Abstract
Disease dilution (reduced disease prevalence with increasing biodiversity) has been described for many different pathogens. Although the mechanisms causing this phenomenon remain unclear, the disassembly of communities to predictable subsets of species, which can be caused by changing climate, land use or invasive species, underlies one important hypothesis. In this case, infection prevalence could reflect the competence of the remaining hosts. To test this hypothesis, we measured local host species abundance and prevalence of four generalist aphid-vectored pathogens (barley and cereal yellow dwarf viruses) in a ubiquitous annual grass host at 10 sites spanning 2000 km along the North American West Coast. In laboratory and field trials, we measured viral infection as well as aphid fecundity and feeding preference on several host species. Virus prevalence increased as local host richness declined. Community disassembly was non-random: ubiquitous hosts dominating species-poor assemblages were among the most competent for vector production and virus transmission. This suggests that non-random biodiversity loss led to increased virus prevalence. Because diversity loss is occurring globally in response to anthropogenic changes, such work can inform medical, agricultural and veterinary disease research by providing insights into the dynamics of pathogens nested within a complex web of environmental forces.
Keywords: barley and cereal yellow dwarf viruses, Bromus hordeaceus, disease dilution, nestedness, vector-borne pathogen
1. Introduction
Traditionally, research on infectious diseases has taken place within isolated disciplines such as plant pathology, human medicine and veterinary medicine, and, within these disciplines, research laboratories and funding are often tied to specific hosts and pathogens [1]. However, many important pathogens are host generalists and host–pathogen interactions can be governed by variable and changing environmental conditions such as temperature, rainfall and nutrient supplies or the presence of alternative hosts [2–5].
Recent research in community and theoretical ecology has benefited from theoretical foundations in statistical physics and mechanics, and thus has provided important insights into the dynamics of species distribution and interactions in complex systems [6–8]. In disease ecology, such integrative work on host–pathogen interactions can be translated into better forecasting and management of infectious disease [1], especially in the face of human alteration of the global ecosystem. Importantly, altered abiotic regimes including global biogeochemical cycles and biotic interactions including the spread of invasive species have led to global and local declines in species diversity [9,10]. The simultaneous trends of accelerating loss of native biodiversity [11,12] and emergence of infectious diseases [13–15] has spurred investigations into the role that host species diversity can play in mediating host–parasite interactions [2,16,17]. Biodiversity can modify infectious disease prevalence and risk through either an amplification or a dilution effect [2]. Disease dilution, an important ecosystem service, has been reported for a variety of systems from vector-borne zoonoses (i.e. diseases transmitted from animals to humans) to directly transmitted plant pathogens [16,18–22]. However, a variety of mechanisms might underlie this common pattern, and the effects of biodiversity on disease prevalence are still in debate [23–25]. Examination of theoretical and empirical studies has revealed the importance of specific characteristics of the hosts and parasites involved, of the type of metric used to assess disease risk, of the spatial scale used to assess the biodiversity–disease relationship, and of changes in host community structure versus diversity [23,26–28].
When pathogen transmission is density dependent, as is typical for directly transmitted diseases with a narrow host range and diseases transmitted by ‘sit-and-wait’ vectors (e.g. ticks), biodiversity can alter infection prevalence through a change in the absolute abundance of hosts and vectors [22,29–31]. The prevalence of generalist parasites with vectors that disperse over long distances, thereby reducing the density dependence of transmission, may also depend on biodiversity under certain conditions [16,23,29,32]. In particular, reduced encounter probability between susceptible hosts and infected vectors can reduce infection risk with increasing biodiversity when host behaviour or vector search efficiency is altered [2,33]. Disease prevalence can also be affected by heterogeneity in the competence of host reservoirs for parasite transmission [32,34]. Taken together, this body of work points to the importance of host biodiversity for controlling disease spread via interspecific variability in host competence and vector search efficiency [2,26,32].
