem cells [15,49,56] or GF [22,24,49,51,57] in a in vivo approach (to permit the self-regeneration of small tissue lesions) or used alone [58–60] in an ex vivo approach, designed to produce functional tissue that can be implanted in the body. The ideal scaffold for tendon engineering must retain the basic structure of the tendon, mimic native extracellular matrix (ECM) and competence for cell seeding [61]. Reports on the use of several scaffolds (e.g. silk fibroin [54], collagen [45,58], chitosan-based [53] or poly(ethylene glycol) diacrylate hydrogel [62]) combined with adult mesenchymal stem cells (MSCs) demonstrated that differentiation of MSCs into tenocyte-like cells can occur in response to chemical factors, including BMPs, TGF-β and FGF [46,49].
1.1.3. Applications of tissue engineering and regenerative medicine strategies to bone repair
Bone defects and bone reconstruction are, probably, two of the most important issues in a TERM perspective, with several proposals advanced over the years [7,29,53,62,63]. Some injuries in anatomic areas such as distal tibia, talus or calcaneus, given their difficult irrigation and scarce soft tissue protection, usually are difficult to consolidate. This is a particularly critical problem in patients with a clinical history of multiple surgical interventions [33].
Bone grafts can cover the basic requirements for bone repair as they combine a scaffold, GFs and cells with osteogenic potential. Yet, the use of bone grafts is associated with several complications, i.e. non-unions [64], incomplete filling of the defect and late graft fracture [63]. Furthermore, harvesting of autologous bone often results in donor site morbidity, which may vary with the location site and the applied technique [65].
Some technologies combining the use of GFs (namely BMPs) [7,28,29], cells [16] and/or scaffolds [66,67], adapted or not to a surgical intervention have achieved promising results in cases where several previous surgeries have failed systematically [3,33,68].
BMPs, specifically BMP-2, BMP-4 and BMP-7, have been known for over a decade for inducing osteogenic cell differentiation in vitro and in vivo [68]. The value of recombinant human BMP-2 (rhBMP-2) has been evaluated in a prospective study for treating open tibial shaft fractures [69]. A significant reduction of a secondary intervention was observed in the rhBMP-2 group as compared with the standard care group, suggesting that the use of GFs could accelerate healing of fractures and soft tissue, reduce hardware failure, and thus re-operation owing to delayed healing/non-union. Still, there are only few available GFs for clinical use in bone regeneration besides BMP-2, BMP-7 and growth and differentiation factor-5 (GDF-5) [70]. Recently, Kleinschmidt et al. [70] reported that the use of a mutant GDF-5 (obtained by introducing BMP-2 residues into GDF-5) demonstrated enhanced osteogenesis and long bone formation capacity [70]. When the use of GFs alone is not recommended, as in the treatment of large bone defects, stem cells and scaffolds are a very promising alternative to standard procedure. Stem cell-based TERM strategies require three main steps: (i) cells are harvested, isolated and expanded, (ii) scaffolds are seeded with the induced cells, and (iii) cell-seeded scaffolds are re-implanted in vivo [68]. The aim of TERM is the substitution of the missing tissue with the ex vivo tissue-engineered construct. There are several reports [71,72] on the application of different scaffolds combined with stem cells (mostly MSCs derived from bone marrow or adipose tissue). These have shown favourable autogenous bone grafting and no donor site morbidity [68]. Scaffold choice is still under investigation in order to be standardized. Biodegradable synthetic polyesters [73], calcium phosphate ceramics [74,75] and chitosan–alginate [76] are some of the scaffolds that have proved to have significant value in the treatment of bone defects.
Cancedda et al. [63] have provided relevant information and new insights on the importance of scaffold architecture towards enhancing de novo bone formation within scaffolds in vivo.
Kokemueller et al. [77] have been also investigating the vascularization of seeded scaffolds required for clinical application in reconstructive cranio-maxillofacial surgery. The authors reported that prefabrication of vascularized bioartificial bone grafts in vivo might be an alternative to in vitro tissue engineering techniques as it presented minimal donor site morbidity and no shape or volume limitations.
More recently, Nagata et al. [78] reported the use of cultured autogenous periosteal cells (CAPCs) in alveolar bone regeneration. CAPCs were mixed with particulate autogenous bone and PRP and grafted into the injury sites. Results have shown that CAPC grafting enhances recruitment of both osteoblasts and osteoclasts, accompanied by angiogenesis and leading to satisfactory bone regeneration.
Oliveira et al. [79] proposed the combination of nanotechnology tools and tissue engineering approaches for pre-programming the fate of bone marrow stromal cells (BMSCs) towards promoting superior de novo bone formation. The authors have shown that BMSCs cultured in vitro (figure 5) with a dendron-like nanoparticles system that delivers dexamethasone intracellularly, seeded onto starch–polycaprolactone (SPCL) scaffolds (figure 5a) and implanted subcutaneously were able to differentiate and produce new bone, in vivo (figure 5c). That work clearly evidenced the advantages of using intracellular tools, for example the dendronized nanoparticles, for tuning stem cells in vivo.
Figure 5.
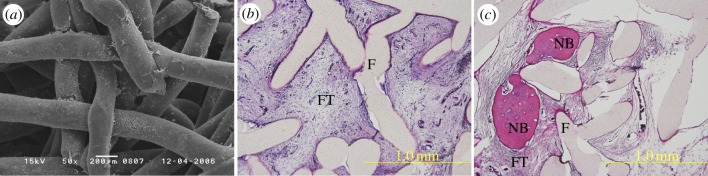
(a) Scanning electron microscopy image of MSCs seeded onto SPCL scaffolds and maintained in a standard osteogenic culture medium, after 14 days of culturing. Microscopy images of histological sections (haematoxylin and eosin staining) of (b) SPCL scaffold controls and (c) MSCs/SPCL construct explants after four weeks of implantation (Fischer rats subcutaneous model). Newly bone formed (NB), SPCL fibres (F) and fibrous tissue (FT).
2. Osteochondral ankle lesions
Osteochondral defects (OCDs) and osteoarthritis in lower limb have a relevant socio-economic impact with significant therapeutic investments and absence from work-related costs [80,81]. OCDs are defined as lesions of any origin that involve the articular surface and/or subchondral region, thus affecting cartilage, bone or both [81]. Suggested causes of ankle OCDs include local avascular necrosis, systemic vasculopathies, acute trauma, chronic microtrauma, endocrine or metabolic factors, degenerative joint disease and genetic predisposition [82].
Asymptomatic OCD patients can be treated non-operatively, with rest, ice application and immobilization or temporarily reduced weight bearing, even though this management has shown relatively high rates of failure [83].
Symptomatic patients with OCDs should be treated surgically. The main aim is to promote re-vascularization of the bone defect [84–86]. This goal is achieved applying three principles [87]: (i) debridement and bone marrow stimulation (e.g. microfracture, drilling and abrasion arthroplasty), (ii) securing a lesion to the talar dome (e.g. fragment fixation, retrograde drilling and bone grafting), and (iii) development or replacement of hyaline cartilage (e.g. autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OAT), mosaicplasty and allografts) [88].
Articular hyaline cartilage is avascular and it has poor regenerative capability [89,90]. When repair involves the formation of fibrous cartilage, the newly formed tissue will lack favourable biomechanical properties and it can fail [90]. Therefore, the damaged tissue should be replaced with a tissue that best resembles the native hyaline cartilage [81,88]. For this reason, significant economic and scientific investments have been made on TERM applications in the treatment and prevention of cartilage defects and joint degradation [33]. Minimally invasive methods that can facilitate their use have also attracted much attention [81,88,91,92]. Besides, prolotherapy and arthroscopic/endoscopic procedures have a lower risk of complications. These procedures facilitate and decrease rehabilitation time, thus they help fight absence from work and promote return to athletic activity [88,92]. TERM strategies have been developed or adapted to promote this kind of application/delivery [81,88,92].
2.1. Applications of tissue engineering and regenerative medicine strategies to ankle osteochondral lesions repair
2.1.1. Applications of isolated growth factors
Debridement and bone marrow stimulation have been used as surgical approaches for partially destroying the calcified zone that is often present in OCDs and to create multiple openings into the subchondral bone [87,89]. As a consequence of these interventions, intra-osseous blood vessels are disrupted, and the release of GFs can lead to the formation of a fibrin clot and fibro-cartilaginous tissue formation. These approaches have proven to be one of the most effective treatments for OCDs of the talus, especially in a small lesion (less than 6 mm), with minimal subchondral bone involvement [81,87].
Based on this surgical treatment, the use of isolated GFs in the treatment of symptomatic OCDs has undergone a huge expansion over the last few years [33]. In the body's natural response to injury, a complex healing process is initiated. Platelets participate in this process, as they are responsible for stopping bleeding and for haemostasis [22]. Once they are activated by mediators at the site of injury, they undertake degranulation, releasing GFs that will help the wound-healing process. Examples of these GFs are TGF-β, IGF-1 and IGF-2, FGF, all of which have been shown in experimental settings to promote healing and the formation of the new tissue [8].
The short half-life of these proteins, the difficulty in keeping them within the area of the defect and the low mitotic rate of chondrocytes, among several other issues, make it hard or even impossible to predict, from a theoretical perspective, the complete repair of a chondral defect or OCD using this approach [28]. Moreover, results available in the literature are controversial, with some series reporting significant clinical or symptomatic improvement [17,24], while other studies conclude that there is not enough evidence to support their use with this objective [8]. Two recent reports have used TGF-β, IGF-1 and BMP-2 associated with scaffolds and have reported promising results for the repair of OCDs and cartilages [93,94]. Although the anabolic effect of these GFs cannot be questioned, as has been demonstrated and confirmed in vitro and in vivo [95,96], the original tissue replacement for fifibrous tissue is commonly observed in the neo-surface of the OCDs [2,94].
It is consistently recognized that most of the published studies have a low methodological quality in this matter, i.e. besides the absence of uniform criteria in outcome assessment, most of them also do not consider or not specify the different GFs applied, their quantities, isolation methods, simultaneous presence or absence of other proteins (e.g. metalloproteases) or cells (e.g. leucocytes) [22,25,97]. It becomes obvious that the improper early use of a promising technique will lead to obstacles in its correct improvement which creates higher resistance to its future application. However, tissue repair and homeostasis depend on a multitude of factors (the TERM triad) and should not be lightly simplified this way. Research must still progress considerably to gain deeper knowledge on the GFs application and their effects on different tissues and clinical situations. Thus, GFs are probably not expected to be a panacea, being able to solve all our problems independently of the way they are produced, stabilized and administered to the patient.
Besides the previously stated, one cannot ignore the analgesic effects which simple platelet-derived GF methods have shown in several clinical trials [8,22], particularly among high-level athletes.
2.1.2. Applications of isolated cells
MSCs have demonstrated their high potential for clinical use as therapeutic agents with several possible RM applications including orthopaedics and percutaneous (injectable) techniques [98].
The rehabilitation of injured/degraded cartilage through the degenerative process leading to osteoarthritis remains the main challenge that clinicians and researchers have been facing. Several researchers have tested the use of MSCs instead of chondrocytes in the attempt to repair cartilage defects and defend joint homeostasis [71,99,100].
MSCs have the capacity to modulate the immune response of the individual and positively influence the microenvironment of pluripotent cells already present in native injured tissue. Through direct cell-to-cell interactions or by secreting a number of different proteins, MSCs can promote the endogenous regenerative mechanisms still present in an arthritic joint [101].
Gene therapy with modified MSCs might increase this therapeutic field in the near future [68,96,101]. Besides their isolated application, MSCs’ chondrogenic differentiation can be induced at the target tissue or in combination with an adequate support scaffold [99]. This may obviate the limited lifespan of chondrocytes that is an obstacle in the treatment of large OCDs [102].
Another therapeutic possibility makes use of cultured chondrocytes, which are expanded and finally implanted at the defect site [103]. ACI is an alternative to OAT and it involves harvesting a small amount of cartilage for chondrocyte isolation and culturing (in vitro), usually from a knee ipsilateral to the ankle injury [87,88,103]. Cell-based techniques have gained relevance in OCDs because, unlike bone-marrow-stimulation methods, where fibrocartilage fills the defect, cells can potentially induce regeneration and produce a ‘hyaline-like cartilage’ [104]. Nevertheless, a recent study [105] has shown that chondrocytes from the injured zone in the ankle have poorer regenerative capacities as compared with normal tissue, stating some reservations to their use in the therapeutic field. Thus, it seems that the source for harvesting cells should be a normal, healthy tissue, requiring one additional surgical procedure and limited associated morbidity.
On the other hand, the differentiated cells are sensitive and can present biochemical changes or diminished viability during the processes of harvesting, culturing, expansion or re-implantation in the defect zone [6].
The potential of ACI in the treatment of OCDs has been the source of great enthusiasm since the study performed by Brittberg et al. [103]. After 3 years of follow-up, the transplants restored considerable knee function in 14 of the 16 patients with femoral defects. The treatment resulted in the formation of new cartilage that was similar to normal cartilage in that it had an abundance of type II collagen and metachromatically stained matrix, similar as in original cartilage.
Still, despite several successes reported by the followers of this technique [106], up to now there is no evidence-based medicine to support their use, with no proven cost-effective advantages as compared to ‘classic’ treatment options such as microfractures or osteochondral grafting techniques (OAT, mosaicplasty) [107–111].
Some advocate specific conditions for its use, for example a defect area more than 4 cm2 (factor predictor of a better outcome with ACI), reinstating the existence of specific injury and individual's conditions which might play a determinant role in outcome [112]. As aforementioned, gene therapy can enhance the clinical application of differentiated cells as stated by Orth et al. [113]. That study demonstrated that chondrocytes modified for higher co-expression of IGF-1 and FGF-2 hold an increased chondrogenic capacity in vivo.
2.1.3. Applications of biomaterials
Hyaline cartilage serves as a low-friction surface with high wear resistance for weight-bearing joints. Unfortunately, it possesses an avascular and alymphatic profile which limits its autonomous regenerative capacity. The application of differentiated cells in the clinic presents additional problems such as cells' tendency towards losing their differentiated phenotype in a two-dimensional culture (e.g. chondrocytes) and to differentiate towards a fibroblast-like phenotype [114]. To overcome this problem in the treatment of cartilage lesions, different scaffolds have been developed for supporting cell adhesion, proliferation and maintenance of phenotype in an effective manner [4,115].
Among the several scaffolds proposed in an attempt to better fulfil the requirements of cartilage regeneration process, there are substantial differences regarding the materials chosen and their physical forms (i.e. fibers, meshes and gels). Solid scaffolds provide a substrate on which cells can adhere, whereas gel scaffolds physically entrap the cells [116]. The biomaterials used can be classified as synthetic or natural. Synthetic matrices present mechanical properties and degradation rates more easily tuned as compared with that of natural polymers, but some biocompatibility concerns might be raised owing to their degradation products and potential effect on native tissue and implanted cells. However, innovations in chemistry and materials science have been improving their biocompatibility [116]. Among the natural and synthetic materials that have been investigated (e.g. gellan gum, alginate, silk fibroin, chitosan, hydroxyapatite, collagen, hyaluronic acid (HA), polyglycolic acid and polylactic acid) [117], few have been used in ankle lesions, probably due to the lack of studies in the field of ankle tissue regeneration. Table 1 [5,53,93,118–127,129,130,132–140,142–145,147–151] summarizes the most important reports on polymers, ceramics and composites that have been used as scaffolds for osteochondral tissue regeneration.
Table 1.
Biomaterials used in the preparation of scaffolds for osteochondral tissue regeneration.
| repeating unit | properties | examples of proposed applications | |
|---|---|---|---|
| natural polymers | |||
| collagen | 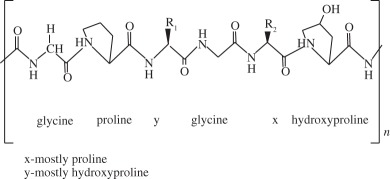 |
it is the most abundant protein in the body. It possesses high mechanical strength, good biocompatibility and low antigenicity, which make it suitable for tissue engineering. Combinations of other materials are also described, as well as GFs or cell implantation | atelocollagen gel was reported to be successfully used on OCDs on talar dome [118] collagen bioscaffold seeded with autologous chondrocyte for the treatment of OCDs in rabbit knee [119] collagen biphasic-based scaffolds were used in OCDs of the goat and compared to PLGA. Both provide indications of satisfactory development of a structural repair [120] |
| silk fibroin | 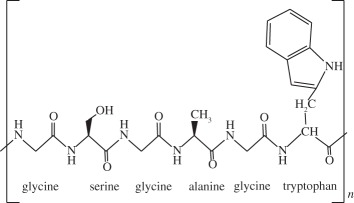 |
it contains a highly repetitive primary sequence that leads to a high content of β-sheets, responsible for the good mechanical properties of silk fibres. It has been shown to be a biocompatible material that allows good cell attachment, providing an adequate three-dimensional porous structure and the necessary mechanical support for bone and cartilage tissue generation | porous silk scaffolds, bioreactors and BMSCs were used to engineer cartilage- or bone-like tissue constructs [121] silk fibroin scaffolds were reported to be suitable for use in meniscus and cartilage tissue-engineered scaffolding [5] rabbit BMSC/silk fibroin scaffold-based co-culture approach was used to generate tissue-engineered osteochondral grafts [122] |
| alginate | 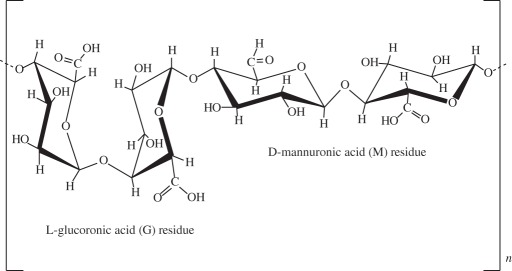 |
it is non-toxic, biocompatible and biodegradable natural polymer that is widely applied in drug and cell delivery systems. Hydrogel formation can be obtained by interactions of anionic alginates with multivalent inorganic cations by simple ionotropic gelation method. Hydrophilic polymeric network of three-dimensional cross-linked structures of hydrogels absorbs substantial amount of water or biological fluids | alginate droplets were gelated to form a highly organized scaffold and the feasibility of the use of this scaffold in cartilage tissue engineering was demonstrated [123] alginate-based bilayered scaffolds loaded with GFs on rabbit knee [93] |
| chitosan | 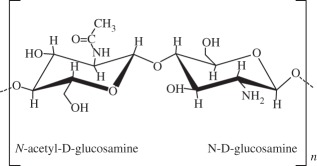 |
it is a derivative of chitin and partially de-acetylated. Structurally, chitosan is a linear polysaccharide that shares some characteristics with various glycosaminoglycans and hyaluronic acid present in articular cartilage, composed of glucosamine and N-acetyl glucosamine. Some important properties are its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity and wound-healing ability | development of novel hydroxyapatite/chitosan bilayered scaffold that shows potential for being used in TE of OCDs [124] appropriate chitosan properties were evaluated for an in vivo osteochondral tissue regeneration on rabbit knee [125] |
| hyaluronic acid | 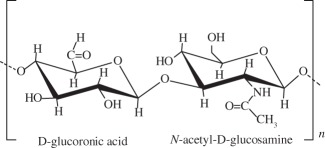 |
one of the most important components of the ECM. Is soluble in water and can form hydrogels by covalent and photo-cross-linking, esterification and annealing. It is enzymatically degraded by hyaluronidase. The degradation products of hyaluronan, the oligosaccharides and very low-molecular-weight hyaluronan exhibit pro-angiogenic properties and can induce inflammatory responses in macrophages and dendritic cells in injured tissues |
in situ photo-cross-linkable hyaluronan was developed and evaluated as a scaffold for articular cartilage repair in vitro [126] MSCs were seeded in a hyaluronan scaffold for repair of an OCD in rabbit knee [127] |
| gellan gum | 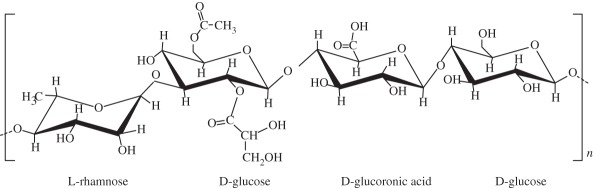 |
it forms thermoreversible gels possessing mechanical properties varying from soft to elastic. Presents no toxicity and it could be used in a non-invasive manner. Similar structure to native cartilage glycosaminoglycans | gellan gum adequately supported the growth and ECM deposition of human articular chondrocytes implanted subcutaneously in nude mice [128] successful encapsulation of human nasal chondrocytes on gellan gum [129] gellan gum hydrogels seeded with autologous cells proved to be a promising approach in treatment of cartilage defects in rabbit knee [129,130] |
| synthetic polymers | |||
| poly(ethylene glycol) derivatives | 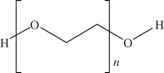 |
synthetic hydrogels are water-swollen polymeric networks, usually consisting of cross-linked hydrophilic polymers that can swell, but do not dissolve in water. This ability to swell under biological conditions makes them an ideal class of materials for biomedical applications, such as drug delivery systems and tissue engineering scaffolds for cell encapsulation. Hydrogels possess a three-dimensional network structure, cross-linked together either physically or chemically. This insoluble cross-linked structure allows effective immobilization and release of active agents and biomolecules or even cells. Generally exhibit good biocompatibility and high permeability to gases, nutrients and other water-soluble metabolites, making them attractive scaffolds | poly(ethylene glycol)-based hydrogels used in osteochondral knee defect in rats [132] oligo[poly(ethylene glycol) fumarate] hydrogel alone or loaded with BMSCs to endorse fully repair of OCDs on porcine model [133,134] |
| PLGA | 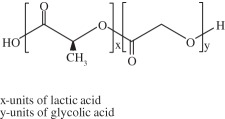 |
biodegradable and biocompatible and having mechanical strength, suitable for cartilage repair. It can be tuned with different pore size along the scaffold and combined with other polymers, for example polyurethane. It is suitable for seeding with BMSCs and GFs | biphasic cylindrical porous plug of PGLA with β-tricalcium phosphate was used to repair articular cartilage in porcine model [135] PLGA scaffold was implanted into OCDs on femoral trochlea of rabbits [136] bilayered porous scaffolds seeded with BMSCs for regeneration of OCDs on rabbit knee [137] PLGA-based bilayered scaffolds loaded with GFs on rabbit knee [138] |
| poly(l-lactic acid) (PLLA) | 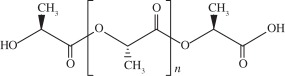 |
biodegradable polyester that exhibits mechanical properties suitable for bone tissue regeneration. It degrades by hydrolytic scission of its ester bonds, yielding the physiologic molecule lactic acid. As a biodegradable material, it is suitable for tissue engineering, owing to the fact that the newly formed tissue can invade the space while the material degrades | PLLA-based scaffold incorporated with GFs was used to repair articular cartilage defect in a rabbit model [139] PLLA/hydroxyapatite nanocomposites induced differentiation of hMSCs in a chondrocyte-like phenotype with generation of a proteoglycan-based matrix [140] optimization of the mineralization process on a PLLA macroporous scaffold on OCDs performed in the medial femoral condyle of healthy sheep [141] |
| polycaprolactone (PCL) | 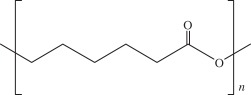 |
it is one of the most widely used biodegradable polyesters for medical application owing to its slow biodegradability, biocompatibility, mechanical properties and structural flexibility. PCL expresses slow degradation kinematics and its degradation products are harmlessly metabolized in the tricarboxylic acid cycle | three-dimensional PCL scaffolds with BMP-2 were applied to investigate the influence of BMP-2 on cartilage matrix and bone matrix production [142] nanostructured porous PCL scaffold was developed to stimulate articular cartilage repair. It improved chondrocytic differentiation to produce more hyaline-like tissue [143] |
| ceramics | chemical structure | properties | examples of proposed applications |
| hydroxyapatite | Ca10(PO4)6(OH)2 | it presents high biocompatibility, but low strength and fracture toughness, which may be a problem in OCD engineering. The osteocondutive properties of hydroxyapatite-based materials can be improved by manipulation of the structural characteristics | implants load with BMSCs have proved to be useful in bone repair of sheep long bones [144] trilayered scaffold with collagen and hydroxyapatite used on osteochondral regeneration in the femoral condyles of the sheep [145,146] composed with zirconia has been proved to be an effective scaffold for cartilage tissue engineering [147] |
| aragonite | Ca(CO3) | it is a biological material very similar to bone, including its three-dimensional structure and pore interconnections that confer osteoconductive ability. Nevertheless, the native material does not regenerate hyaline cartilage | aragonite–hyaluronate bi-phasic scaffold showed cartilage regenerative potential in a goat model [148] |
| tricalcium phosphate | Ca3(PO4)2 | it is a calcium salt of phosphoric acid, widely used as a synthetic alternative owing to their chemical similarity to the mineral part of the bone. Presents a high osteoconductivity and a cell-mediated resorption. Calcium and phosphate ions released during the resorption can be used to mineralize new bone in the bone remodelling process. It may be used alone or in combination with a biodegradable and resorbable polymer, for example polyglycolic acid | tricalcium phosphate-based scaffold loaded with GFs was reported to induce chondrogenic differentiation, tissue formation and differentiation in a mini-pig model [149] microporous three-dimensional calcium phosphate was seeded with autologous chondrocytes and implanted in femoral condyle of ovine knees [150,151] |
Biomaterials including ceramics and polymers, such as aragonite [148], silk fibroin [5,121] or tricalcium phosphate [149–151], are some of the most promising materials for OCD regeneration, alone or alternatively blended with other materials.
The application of an injectable biomaterial with bioadhesive properties, for example gellan gum (figure 6a), for regeneration of cartilage has been proposed for the first time by Oliveira et al. [129]. The gellan gum hydrogel was shown to efficiently sustain the delivery and growth of human articular chondrocytes and support the deposition of a hyaline-like ECM [128], leading to the formation of a functional cartilage. The use of biocompatible gellan gum-based hydrogels (e.g. methacrylated gellan gum, GG-MA) is also justified due to their many advantages such as improved biostability, tuneable degradability, mechanical properties and bioadhesiveness [52,130,131]. The versatility of the injectable gellan gum hydrogels and functionalized derivatives allowed the development of ionic- and photo-cross-linked GG-MA hydrogels, with improved mechanical properties for in situ gelation, within seconds to a few minutes [152,153]. Besides being able to serve as carriers of GFs/drugs and/or cells and promote ECM production, in another study [154], GG-MA hydrogels have been shown to possibly enable the control of the neovascularization process. In other words, one can use two different forms of gellan gum-based hydrogels to transport different cells: (i) in a given zone, facilitate vascular ingrowth (e.g. area to integrate in subchondral bone in a grade IV injury according to International Cartilage Repair Society) and (ii) in another area, prevent neovascularization and re-innervation by the presence of the hydrogel itself while it can also transport chondrocytes to the region that will replace hyaline cartilage [154]. That important work brings new insights to mimicking more precisely the native properties of tissue, because different tissues require neovascularization for regeneration, as in others vascularization and re-innervation is associated with pain and degeneration [155]. In fact, one of the goals of TERM is, precisely, to maintain the human characteristics of the natural tissue and so the knowledge of physiology of the original tissue is crucial.
Figure 6.
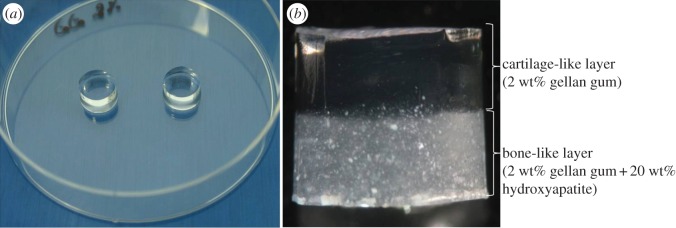
Photographs of gellan gum hydrogels: (a) single and (b) bilayered.
Another biomaterial that has been tested, including in talar dome resurfacing, is collagen in its many presentations [66]. Besides its biocompatibility and positive results for the management of painful post-traumatic of the ankle joint, the biomechanical properties and stability remains an issue in several of its applications [66,118].
Hydrogel systems have been developed to obtain optimal nutrient diffusion [40,49], connectivity with host matrix, adequate biodegradability, solubility and mechanical properties to facilitate the production and organization of the matrix [14]. Several improvements have been achieved with several former systems, but the ‘ideal’ one remains to be established [156]. One of the most studied hydrogels is based in HA. The use of HA as adjuvant of microfractures surgical treatment (i.e. bone-marrow-stimulation techniques) seems to improve the results of microfractures alone, taking advantages of HA's rheological properties [157].
Since a treatment that focuses exclusively on articular cartilage is likely to fail [90], it has been suggested that treatment strategies should be designed with the entire osteochondral unit (articular cartilage and subchondral bone) [90]. Therefore, bilayered porous scaffolds with poly(lactide-co-glycolic) (PLGA) seeded with BMSCs [137] or with GFs [138] were reported to simultaneously regenerate cartilage and subchondral bone of rabbit knee. Porous PLGA–calcium sulfate biopolymer (TruFit by Smith and Nephew, London, UK) is one of the most popular commercially available devices (probably the most clinically tested) [135,136], and it has been applied from mono- to bilayered presentations (figure 7). Jiang et al. [135] observed bone formation in the osseous phase, with evident subchondral remodelling, as well as normal hyaline cartilage, in a mini-pig model, when cell suspension (composed of the harvested autogenous cartilage) was injected into the chondral phase of the PLGA scaffold. More recently, this device is also available in a shape adapted to the anteromedial talar corner. However, there is still little evidence-based medical data supporting its use in either acellular or cellular strategies, besides the existence of some concerns with polyglycolic acid biocompatibility [90,158].
Figure 7.
(a) Photograph of TruFit PLGA-based scaffold delivery device, (b) defect zone prepared to receive the plug and (c) arthroscopically implanted device to resurface the defect preserving joint congruency.
In the field of ceramic polymers, hydroxyapatite is one of the most used implant materials for medical applications owing to its high biocompatibility [144]. It seems to be the most appropriate ceramic material for cartilage tissue engineering. However, owing to low strength and fracture toughness of the material, new approaches have been reported [147] in order to achieve a scaffold with the most suitable properties for cartilage tissue engineering. Sotoudeh et al. [147] reported that a composite of zirconia and hydroxyapatite would be an effective scaffold for cartilage regeneration.
The use of bilayered scaffolds (figure 6b) that combine different materials in the same implant constitutes a natural evolution in OCD treatment, in an attempt to combine favourable properties to both bone integration and cartilage repair [124,159]. In fact, it has been shown that the hydroxyapatite layer permits adhesion and proliferation of MSCs and osteogenic differentiation in vitro [124], while facilitating new bone formation in vivo [72]. By its turn, the cartilage-like layer is also able to support the adhesion of MSCs and can promote chondrogenesis, in vitro.
Another important commercially available product is MaioRegen (Fin-Ceramica SpA, Faenza, Italy) [146,160], which is a trilayered scaffold for treatment of OCDs. The deepest layer is composed of hydroxyapatite, the intermediate layer is a mixture of type I collagen and hydroxyapatite and the superficial layer consists of type I collagen only. In a previous study performed in vitro and in vivo, Kon et al. [145] obtained similar results when the scaffold was loaded with autologous chondrocytes or when it was used alone. The ability of the scaffold to induce OCD repair without the seeding of autologous cells makes it very attractive [146]. Comparative studies with OAT, ACI and bone-marrow-stimulation techniques are needed to establish the clinical outcome of this procedure.
2.1.4. Applications of advanced tissue engineering and regenerative medicine strategies
The requirement for full OCD repair has been approached considering the heterogeneity of different tissues, different components and layers (including subchondral bone plate and different hyaline cartilage layers). This is also part of the underlying principle for OAT. Although some attempts have been made to overcome one of the most relevant problem of OAT [107], relevant morbidity related to donor zone in knee-to-ankle transplantation has been demonstrated [110,161]. Furthermore, other problems persist with these techniques including graft's source, achievement of joint congruence and interface between graft plugs and between grafts and native cartilage. It is generally accepted that the use of a lower number of plugs is a predictor of a better mid- to long-term outcome [107].
Table 2 [162–165] summarizes the most important clinical studies related to TERM strategies for treatment of ankle lesions. Those studies have tested two main biomaterials, i.e. collagen and hyaluronan-based scaffolds/membranes, with matrix-induced ACI (MACI, Verigen, Leverkusen, Germany) being the most used approach. This technique can be considered as an evolution of conventional ACI and it makes use of processed cells that are harvested and isolated from the patient and expanded in vitro. Once grown, the chondrocytes are seeded between layers of a bilayered collagen scaffold in the operating room, prior to implantation of cell–scaffold construct into the defect area.
Table 2.
Clinical studies on TE of cartilage/OCD of the ankle.
| references | biomaterial/treatment approach | defect area/follow-up | procedure | outcome |
|---|---|---|---|---|
| Giannini et al. [162] | Hyalograft C scaffold seeded with human autologous chondrocytes | ankle/12 and 36 months | patients (n = 46) with a mean age of 31.4 years, post-traumatic talar dome lesions. First procedure: ankle arthroscopy to harvest cartilage. Chondrocytes were cultured on Hyalograft C scaffold. In the second step, the construct was arthroscopically implanted into the lesion site. Patients were evaluated by AOFAS score pre-operatively and at 12 and 36 months post-surgery | the mean pre-operative AOFAS score was 57.2 ± 14.3. After 12 and 36 months, the scores were 86.8 ± 13.4 and 89.5 ± 13.4, respectively. Clinical results were significantly related to the age of patients and to previous operations for cartilage repair. Histological stainings have revealed that hyaline-like cartilage was formed |
| Giannini et al. [163] | collagen powder/hyaluronan membrane loaded with concentrated BMDCs | ankle/6, 12, 18 and 24 months | patients (n = 23) used collagen/MSCs, and 25 patients used hyaluronan/MSCs for the treatment. Porcine collagen powder (Spongostan Powder) and hyaluronic acid membrane (HYAFF-11) were used. At first, bone marrow was harvested and concentrated. Then, the collagen powder or hyaluronan membrane was mixed with bone marrow and platelet-rich fibrin gel and composites were implanted | for the collagen powder group, the mean AOFAS scores of pre-operation and 24 months post-operation were 62.5 ± 18 and 89.8 ± 9.8, respectively. In the hyaluronic acid group, the scores increased from 66.2 ± 10.5 to 92.8 ± 5.3, 24 months after the surgery. At 2 years follow-up, MRIs showed the restoration of the cartilage layer and subchondral bone of the patients |
| Giza et al. [164] | collagen type I/III bilayered membrane with autologous chondrocytes | ankle/1 and 2 years | patients (n = 10) with average age of 40.2 years. The size and location of the defects were analysed by ankle arthroscopy, and cartilage was also harvested from the border or the lesion. Expanded chondrocytes were seeded into the collagen membrane. The joint was exposed with a small anterolateral or anteromedial approach, without malleolar osteotomy. The graft was cut and placed into the defect on top of a layer of fibrin sealant | the AOFAS hindfoot scores increased from 61.2 (pre-operative, ranged from 42 to 76) to 74.7 (1 year post-operative, ranged from 46 to 87) and 73.3 (2 year post-operative, ranged from 42 to 90). At 19 months post-operation, MRIs showed the regeneration of articular cartilage and subchondral bone |
| Aurich et al. [165] | collagen type I scaffold with autologous chondrocytes (MACI) | ankle/mean follow-up 24.5 months | patients (n = 18, with a total of 19 defects) with mean age of 29.2. Arthroscopy was used for the evaluation and debridement on the defects, as well as the harvest of cartilage. Cultured chondrocytes were seeded into the collagen membrane and implanted in the defects, with fibrin as the glue. MOCART score, the pain and disability module of the foot function index (FFI), AOFAS score and the core scale of the foot and ankle module of the American Academy of Orthopaedic Surgeons (AAOS) lower limb outcomes assessment instruments were used | according to AOFAS hindfoot score, 64% were rated as excellent and good, whereas 36% were rated fair and poor. The results correlated with the age of the patient and the duration of symptoms, but not with the size of the lesion. Mean MOCART score was 62.4 ± 15.8 points. There was no relation between MOCART score and the clinical outcome |
The studies that have been reported demonstrate [119,121,133,151] that combination of scaffolds and autologous cells can enhance the regeneration outcome, using scores adopted either by American Orthopaedic Foot and Ankle Society (AOFAS) or Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART).
Cellular-based techniques, such as ACI and MACI, require a two-stage operative procedure, where initial harvesting of cartilage is followed by culturing and subsequent implantation of the cultured tissue. In fact, this issue has been considered one of the major drawbacks of ACI. This has been the driving force for the search for new treatment methods [166] and development of novel and bioactive scaffolds, which can be easily implanted and fixed, and best mimic the native tissue to be repaired. The use of bilayered tridimensional porous scaffolds enhanced by MSCs requires several years of preclinical research [124]. Still, it remains a trend with high interest and investment from the scientific community. The histological results are available only in animal studies, but are indeed very encouraging [145]. Clinically, they have been applied up to now only in the knee, but they may represent a solution for the repair of deep OCDs even in the ankle [100,146]. The development of the ideal scaffold has been performed in a stepwise manner and is dependent on the knowledge gained in the last few years, in what concerns the biomechanical and biological properties of native tissues [5].
MSCs are emerging as a powerful tool for treatment of cartilage lesions, thanks to their ability to differentiate into various lineages [167]. In particular, the use of concentrated bone marrow instead of chondrocytes, in order to provide MSCs to be seeded onto the scaffold, has been recently introduced in clinical practice as a one-step procedure for the treatment of OCDs.
Giannini et al. [163] described their experience with bone-marrow-derived cells (BMDCs) implanted in talar dome focal OCDs. Two types of scaffolds were tested. Both collagen powder and hyaluronic acid membrane showed similar clinical improvement at 2 years in AOFAS score and a good MRI. Recently, the same group [168] compared the clinical outcome in focal osteochondral monolateral talar dome lesions after three different surgical approaches: (i) open fifirst generation ACI, (ii) arthroscopic Hyalograft C (Fidia Advanced Biopolymers Laboratories, Padova, Italy) implantation, and (iii) arthroscopic repair by BMDC implantation on a hyaluronic acid membrane. Although similar pattern of improvement was found at 3 years follow-up in all groups regarding collagen type II and proteoglycan expression, BMDCs showed a marked reduction in procedure morbidity and costs, demonstrating it to be a one-step technique able to overcome most of the drawbacks of previous techniques. Nearly complete integration of the regenerated tissue with the surrounding cartilage was demonstrated in 76% of the cases. In addition, histological analysis highlighted the presence of all components of hyaline cartilage in repaired tissue, which showed various degrees of remodelling.
Finally, Battaglia et al. [169] confirmed the good results of BMDC transplantation, with 85% of good to excellent clinical outcome, and demonstrated the ability to regenerate hyaline cartilage but not the capability of osteogenesis in OCD repair. In fact, regenerated mature bone was evident only in two cases and in less than 8% of regenerated volume. It must also be kept in mind that the phenotypic preservation of chondrocytes and/or adequate manipulation of MSC differentiation process in different tissues remain as challenging unsolved issues. Chondrocytes are ‘fragile’ cells, exposed to de-differentiation during laboratory manipulation (loss of original phenotype) [59,68,111]. The differentiation of MSCs into chondrocytes is a multi-factorial, complex target which requires, in vitro, the contemplation of simulators of biophysical stimulus present in normal tissues—bioreactors [26,135,136,158,170]. Both cell types remain under preclinical investigation and the bench-to-bedside transfer is still an unclosed matter.
The treatment of different focal OCDs by means of using autologous chondrocyte transplantation in tridimensional support scaffolds has been recently attempted [10,108,112,164]. Aiming to enhance this therapeutic strategy, the simultaneous application of GFs has also been evaluated, attempting to favour local environment for short-term integration and promote differentiation [10,11].
A recent study comparing two commercially available methods, (i) Hyalograft C (used by arthroscopic application) and (ii) Chondro-Gide MACI (open surgery application), concluded that both methods led to positive results, but the method of application influenced short-term results [171]. Arthroscopic application seems to provide faster rehabilitation, despite no significant differences being noted at 2 years follow-up. The reported failure rate was globally 20% highlighting the need for improvement of both techniques. The authors considered results as fair/good and recommended consideration of these techniques when debridement and bone marrow stimulation fail [171].
Gene therapy can provide some new answers to previously described pitfalls and limitations, but it might raise a different level of concern. The use of chondrocytes genetically transfected to increase the expression of BMP-7 inoculated into a fibrin–collagen scaffold provided better histological results as compared with controls (rabbit model) [18].
TERM applications have not only been attempted in focal defects but also in global joint degeneration, i.e. arthritis. Joint replacement using biological tissue modified using TERM principles to mimic osteochondral tissue has been attempted [172]. In addition, the use of synthetic materials (e.g. ceramics) enhanced by MSCs aiming at future application in patients presently referred to fusion or total ankle arthroplasty has been evaluated [173].
Concerning focal defects, a non-biological solution developed by van Dijk's group [174] presented promising results by means of contoured focal metallic replacement (figure 8), despite the lack of mid- to long-term follow-up in larger series.
Figure 8.
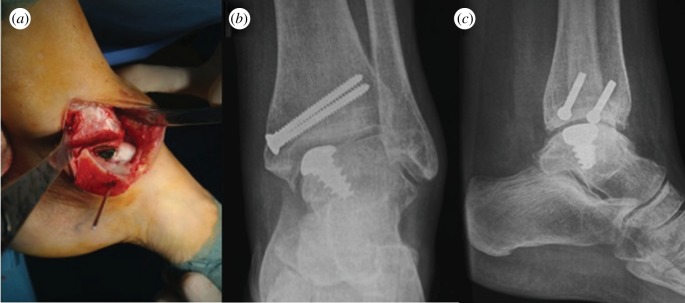
(a) Per-operative photograph of Hemicap ankle implant after tibial osteotomy and control X-ray in (b) frontal and (c) lateral views at 1 year follow-up.
An important issue regarding the applications of biomaterials is the implant–tissue interface. Because of the geometric complexity of the ankle and the relative thickness of its cartilage, the use of focal resurfacing implants to treat talar OCDs, as well as biomaterials, presents challenges with regard to implant/biomaterial design, selection and surgical placement [175]. Considering the basic principles of TERM, besides biological conditions, ankle biomechanics must be taken into account [91] since it is a more congruent joint compared with the knee [176]. A congruent joint surface, for example the ankle, is usually covered with thinner hyaline cartilage compared with incongruent ones that possess thicker cartilage, for example in the knee. The diminishing of articular congruence produces higher contact pressure per joint area. Higher loss of congruence or malalignment will lead to growing contact pressure with all its implications [91,177,178]. Injured subchondral bone, as in OCDs, is less effective in supporting the overlying cartilage, and this might be one of the reasons explaining the greater difficulty for cartilage repair in these situations [179,180].
Becher et al. [181] measured contact stress redistribution in the human knee after implantation of a metallic resurfacing cap, and reported elevated contact stresses associated with device implants. Also, Custers et al. [182] stated that implants seem to cause considerable degeneration of the directly articulating cartilage in the knee. In the case of biomaterials, owing to their biocompatibility, integration into the surrounding cartilage is usually observed [183]. This way, the stress level changes on the joint are minor. However, the size and shape of the OCDs must be taken into account, to ensure that the biomaterial is as similar as possible, in order to completely fulfil the injured area.
3. Final considerations
The appropriate treatment for OCD repair is still controversial. The ideal technique would regenerate a tissue with biomechanical properties similar to normal hyaline articular cartilage, with reduced morbidity and costs. The excellent durability of results obtained by ACI or MACI over time is well established and contrasts sharply with the long-term results reported for bone-marrow-stimulating techniques (such as abrasion, drilling or microfractures).
A variety of biomaterials including polymers and ceramics have been proposed for regeneration of the cartilage of OCDs, and composite scaffolds (e.g. polymers combined with ceramics), especially if seeded with autologous cells and/or GFs, seem to improve biomechanical results.
Up to now only a few clinical trials on ankle healing have been described, whereas a scaffold approach to the treatment of knee chondral lesions has been largely used in clinical practice, with excellent or good clinical results largely documented in the literature. New approaches must be considered to talus osteocondral defects in order to improve restoration. Although there are particularities of such area, other biomaterials with significant results in knee OCDs may be applied to the ankle lesions.
TERM approaches are changing the paradigms of medicine and surgical practice. However, the success of these technologies at present and in future demands deep knowledge of native tissue biology and understanding of its repair mechanisms and response to injury, as well as the new biomaterials under consideration. Basic rules of biology and other ‘basic sciences’ (understanding basic only as fundamental, never as simple) must be well known by all surgeons since only in this way will they be able to understand, adapt and assist in the development of this knowledge to clinical practice.
TERM approaches have proven efficacy in clinical cases and problems which used selection criteria not previously solved by ‘conventional’ therapeutic repair and/or replacement options. However, undiscriminating use of any promising technique is one of the most effective ways to impair or even block its proper development.
Funding statement
The authors acknowledge the Portuguese Foundation for Science and Technology (FCT) through the POCTI and FEDER programmes, including Project OsteoCart (grant no. PTDC/CTM-BPC/115977/2009) for providing funds.
References
- 1.Langer R, Vacanti J. 1993. Tissue engineering. Science 260, 920–926. ( 10.1126/science.8493529) [DOI] [PubMed] [Google Scholar]
- 2.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noel D. 2009. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 27, 307–314. ( 10.1016/j.tibtech.2009.02.005) [DOI] [PubMed] [Google Scholar]
- 3.Rush SM. 2010. Trinity evolution: mesenchymal stem cell allografting in foot and ankle surgery. Foot Ankle Spec. 3, 140–143. ( 10.1177/1938640010369638) [DOI] [PubMed] [Google Scholar]
- 4.Ducheyne P, Mauck RL, Smith DH. 2012. Biomaterials in the repair of sports injuries. Nat. Mater. 11, 652–654. ( 10.1038/nmat3392) [DOI] [PubMed] [Google Scholar]
- 5.Yan LP, Oliveira JM, Oliveira AL, Caridade SG, Mano JF, Reis RL. 2012. Macro/microporous silk fibroin scaffolds with potential for articular cartilage and meniscus tissue engineering applications. Acta Biomater. 8, 289–301. ( 10.1016/j.actbio.2011.09.037) [DOI] [PubMed] [Google Scholar]
- 6.Balash P, Kang RW, Schwenke T, Cole BJ, Wimmer MA. 2010. Osteochondral tissue cell viability is affected by total impulse during impaction grafting. Cartilage 1, 270–275. ( 10.1177/1947603510367913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Amin SF, Hogan MV, Allen AA, Hinds J, Laurencin CT. 2010. The indications and use of bone morphogenetic proteins in foot, ankle, and tibia surgery. Foot Ankle Clin. 15, 543–551. ( 10.1016/j.fcl.2010.08.001) [DOI] [PubMed] [Google Scholar]
- 8.Engebretsen L, et al. 2010. IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br. J. Sports Med. 44, 1072–1081. ( 10.1136/bjsm.2010.079822) [DOI] [PubMed] [Google Scholar]
- 9.Furth ME, Atala A, Van Dyke ME. 2007. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 28, 5068–5073. ( 10.1016/j.biomaterials.2007.07.042) [DOI] [PubMed] [Google Scholar]
- 10.Dhollander AA, De Neve F, Almqvist KF, Verdonk R, Lambrecht S, Elewaut D, Verbruggen G, Verdonk PC. 2011. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg. Sports Traumatol. Arthrosc. 19, 536–542. ( 10.1007/s00167-010-1337-4) [DOI] [PubMed] [Google Scholar]
- 11.Kreuz PC, Muller S, Freymann U, Erggelet C, Niemeyer P, Kaps C, Hirschmuller A. 2011. Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. Am. J. Sports Med. 39, 1697–1705. ( 10.1177/0363546511403279) [DOI] [PubMed] [Google Scholar]
- 12.Freed LE, Vunjak-Novakovic G, Langer R. 1993. Cultivation of cell-polymer cartilage implants in bioreactors. J. Cell Biochem. 51, 257–264. ( 10.1002/jcb.240510304) [DOI] [PubMed] [Google Scholar]
- 13.Brittberg M. 2010. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am. J. Sports Med. 38, 1259–1271. ( 10.1177/0363546509346395) [DOI] [PubMed] [Google Scholar]
- 14.Gurkan UA, Tasoglu S, Kavaz D, Demirci U. 2012. Emerging technologies for assembly of microscale hydrogels. Adv. Healthc. Mater. 1, 149–158. ( 10.1002/adhm.201200011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen S, Leshansky L, Zussman E, Burman M, Srouji S, Livne E, Abramov N, Itskovitz-Eldor J. 2010. Repair of full-thickness tendon injury using connective tissue progenitors efficiently derived from human embryonic stem cells and fetal tissues. Tissue Eng. A 16, 3119–3137. ( 10.1089/ten.TEA.2009.0716) [DOI] [PubMed] [Google Scholar]
- 16.Atesok K, Matsumoto T, Karlsson J, Asahara T, Atala A, Doral MN, Verdonk R, Li R, Schemitsch E. 2012. An emerging cell-based strategy in orthopaedics: endothelial progenitor cells. Knee Surg. Sports Traumatol. Arthrosc. 20, 1366–1377. ( 10.1007/s00167-012-1940-7) [DOI] [PubMed] [Google Scholar]
- 17.DeChellis DM, Cortazzo MH. 2011. Regenerative medicine in the field of pain medicine: prolotherapy, platelet-rich plasma therapy, and stem cell therapy: theory and evidence. Tech. Reg. Anesth. Pain Manag. 15, 74–80. ( 10.1053/j.trap.2011.05.002) [DOI] [Google Scholar]
- 18.Che JH, Zhang ZR, Li GZ, Tan WH, Bai XD, Qu FJ. 2010. Application of tissue-engineered cartilage with BMP-7 gene to repair knee joint cartilage injury in rabbits. Knee Surg. Sports Traumatol. Arthrosc. 18, 496–503. ( 10.1007/s00167-009-0962-2) [DOI] [PubMed] [Google Scholar]
- 19.Badylak SF, Nerem RM. 2010. Progress in tissue engineering and regenerative medicine. Proc. Natl Acad. Sci. USA 107, 3285–3286. ( 10.1073/pnas.1000256107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zengerink M, van Dijk CN. 2012. Complications in ankle arthroscopy. Knee Surg. Sports Traumatol. Arthrosc. 20, 1420–1431. ( 10.1007/s00167-012-2063-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeCarbo WT. 2009. Special segment: soft tissue matrices—Apligraf bilayered skin substitute to augment healing of chronic wounds in diabetic patients. Foot Ankle Spec. 2, 299–302. ( 10.1177/1938640009354041) [DOI] [PubMed] [Google Scholar]
- 22.Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, Ayeni OR, Bhandari M. 2012. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J. Bone Joint Surg. Am. 94, 298–307. ( 10.2106/JBJS.K.00154) [DOI] [PubMed] [Google Scholar]
- 23.Schliephake H. In press. Clinical efficacy of growth factors to enhance tissue repair in oral and maxillofacial reconstruction: a systematic review. Clin. Implant Dent. Relat. Res. ( 10.1111/cid.12114) [DOI] [PubMed] [Google Scholar]
- 24.Soomekh DJ. 2011. Current concepts for the use of platelet-rich plasma in the foot and ankle. Clin. Podiatr. Med. Surg. 28, 155–170. ( 10.1016/j.cpm.2010.09.001) [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. 2010. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy 26, 269–278. ( 10.1016/j.arthro.2009.11.015) [DOI] [PubMed] [Google Scholar]
- 26.Ruszczak Z, Friess W. 2003. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv. Drug Deliv. Rev. 55, 1679–1698. ( 10.1016/j.addr.2003.08.007) [DOI] [PubMed] [Google Scholar]
- 27.Bandyopadhyay A, Yadav PS, Prashar P. 2013. BMP signaling in development and diseases: a pharmacological perspective. Biochem. Pharmacol. 85, 857–864. ( 10.1016/j.bcp.2013.01.004) [DOI] [PubMed] [Google Scholar]
- 28.Bessa PC, Casal M, Reis RL. 2008. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts). J. Tissue Eng. Regen. Med. 2, 1–13. ( 10.1002/term.63) [DOI] [PubMed] [Google Scholar]
- 29.Urist MR. 1965. Bone: formation by autoinduction. Science 150, 893–899. ( 10.1126/science.150.3698.893) [DOI] [PubMed] [Google Scholar]
- 30.Yamada N, Uchinuma E, Kuroyanagi Y. 2008. Clinical trial of allogeneic cultured dermal substitutes for intractable skin ulcers of the lower leg. J. Artif. Organs 11, 100–103. ( 10.1007/s10047-008-0406-7) [DOI] [PubMed] [Google Scholar]
- 31.Winters CL, Brigido SA, Liden BA, Simmons M, Hartman JF, Wright ML. 2008. A multicenter study involving the use of a human acellular dermal regenerative tissue matrix for the treatment of diabetic lower extremity wounds. Adv. Skin Wound Care 21, 375–381. ( 10.1097/01.ASW.0000323532.98003.26) [DOI] [PubMed] [Google Scholar]
- 32.Brigido SA. 2006. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int. Wound J. 3, 181–187. ( 10.1111/j.1742-481X.2006.00209.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weil L., Jr 2011. Biologics in foot and ankle surgery. Foot Ankle Spec. 4, 249–252. ( 10.1177/1938640011415373) [DOI] [PubMed] [Google Scholar]
- 34.van Dijk CN, van Sterkenburg MN, Wiegerinck JI, Karlsson J, Maffulli N. 2011. Terminology for Achilles tendon related disorders. Knee Surg. Sports Traumatol. Arthrosc. 19, 835–841. ( 10.1007/s00167-010-1374-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longo UG, Garau G, Denaro V, Maffulli N. 2008. Surgical management of tendinopathy of biceps femoris tendon in athletes. Disabil. Rehabil. 30, 1602–1607. ( 10.1080/09638280701786120) [DOI] [PubMed] [Google Scholar]
- 36.Ebinesan AD, Sarai BS, Walley GD, Maffulli N. 2008. Conservative, open or percutaneous repair for acute rupture of the Achilles tendon. Disabil. Rehabil. 30, 1721–1725. ( 10.1080/09638280701786815) [DOI] [PubMed] [Google Scholar]
- 37.Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, Denaro V. 2009. Characteristics at haematoxylin and eosin staining of ruptures of the long head of the biceps tendon. Br. J. Sports Med. 43, 603–607. ( 10.1136/bjsm.2007.039016) [DOI] [PubMed] [Google Scholar]
- 38.Maffulli N, Ajis A, Longo UG, Denaro V. 2007. Chronic rupture of tendo Achillis. Foot Ankle Clin. 12, 583–596. ( 10.1016/j.fcl.2007.07.007) [DOI] [PubMed] [Google Scholar]
- 39.Sharma P, Maffulli N. 2005. Tendon injury and tendinopathy: healing and repair. J. Bone Joint Surg. 87, 187–202. ( 10.2106/jbjs.d.01850) [DOI] [PubMed] [Google Scholar]
- 40.Longo UG, Lamberti A, Maffulli N, Denaro V. 2011. Tissue engineered biological augmentation for tendon healing: a systematic review. Br. Med. Bull. 98, 31–59. ( 10.1093/bmb/ldq030) [DOI] [PubMed] [Google Scholar]
- 41.Reverchon E, Baldino L, Cardea S, De Marco I. 2012. Biodegradable synthetic scaffolds for tendon regeneration. Muscles Ligaments Tendons J. 2, 181–186. [PMC free article] [PubMed] [Google Scholar]
- 42.Moshiri A, Oryan A, Meimandi-Parizi A. 2013. Role of tissue-engineered artificial tendon in healing of a large Achilles tendon defect model in rabbits. J. Am. Coll. Surg. 217, 421–441. ( 10.1016/j.jamcollsurg.2013.03.025) [DOI] [PubMed] [Google Scholar]
- 43.Smith L, Xia Y, Galatz LM, Genin GM, Thomopoulos S. 2012. Tissue-engineering strategies for the tendon/ligament-to-bone insertion. Connect. Tissue Res. 53, 95–105. ( 10.3109/03008207.2011.650804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang J. 2012. Studies in flexor tendon reconstruction: biomolecular modulation of tendon repair and tissue engineering. J. Hand Surg. 37, 552–561. ( 10.1016/j.jhsa.2011.12.028) [DOI] [PubMed] [Google Scholar]
- 45.Kew SJ, et al. 2011. Regeneration and repair of tendon and ligament tissue using collagen fibre biomaterials. Acta Biomater. 7, 3237–3247. ( 10.1016/j.actbio.2011.06.002) [DOI] [PubMed] [Google Scholar]
- 46.Kryger GS, Chong AKS, Costa M, Pham H, Bates SJ, Chang J. 2007. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J. Hand Surg. 32, 597–605. ( 10.1016/j.jhsa.2007.02.018) [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Bogdanowicz D, Erisken C, Lee NM, Lu HH. 2012. Biomimetic scaffold design for functional and integrative tendon repair. J. Shoulder Elbow Surg. 21, 266–277. ( 10.1016/j.jse.2011.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Sterkenburg MN, van Dijk CN. 2011. Injection treatment for chronic midportion Achilles tendinopathy: do we need that many alternatives? Knee Surg. Sports Traumatol. Arthrosc. 19, 513–515. ( 10.1007/s00167-011-1415-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JY, et al. 2011. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS ONE 6, e17531 ( 10.1371/journal.pone.0017531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurtz CA, Loebig TG, Anderson DD, DeMeo PJ, Campbell PG. 1999. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am. J. Sports Med. 27, 363–369. [DOI] [PubMed] [Google Scholar]
- 51.de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, van Osch GJ. 2008. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am. J. Sports Med. 36, 1171–1178. ( 10.1177/0363546508314430) [DOI] [PubMed] [Google Scholar]
- 52.Pereira DR, et al. 2011. Development of gellan gum-based microparticles/hydrogel matrices for application in the intervertebral disc regeneration. Tissue Eng. C 17, 961–972. ( 10.1089/ten.tec.2011.0115) [DOI] [PubMed] [Google Scholar]
- 53.Oliveira JM, Sousa RA, Kotobuki N, Tadokoro M, Hirose M, Mano JF, Reis RL, Ohgushi H. 2009. The osteogenic differentiation of rat bone marrow stromal cells cultured with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles. Biomaterials 30, 804–813. ( 10.1016/j.biomaterials.2008.10.024) [DOI] [PubMed] [Google Scholar]
- 54.Sahoo S, Toh SL, Goh JCH. 2010. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials 31, 2990–2998. ( 10.1016/j.biomaterials.2010.01.004) [DOI] [PubMed] [Google Scholar]
- 55.Fang Q, Chen D, Yang Z, Li M. 2009. In vitro and in vivo research on using Antheraea pernyi silk fibroin as tissue engineering tendon scaffolds. Mater. Sci. Eng. C 29, 1527–1534. ( 10.1016/j.msec.2008.12.007) [DOI] [Google Scholar]
- 56.Yin Z, Chen X, Chen JL, Ouyang HW. 2010. Stem cells for tendon tissue engineering and regeneration. Expert Opin. Biol. Ther. 10, 689–700. ( 10.1517/14712591003769824) [DOI] [PubMed] [Google Scholar]
- 57.de Jonge S, de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Tol JL. 2011. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am. J. Sports Med. 39, 1623–1629. ( 10.1177/0363546511404877) [DOI] [PubMed] [Google Scholar]
- 58.Enea D, et al. 2012. Collagen fibre implant for tendon and ligament biological augmentation. In vivo study in an ovine model. Knee Surg. Sports Traumatol. Arthrosc. 21, 1783–1793. ( 10.1007/s00167-012-2102-7) [DOI] [PubMed] [Google Scholar]
- 59.Longo UG, Lamberti A, Petrillo S, Maffulli N, Denaro V. 2012. Scaffolds in tendon tissue engineering. Stem Cells Int. 2012, 517165 ( 10.1155/2012/517165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hogan MV, Bagayoko N, James R, Starnes T, Katz A, Chhabra AB. 2011. Tissue engineering solutions for tendon repair. J. Am. Acad. Orthop. Surg. 19, 134–142. [DOI] [PubMed] [Google Scholar]
- 61.Omae H, Zhao C, Sun YL, An K-N, Amadio PC. 2009. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J. Orthopaed. Res. 27, 937–942. ( 10.1002/jor.20823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paxton JZ, Donnelly K, Keatch RP, Baar K. 2009. Engineering the bone-ligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng. A 15, 1201–1209. ( 10.1089/ten.tea.2008.0105) [DOI] [PubMed] [Google Scholar]
- 63.Cancedda R, Giannoni P, Mastrogiacomo M. 2007. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 28, 4240–4250. ( 10.1016/j.biomaterials.2007.06.023) [DOI] [PubMed] [Google Scholar]
- 64.Muramatsu K, Doi K, Ihara K, Shigetomi M, Kawai S. 2003. Recalcitrant posttraumatic nonunion of the humerus. Acta Orthop. Scand. 74, 95–97. ( 10.1080/00016470310013734) [DOI] [PubMed] [Google Scholar]
- 65.Chou LB, Mann RA, Coughlin MJ, McPeake WT, Mizel MS. 2007. Stress fracture as a complication of autogenous bone graft harvest from the distal tibia. Foot Ankle Int. 28, 199–201. ( 10.3113/fai.2007.0199) [DOI] [PubMed] [Google Scholar]
- 66.Ramanujam CL, Sagray B, Zgonis T. 2010. Subtalar joint arthrodesis, ankle arthrodiastasis, and talar dome resurfacing with the use of a collagen–glycosaminoglycan monolayer. Clin. Podiatr. Med. Surg. 27, 327–333. ( 10.1016/j.cpm.2009.12.004) [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Bloemen V, Impens S, Moesen M, Luyten FP, Schrooten J. 2011. Characterization and optimization of cell seeding in scaffolds by factorial design: quality by design approach for skeletal tissue engineering. Tissue Eng. C 17, 1211–1221. ( 10.1089/ten.tec.2011.0092) [DOI] [PubMed] [Google Scholar]
- 68.Khaled EG, Saleh M, Hindocha S, Griffin M, Khan WS. 2011. Tissue engineering for bone production: stem cells, gene therapy and scaffolds. Open Orthop. J. 5(Suppl. 2), 289–295. ( 10.2174/1874325001105010289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nordsletten L. 2006. Recent developments in the use of bone morphogenetic protein in orthopaedic trauma surgery. Curr. Med. Res. Opin. 22, S13–S17. ( 10.1185/030079906X80585) [DOI] [PubMed] [Google Scholar]
- 70.Kleinschmidt K, Ploeger F, Nickel J, Glockenmeier J, Kunz P, Richter W. 2013. Enhanced reconstruction of long bone architecture by a growth factor mutant combining positive features of GDF-5 and BMP-2. Biomaterials 34, 5926–5936. ( 10.1016/j.biomaterials.2013.04.029) [DOI] [PubMed] [Google Scholar]
- 71.Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, Toda K. 2006. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg. Sports Traumatol. Arthrosc. 14, 1307–1314. ( 10.1007/s00167-006-0124-8) [DOI] [PubMed] [Google Scholar]
- 72.Oliveira JM, Kotobuki N, Tadokoro M, Hirose M, Mano JF, Reis RL, Ohgushi H. 2010. Ex vivo culturing of stromal cells with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles promotes ectopic bone formation. Bone 46, 1424–1435. ( 10.1016/j.bone.2010.02.007) [DOI] [PubMed] [Google Scholar]
- 73.Abukawa H, Shin M, Williams WB, Vacanti JP, Kaban LB, Troulis MJ. 2004. Reconstruction of mandibular defects with autologous tissue-engineered bone. J. Oral Maxillofac. Surg. 62, 601–606. ( 10.1016/j.joms.2003.11.010) [DOI] [PubMed] [Google Scholar]
- 74.Mankani MH, Kuznetsov SA, Shannon B, Nalla RK, Ritchie RO, Qin Y, Robey PG. 2006. Canine cranial reconstruction using autologous bone marrow stromal cells. Am. J. Pathol. 168, 542–550. ( 10.2353/ajpath.2006.050407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H, Zhi W, Lu X, Duan K, Duan R, Mu Y, Weng J. 2013. Comparative studies on ectopic bone formation in porous HA scaffolds with complementary pore structures. Acta Biomater. 9, 8413–8421. ( 10.1016/j.actbio.2013.05.026) [DOI] [PubMed] [Google Scholar]
- 76.Florczyk S, Leung M, Li Z, Huang J, Hopper R, Zhang M. 2013. Evaluation of three-dimensional porous chitosan–alginate scaffolds in rat calvarial defects for bone regeneration applications. J. Biomed. Mater. Res. A 101, 2974–2983. ( 10.1002/jbm.a.34593) [DOI] [PubMed] [Google Scholar]
- 77.Kokemueller H, Spalthoff S, Nolff M, Tavassol F, Essig H, Stuehmer C, Bormann KH, Rücker M, Gellrich NC. 2010. Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: experimental pilot study in sheep and first clinical application. Int. J. Oral Maxillofac. Surg. 39, 379–387. ( 10.1016/j.ijom.2010.01.010) [DOI] [PubMed] [Google Scholar]
- 78.Nagata M, et al. 2012. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: coordinated activation of bone formation and resorption. Bone 50, 1123–1129. ( 10.1016/j.bone.2012.02.631) [DOI] [PubMed] [Google Scholar]
- 79.Oliveira JM, Sousa RA, Malafaya PB, Silva SS, Kotobuki N, Hirose M, Ohgushi H, Mano JF, Reis RL. 2011. In vivo study of dendronlike nanoparticles for stem cells ‘tune-up’: from nano to tissues. Nanomedicine 7, 914–924. ( 10.1016/j.nano.2011.03.002) [DOI] [PubMed] [Google Scholar]
- 80.Bitton R. 2009. The economic burden of osteoarthritis. Am. J. Manag. Care 15, 230–235. [PubMed] [Google Scholar]
- 81.O'Loughlin PF, Heyworth BE, Kennedy JG. 2010. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am. J. Sports Med. 38, 392–404. ( 10.1177/0363546509336336) [DOI] [PubMed] [Google Scholar]
- 82.Bhosale AM, Richardson JB. 2008. Articular cartilage: structure, injuries and review of management. Br. Med. Bull. 87, 77–95. ( 10.1093/bmb/ldn025) [DOI] [PubMed] [Google Scholar]
- 83.Shearer C, Loomer R, Clement D. 2002. Nonoperatively managed stage 5 osteochondral talar lesions. Foot Ankle Int. 23, 651–654. ( 10.1177/107110070202300712) [DOI] [PubMed] [Google Scholar]
- 84.Giannini S, Buda R, Faldini C, Vannini F, Bevoni R, Grandi G, Grigolo B, Berti L. 2005. Surgical treatment of osteochondral lesions of the talus in young active patients. J. Bone Joint Surg. 87, 28–41. ( 10.2106/jbjs.e.00516) [DOI] [PubMed] [Google Scholar]
- 85.Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. 2006. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy 22, 1085–1092. ( 10.1016/j.arthro.2006.05.016) [DOI] [PubMed] [Google Scholar]
- 86.Savva N, Jabur M, Davies M, Saxby T. 2007. Osteochondral lesions of the talus: results of repeat arthroscopic debridement. Foot Ankle Int. 28, 669–673. ( 10.3113/fai.2007.0669) [DOI] [PubMed] [Google Scholar]
- 87.Zengerink M, Struijs PA, Tol JL, van Dijk CN. 2010. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg. Sports Traumatol. Arthrosc. 18, 238–246. ( 10.1007/s00167-009-0942-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Dijk CN, van Bergen CJ. 2008. Advancements in ankle arthroscopy. J. Am. Acad. Orthop. Surg. 16, 635–646. [DOI] [PubMed] [Google Scholar]
- 89.Chen H, Chevrier A, Hoemann CD, Sun J, Ouyang W, Buschmann MD. 2011. Characterization of subchondral bone repair for marrow-stimulated chondral defects and its relationship to articular cartilage resurfacing. Am. J. Sports Med. 39, 1731–1740. ( 10.1177/0363546511403282) [DOI] [PubMed] [Google Scholar]
- 90.Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, Kon E. 2010. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg. Sports Traumatol. Arthrosc. 18, 434–447. ( 10.1007/s00167-010-1072-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. 2010. Osteochondral defects in the ankle: why painful? Knee Surg. Sports Traumatol. Arthrosc. 18, 570–580. ( 10.1007/s00167-010-1064-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martel-Pelletier J, Wildi LM, Pelletier JP. 2012. Future therapeutics for osteoarthritis. Bone 51, 297–311. ( 10.1016/j.bone.2011.10.008) [DOI] [PubMed] [Google Scholar]
- 93.Reyes R, Delgado A, Sánchez E, Fernández A, Hernández A, Evora C. In press. Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFβ1 from a bilayered alginate–PLGA scaffold. J. Tissue Eng. Regen. Med. ( 10.1002/term.1549) [DOI] [PubMed] [Google Scholar]
- 94.Holland TA, Bodde EWH, Cuijpers VMJI, Baggett LS, Tabata Y, Mikos AG, Jansen JA. 2007. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage 15, 187–197. ( 10.1016/j.joca.2006.07.006) [DOI] [PubMed] [Google Scholar]
- 95.Kubota K, Iseki S, Kuroda S, Oida S, Iimura T, Duarte WR, Ohya K, Ishikawa I, Kasugai S. 2002. Synergistic effect of fibroblast growth factor-4 in ectopic bone formation induced by bone morphogenetic protein-2. Bone 31, 465–471. ( 10.1016/s8756-3282(02)00852-9) [DOI] [PubMed] [Google Scholar]
- 96.Kanitkar M, Tailor HD, Khan WS. 2011. The use of growth factors and mesenchymal stem cells in orthopaedics. Open Orthop. J. 5(Suppl. 2), 271–275. ( 10.2174/1874325001105010271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arnoczky SP, Delos D, Rodeo SA. 2011. What is platelet-rich plasma? Oper. Tech. Sports Med. 19, 142–148. ( 10.1053/j.otsm.2010.12.001) [DOI] [Google Scholar]
- 98.Centeno CJ, Schultz JR, Cheever M, Robinson B, Freeman M, Marasco W. 2010. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr. Stem Cell Res. Ther. 5, 81–93. ( 10.2174/157488810790442796) [DOI] [PubMed] [Google Scholar]
- 99.Qi Y, Feng G, Yan W. 2012. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol. Biol. Rep. 39, 5683–5689. ( 10.1007/s11033-011-1376-z) [DOI] [PubMed] [Google Scholar]
- 100.Qi Y, Du Y, Li W, Dai X, Zhao T, Yan W. In press. Cartilage repair using mesenchymal stem cell (MSC) sheet and MSCs-loaded bilayer PLGA scaffold in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. ( 10.1007/s00167-012-2256-3) [DOI] [PubMed] [Google Scholar]
- 101.Chung R, Foster BK, Xian CJ. 2011. Preclinical studies on mesenchymal stem cell-based therapy for growth plate cartilage injury repair. Stem Cells Int. 2011, 570125 ( 10.4061/2011/570125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steinert A, Ghivizzani S, Rethwilm A, Tuan R, Evans C, Noth U. 2007. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. Ther. 9, 213 ( 10.1186/ar2195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. 1994. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New Engl. J. Med. 331, 889–895. ( 10.1056/NEJM199410063311401) [DOI] [PubMed] [Google Scholar]
- 104.Johnson B, Lever C, Roberts S, Richardson J, McCarthy H, Harrison P, Laing P, Makwana N. 2013. Cell cultured chondrocyte implantation and scaffold techniques for osteochondral talar lesions. Foot Ankle Clin. 18, 135–150. ( 10.1016/j.fcl.2012.12.008) [DOI] [PubMed] [Google Scholar]
- 105.Candrian C, et al. 2010. Are ankle chondrocytes from damaged fragments a suitable cell source for cartilage repair? Osteoarthritis Cartilage 18, 1067–1076. ( 10.1016/j.joca.2010.04.010) [DOI] [PubMed] [Google Scholar]
- 106.Brittberg M, Winalski CS. 2003. Evaluation of cartilage injuries and repair. J. Bone Joint Surg. Am. 85A(Suppl. 2), 58–69. [DOI] [PubMed] [Google Scholar]
- 107.Espregueira-Mendes J, Pereira H, Sevivas N, Varanda P, da Silva MV, Monteiro A, Oliveira JM, Reis RL. 2012. Osteochondral transplantation using autografts from the upper tibio-fibular joint for the treatment of knee cartilage lesions. Knee Surg. Sports Traumatol. Arthrosc. 20, 1136–1142. ( 10.1007/s00167-012-1910-0) [DOI] [PubMed] [Google Scholar]
- 108.Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, Jobanputra P, Waugh N. 2005. Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation. Health Technol. Assess. 9, 1–82. [DOI] [PubMed] [Google Scholar]
- 109.Vasiliadis HS, Wasiak J. 2010. Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst. Rev. CD003323. ( 10.1002/14651858.CD003323.pub3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. 2009. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am. J. Sports Med. 37(Suppl. 1), 105–111. ( 10.1177/0363546509351481) [DOI] [PubMed] [Google Scholar]
- 111.Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. 2010. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population. Am. J. Sports Med. 38, 1125–1133. ( 10.1177/0363546509360405) [DOI] [PubMed] [Google Scholar]
- 112.Harris JD, Siston RA, Pan X, Flanigan DC. 2010. Autologous chondrocyte implantation: a systematic review. J. Bone Joint Surg. Am. 92, 2220–2233. ( 10.2106/JBJS.J.00049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Orth P, Kaul G, Cucchiarini M, Zurakowski D, Menger MD, Kohn D, Madry H. 2011. Transplanted articular chondrocytes co-overexpressing IGF-I and FGF-2 stimulate cartilage repair in vivo. Knee Surg. Sports Traumatol. Arthrosc. 19, 2119–2130. ( 10.1007/s00167-011-1448-6) [DOI] [PubMed] [Google Scholar]
- 114.Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vécsei V, Schlegel J. 2002. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage 10, 62–70. ( 10.1053/joca.2001.0482) [DOI] [PubMed] [Google Scholar]
- 115.Domm C, Schünke M, Christesen K, Kurz B. 2002. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthritis Cartilage 10, 13–22. ( 10.1053/joca.2001.0477) [DOI] [PubMed] [Google Scholar]
- 116.Kon E, Verdonk P, Condello V, Delcogliano M, Dhollander A, Filardo G, Pignotti E, Marcacci M. 2009. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am. J. Sports Med. 37, 156S–166S. ( 10.1177/0363546509351649) [DOI] [PubMed] [Google Scholar]
- 117.Mano JF, et al. 2007. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J. R. Soc. Interface 4, 999–1030. ( 10.1098/rsif.2007.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Agung M, Ochi M, Adachi N, Uchio Y, Takao M, Kawasaki K. 2004. Osteochondritis dissecans of the talus treated by the transplantation of tissue-engineered cartilage. Arthroscopy 20, 1075–1080. ( 10.1016/j.arthro.2004.04.039) [DOI] [PubMed] [Google Scholar]
- 119.Willers C, Chen J, Wood D, Xu J, Zheng MH. 2005. Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits. Tissue Eng. A 11, 1065–1076. ( 10.1089/ten.2005.11.1065) [DOI] [PubMed] [Google Scholar]
- 120.Getgood AMJ, Kew SJ, Brooks R, Aberman H, Simon T, Lynn AK, Rushton N. 2012. Evaluation of early-stage osteochondral defect repair using a biphasic scaffold based on a collagen–glycosaminoglycan biopolymer in a caprine model. Knee 19, 422–430. ( 10.1016/j.knee.2011.03.011) [DOI] [PubMed] [Google Scholar]
- 121.Augst A, et al. 2008. Effects of chondrogenic and osteogenic regulatory factors on composite constructs grown using human mesenchymal stem cells, silk scaffolds and bioreactors. J. R. Soc. Interface 5, 929–939. ( 10.1098/rsif.2007.1302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen K, Shi P, Teh TKH, Toh SL, Goh JCH. In press. In vitro generation of a multilayered osteochondral construct with an osteochondral interface using rabbit bone marrow stromal cells and a silk peptide-based scaffold. J. Tissue Eng. Regen. Med. ( 10.1002/term.1708) [DOI] [PubMed] [Google Scholar]
- 123.Wang C-C, Yang K-C, Lin K-H, Liu H-C, Lin F-H. 2011. A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials 32, 7118–7126. ( 10.1016/j.biomaterials.2011.06.018) [DOI] [PubMed] [Google Scholar]
- 124.Oliveira JM, et al. 2006. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 27, 6123–6137. ( 10.1016/j.biomaterials.2006.07.034) [DOI] [PubMed] [Google Scholar]
- 125.Abarrategi A, Lópiz-Morales Y, Ramos V, Civantos A, López-Durán L, Marco F, López-Lacomba JL. 2010. Chitosan scaffolds for osteochondral tissue regeneration. J. Biomed. Mater. Res. A 95A, 1132–1141. ( 10.1002/jbm.a.32912) [DOI] [PubMed] [Google Scholar]
- 126.Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. 2004. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann. Biomed. Eng. 32, 391–397. ( 10.1023/B:ABME.0000017552.65260.94) [DOI] [PubMed] [Google Scholar]
- 127.Løken S, Jakobsen RB, Årøen A, Heir S, Shahdadfar A, Brinchmann JE, Engebretsen L, Reinholt FP. 2008. Bone marrow mesenchymal stem cells in a hyaluronan scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 16, 896–903. ( 10.1007/s00167-008-0566-2) [DOI] [PubMed] [Google Scholar]
- 128.Oliveira JT, Santos TC, Martins L, Silva MA, Marques AP, Castro AG, Neves NM, Reis RL. 2009. Performance of new gellan gum hydrogels combined with human articular chondrocytes for cartilage regeneration when subcutaneously implanted in nude mice. J. Tissue Eng. Regen. Med. 3, 493–500. ( 10.1002/term.184) [DOI] [PubMed] [Google Scholar]
- 129.Oliveira JT, Santos TC, Martins L, Picciochi R, Marques AP, Castro AG, Neves NM, Mano JF, Reis RL. 2010. Gellan gum injectable hydrogels for cartilage tissue engineering applications: in vitro studies and preliminary in vivo evaluation. Tissue Eng. A 16, 343–353. ( 10.1089/ten.tea.2009.0117) [DOI] [PubMed] [Google Scholar]
- 130.Oliveira JT, Gardel LS, Rada T, Martins L, Gomes ME, Reis RL. 2010. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J. Orthopaed. Res. 28, 1193–1199. ( 10.1002/jor.21114) [DOI] [PubMed] [Google Scholar]
- 131.Oliveira JT, Martins L, Picciochi R, Malafaya PB, Sousa RA, Neves NM, Mano JF, Reis RL. 2010. Gellan gum: a new biomaterial for cartilage tissue engineering applications. J. Biomed. Mater. Res. A 93A, 852–863. ( 10.1002/jbm.a.32574) [DOI] [PubMed] [Google Scholar]
- 132.Miljkovic N, Lin Y-C, Cherubino M, Minteer D, Marra K. 2009. A novel injectable hydrogel in combination with a surgical sealant in a rat knee osteochondral defect model. Knee Surg. Sports Traumatol. Arthrosc. 17, 1326–1331. ( 10.1007/s00167-009-0881-2) [DOI] [PubMed] [Google Scholar]
- 133.Lim CT, Ren X, Afizah MH, Tarigan-Panjaitan S, Yang Z, Wu Y, Chian KS, Mikos AG, Hui JHP. 2013. Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model. Tissue Eng. A 19, 1852–1861. ( 10.1089/ten.tea.2012.0621) [DOI] [PubMed] [Google Scholar]
- 134.Hui J, Ren X, Afizah M, Chian K, Mikos A. 2013. Oligo[poly(ethylene glycol)fumarate] hydrogel enhances osteochondral repair in porcine femoral condyle defects. Clin. Orthop. Relat. Res. 471, 1174–1185. ( 10.1007/s11999-012-2487-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang CC, Chiang H, Liao CJ, Lin YJ, Kuo TF, Shieh CS, Huang YY, Tuan RS. 2007. Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J. Orthop. Res. 25, 1277–1290. ( 10.1002/jor.20442) [DOI] [PubMed] [Google Scholar]
- 136.Nagura I, Fujioka H, Kokubu T, Makino T, Sumi Y, Kurosaka M. 2007. Repair of osteochondral defects with a new porous synthetic polymer scaffold. J. Bone Joint Surg. Br. 89, 258–264. ( 10.1302/0301-620X.89B2.17754) [DOI] [PubMed] [Google Scholar]
- 137.Duan P, Pan Z, Cao L, He Y, Wang H, Qu Z, Dong J, Ding J. 2013. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. J. Biomed. Mater. Res. A 102, 180–192. ( 10.1002/jbm.a.34683) [DOI] [PubMed] [Google Scholar]
- 138.Reyes R, Delgado A, Solis R, Sanchez E, Hernandez A, Roman JS, Evora C. In press Cartilage repair by local delivery of transforming growth factor-β1 or bone morphogenetic protein-2 from a novel, segmented polyurethane/polylactic-co-glycolic bilayered scaffold. J. Biomed. Mater. Res. A. ( 10.1002/jbma.34769) [DOI] [PubMed] [Google Scholar]
- 139.Huang X, Yang D, Yan W, Shi Z, Feng J, Gao Y, Weng W, Yan S. 2007. Osteochondral repair using the combination of fibroblast growth factor and amorphous calcium phosphate/poly(l-lactic acid) hybrid materials. Biomaterials 28, 3091–3100. ( 10.1016/j.biomaterials.2007.03.017) [DOI] [PubMed] [Google Scholar]
- 140.Spadaccio C, Rainer A, Trombetta M, Vadalá G, Chello M, Covino E, Denaro V, Toyoda Y, Genovese J. 2009. Poly-l-lactic acid/hydroxyapatite electrospun nanocomposites induce chondrogenic differentiation of human MSC. Ann. Biomed. Eng. 37, 1376–1389. ( 10.1007/s10439-009-9704-3) [DOI] [PubMed] [Google Scholar]
- 141.Deplaine II, et al. 2013. Biomimetic hydroxyapatite coating on pore walls improves osteointegration of poly(L-lactic acid) scaffolds. J. Biomed Mater. Res. B 101B, 173–186. ( 10.1002/jbm.b.32831) [DOI] [PubMed] [Google Scholar]
- 142.Jeong CG, Zhang H, Hollister SJ. 2012. Three-dimensional polycaprolactone scaffold-conjugated bone morphogenetic protein-2 promotes cartilage regeneration from primary chondrocytes in vitro and in vivo without accelerated endochondral ossification. J. Biomed. Mater. Res. A 100A, 2088–2096. ( 10.1002/jbm.a.33249) [DOI] [PubMed] [Google Scholar]
- 143.Christensen B, Foldager C, Hansen O, Kristiansen A, Le D, Nielsen A, Nygaard J, Bünger C, Lind M. 2012. A novel nano-structured porous polycaprolactone scaffold improves hyaline cartilage repair in a rabbit model compared to a collagen type I/III scaffold: in vitro and in vivo studies. Knee Surg. Sports Traumatol. Arthrosc. 20, 1192–1204. ( 10.1007/s00167-011-1692-9) [DOI] [PubMed] [Google Scholar]
- 144.Kon E, et al. 2000. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J. Biomed. Mater. Res. A 49, 328–337. ( 10.1002/(sici)1097-4636(20000305)49:3%3C328::aid-jbm5%3E3.0.co;2-q) [DOI] [PubMed] [Google Scholar]
- 145.Kon E, et al. 2010. Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J. Orthopaed. Res. 28, 116–124. ( 10.1002/jor.20958) [DOI] [PubMed] [Google Scholar]
- 146.Kon E, Delcogliano M, Filardo G, Pressato D, Busacca M, Grigolo B, Desando G, Marcacci M. 2010. A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury 41, 693–701. ( 10.1016/j.injury.2009.11.014) [DOI] [PubMed] [Google Scholar]
- 147.Sotoudeh A, Jahanshahi A, Takhtfooladi MA, Bazazan A, Ganjali A, Harati MP. 2013. Study on nano-structured hydroxyapatite/zirconia stabilized yttria on healing of articular cartilage defect in rabbit. Acta Cir. Bras. 28, 340–345. ( 10.1590/S0102-86502013000500004) [DOI] [PubMed] [Google Scholar]
- 148.Kon E, Filardo G, Robinson D, Eisman JA, Levy A, Zaslav K, Shani J, Altschuler N. In press. Osteochondral regeneration using a novel aragonite-hyaluronate bi-phasic scaffold in a goat model. Knee Surg. Sports Traumatol. Arthrosc. ( 10.1007/s00167-013-2467-2) [DOI] [PubMed] [Google Scholar]
- 149.Gotterbarm T, Richter W, Jung M, Berardi Vilei S, Mainil-Varlet P, Yamashita T, Breusch SJ. 2006. An in vivo study of a growth-factor enhanced, cell free, two-layered collagen–tricalcium phosphate in deep osteochondral defects. Biomaterials 27, 3387–3395. ( 10.1016/j.biomaterials.2006.01.041) [DOI] [PubMed] [Google Scholar]
- 150.Bernstein A, et al. 2013. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: histological results. Acta Biomater. 9, 7490–7505. ( 10.1016/j.actbio.2013.03.021) [DOI] [PubMed] [Google Scholar]
- 151.Mayr HO, et al. 2013. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: biomechanical results. Acta Biomater. 9, 4845–4855. ( 10.1016/j.actbio.2012.07.040) [DOI] [PubMed] [Google Scholar]
- 152.Silva-Correia J, Oliveira JM, Caridade SG, Oliveira JT, Sousa RA, Mano JF, Reis RL. 2011. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regen. Med. 5, 97–107. ( 10.1002/term.363) [DOI] [PubMed] [Google Scholar]
- 153.Silva-Correia J, Oliveira JM, Oliveira JT, Sousa RA, Reis RL. 2011. Photo-crosslinked gellan gum-based hydrogels: preparation methods and uses thereof Patent no. WO/2011/119059.
- 154.Silva-Correia J, Miranda-Goncalves V, Salgado AJ, Sousa N, Oliveira JM, Reis RM, Reis RL. 2012. Angiogenic potential of gellan-gum-based hydrogels for application in nucleus pulposus regeneration: in vivo study. Tissue Eng. A 18, 1203–1212. ( 10.1089/ten.TEA.2011.0632) [DOI] [PubMed] [Google Scholar]
- 155.Scholz B, Kinzelmann C, Benz K, Mollenhauer J, Wurst H, Schlosshauer B. 2010. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur. Cell. Mater. 20, 24–37. [DOI] [PubMed] [Google Scholar]
- 156.Kim IL, Mauck RL, Burdick JA. 2011. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials 32, 8771–8782. ( 10.1016/j.biomaterials.2011.08.073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Doral MN, et al. 2012. Treatment of osteochondral lesions of the talus with microfracture technique and postoperative hyaluronan injection. Knee Surg. Sports Traumatol. Arthrosc. 20, 1398–1403. ( 10.1007/s00167-011-1856-7) [DOI] [PubMed] [Google Scholar]
- 158.Schlichting K, Schell H, Kleemann RU, Schill A, Weiler A, Duda GN, Epari DR. 2008. Influence of scaffold stiffness on subchondral bone and subsequent cartilage regeneration in an ovine model of osteochondral defect healing. Am. J. Sports Med. 36, 2379–2391. ( 10.1177/0363546508322899) [DOI] [PubMed] [Google Scholar]
- 159.Rodrigues MT, Lee SJ, Gomes ME, Reis RL, Atala A, Yoo JJ. 2012. Bilayered constructs aimed at osteochondral strategies: the influence of medium supplements in the osteogenic and chondrogenic differentiation of amniotic fluid-derived stem cells. Acta Biomater. 8, 2795–2806. ( 10.1016/j.actbio.2012.04.013) [DOI] [PubMed] [Google Scholar]
- 160.Luyten FP, Vanlauwe J. 2012. Tissue engineering approaches for osteoarthritis. Bone 51, 289–296. ( 10.1016/j.bone.2011.10.007) [DOI] [PubMed] [Google Scholar]
- 161.Reddy S, Pedowitz DI, Parekh SG, Sennett BJ, Okereke E. 2007. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am. J. Sports Med. 35, 80–85. ( 10.1177/0363546506290986) [DOI] [PubMed] [Google Scholar]
- 162.Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. 2008. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus. Am. J. Sports Med. 36, 873–880. ( 10.1177/0363546507312644) [DOI] [PubMed] [Google Scholar]
- 163.Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. 2009. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin. Orthop. Relat. Res. 467, 3307–3320. ( 10.1007/s11999-009-0885-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giza E, Sullivan M, Ocel D, Lundeen G, Mitchell ME, Veris L, Walton J. 2010. Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int. 31, 747–753. ( 10.3113/FAI.2010.0747) [DOI] [PubMed] [Google Scholar]
- 165.Aurich M, Bedi HS, Smith PJ, Rolauffs B, Mückley T, Clayton J, Blackney M. 2011. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation. Am. J. Sports Med. 39, 311–319. ( 10.1177/0363546510381575) [DOI] [PubMed] [Google Scholar]
- 166.Carmont MR, Carey-Smith R, Saithna A, Dhillon M, Thompson P, Spalding T. 2009. Delayed incorporation of a TruFit plug: perseverance is recommended. Arthroscopy 25, 810–814. ( 10.1016/j.arthro.2009.01.023) [DOI] [PubMed] [Google Scholar]
- 167.Caplan A. 2005. Review: mesenchymal stem cells: cell based reconstructive therapy in orthopedics. Tissue Eng. 11, 1198–1211. ( 10.1089/ten.2005.11.1198) [DOI] [PubMed] [Google Scholar]
- 168.Giannini S, Buda R, Cavallo M, Ruffilli A, Cenacchi A, Cavallo C, Vannini F. 2010. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: from open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury 41, 1196–1203. ( 10.1016/j.injury.2010.09.028) [DOI] [PubMed] [Google Scholar]
- 169.Battaglia M, Rimondi E, Monti C, Guaraldi F, Sant'Andrea A, Buda R, Cavallo M, Giannini S, Vannini F. 2011. Validity of T2 mapping in characterization of the regeneration tissue by bone marrow derived cell transplantation in osteochondral lesions of the ankle. Eur. J. Radiol. 80, 132–139. ( 10.1016/j.ejrad.2010.08.008) [DOI] [PubMed] [Google Scholar]
- 170.De Napoli IE, Scaglione S, Giannoni P, Quarto R, Catapano G. 2011. Mesenchymal stem cell culture in convection-enhanced hollow fibre membrane bioreactors for bone tissue engineering. J. Membr. Sci. 379, 341–352. ( 10.1016/j.memsci.2011.06.001) [DOI] [Google Scholar]
- 171.Kon E, Filardo G, Condello V, Collarile M, Di Martino A, Zorzi C, Marcacci M. 2011. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am. J. Sports Med. 39, 1668–1675. ( 10.1177/0363546511404675) [DOI] [PubMed] [Google Scholar]
- 172.Koestler W, Sidler R, Gonzalez Ballester MA, Nolte LP, Suedkamp NP, Maier D. 2008. A feasibility study of computer-assisted bone graft implantation for tissue-engineered replacement of the human ankle joint. Comput. Aided Surg. 13, 207–217. ( 10.3109/10929080802210814) [DOI] [PubMed] [Google Scholar]
- 173.Ohgushi H, Kotobuki N, Funaoka H, Machida H, Hirose M, Tanaka Y, Takakura Y. 2005. Tissue engineered ceramic artificial joint: ex vivo osteogenic differentiation of patient mesenchymal cells on total ankle joints for treatment of osteoarthritis. Biomaterials 26, 4654–4661. ( 10.1016/j.biomaterials.2004.11.055) [DOI] [PubMed] [Google Scholar]
- 174.van Bergen C, Reilingh M, van Dijk C. 2011. Tertiary osteochondral defect of the talus treated by a novel contoured metal implant. Knee Surg. Sports Traumatol. Arthrosc. 19, 999–1003. ( 10.1007/s00167-011-1465-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Anderson DD, Tochigi Y, Rudert MJ, Vaseenon T, Brown TD, Amendola A. 2010. Effect of implantation accuracy on ankle contact mechanics with a metallic focal resurfacing implant. J. Bone Joint Surg. 92, 1490–1500. ( 10.2106/jbjs.i.00431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Heijink A, Gomoll AH, Madry H, Drobnic M, Filardo G, Espregueira-Mendes J, Van Dijk CN. 2012. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 20, 423–435. ( 10.1007/s00167-011-1818-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lloyd J, Elsayed S, Hariharan K, Tanaka H. 2006. Revisiting the concept of talar shift in ankle fractures. Foot Ankle Int. 27, 793–796. ( 10.1177/107110070602701006) [DOI] [PubMed] [Google Scholar]
- 178.Radin EL, Burr DB. 1984. Hypothesis: joints can heal. Semin. Arthritis Rheum. 13, 293–302. ( 10.1016/0049-0172(84)90031-3) [DOI] [PubMed] [Google Scholar]
- 179.Qiu YS, Shahgaldi BF, Revell WJ, Heatley FW. 2003. Observations of subchondral plate advancement during osteochondral repair: a histomorphometric and mechanical study in the rabbit femoral condyle. Osteoarthritis Cartilage 11, 810–820. ( 10.1016/S1063-4584(03)00164-X) [DOI] [PubMed] [Google Scholar]
- 180.Schachter AK, Chen AL, Reddy PD, Tejwani NC. 2005. Osteochondral lesions of the talus. J. Am. Acad. Orthop. Surg. 13, 152–158. [DOI] [PubMed] [Google Scholar]
- 181.Becher C, Huber R, Thermann H, Paessler H, Skrbensky G. 2008. Effects of a contoured articular prosthetic device on tibiofemoral peak contact pressure: a biomechanical study. Knee Surg. Sports Traumatol. Arthrosc. 16, 56–63. ( 10.1007/s00167-007-0416-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Custers RJH, Saris DBF, Dhert WJA, Verbout AJ, van Rijen MHP, Mastbergen SC, Lafeber FPJG, Creemers LB. 2009. Articular cartilage degeneration following the treatment of focal cartilage defects with ceramic metal implants and compared with microfracture. J. Bone Joint Surg. 91, 900–910. ( 10.2106/jbjs.h.00668) [DOI] [PubMed] [Google Scholar]
- 183.Zheng MH, Willers C, Kirilak L, Yates J, Wood D, Shimmin A. 2007. Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment. Tissue Eng. 13, 737–746. ( 10.1089/ten.2006.0246) [DOI] [PubMed] [Google Scholar]