The potential for hosts to affect the dynamics of generalist vector-borne parasites (host competence) depends on several key epidemiological parameters, including host susceptibility, vector feeding preference and fecundity, and pathogen transmission, which can vary according to host traits such as life history and physiological phenotype [35–37]. Thus, compositional shifts favouring highly competent species in communities are expected to increase transmission rate leading to disease amplification [35,38], whereas an abundance of non- or poorly competent species would reduce disease incidence leading to dilution [23,32].
The distinction between host diversity, host community composition and trait variability [23] is particularly important for predictions of disease prevalence with changing community composition via reduction of species diversity. Realistic biodiversity losses have been shown to disproportionally involve species characterized by particular sets of functional traits [39–41]. Thus, depending on whether host species’ parameters that enhance pathogen spread covary with the order of host species’ loss, the effects of biodiversity loss on disease prevalence could be predicted by the order of species loss in community disassembly, and by traits of host species left in communities [26]. The detection of a dilution effect for generalist vector-borne diseases would thus require more competent hosts also to be more ubiquitous, i.e. to be present in species-poor sites and to co-occur in species-rich communities with non- or poorly competent species [2,23,29,32]. A relationship between host ubiquity and competence has been demonstrated in some cases [16,42]. However, the generality of this mechanism for promoting disease dilution has yet to be demonstrated in a variety of systems and realistic communities (but see [43]).
We quantified the prevalence of a suite of four generalist vector-transmitted phytoviruses (barley and cereal yellow dwarf viruses, B/CYDVs, Luteoviridae) in a sentinel host (Bromus hordeaceus, Poaceae) as well as the diversity, distribution and local abundance of host and non-host plant species in grasslands at 31 sites in the Pacific coast grasslands of North America. California grasslands have experienced a broad-scale shift in community composition caused by the introduction of annual grasses and forbs from the Mediterranean region that subsequently dominated native perennial species [44,45]. In this region, we observe variation in species and host richness [41], variation in the local abundance of species and trait variability [35], providing a promising system for testing the relationship between host diversity and disease prevalence. In addition to our widespread survey, we measured the competence of 20 different common host species, including the ability to become infected, to re-transmit virus infection and to promote vector reproduction and preference under controlled conditions. We use these data to address the following specific questions:
— Does host species diversity determine pathogen prevalence?
— Is the composition of low-diversity host communities a predictable subset of high-diversity communities?
— Does the species loss order affect disease prevalence, i.e. are more ubiquitous host species more competent for pathogen spread?
2. Material and methods
2.1. Study system
B/CYDVs are RNA plant viruses from the Luteoviridae family. These globally distributed viruses are obligately transmitted from plant to plant via aphid vectors (Aphididae) in a persistent, circulative and non-propagative manner [46]. The B/CYDV group includes members of the genera Luteovirus (e.g. BYDV-PAV and -MAV) and Polerovirus (e.g. CYDV-RPV) as well as unassigned virus species (e.g. BYDV-RMV and -SGV; International Committee on Taxonomy of Viruses, www.ictvdb.org). At least 25 aphid species are known as vectors of B/CYDVs, but the transmission efficiency of vectors for each virus species differs strongly [47]. BYDV-PAV is one of the most frequently detected virus species in both crop and wild plants and is efficiently transmitted by the aphid vector Rhopalosiphum padi [48–51].
B/CYDVs are known to infect at least 150 grass species in the Poaceae family [52]. The replication cycle of B/CYDVs is restricted to host phloem cells and can be associated with symptoms, including dwarfing, yellowing and reddening that can lead to decreased infected host fecundity and lifespan [52,53]. B/CYDVs have contributed to significant agricultural losses [54] and have been recognized as the precursors of a remarkable shift in the plant species composition of natural California grasslands [55,56].
2.2. Field survey of B/CYDV prevalence and plant community structure
We surveyed the distribution and local abundance of both plant and B/CYDV species in natural grassland communities along the North American west coast in 2006. The study sites included 31 plant communities in 10 locations in British Columbia, Oregon and California (figure 1 and the electronic supplementary material, table S1). Sites spanned 15 degrees of latitude (33.75–48.81°N) and 2000 km.
Figure 1.
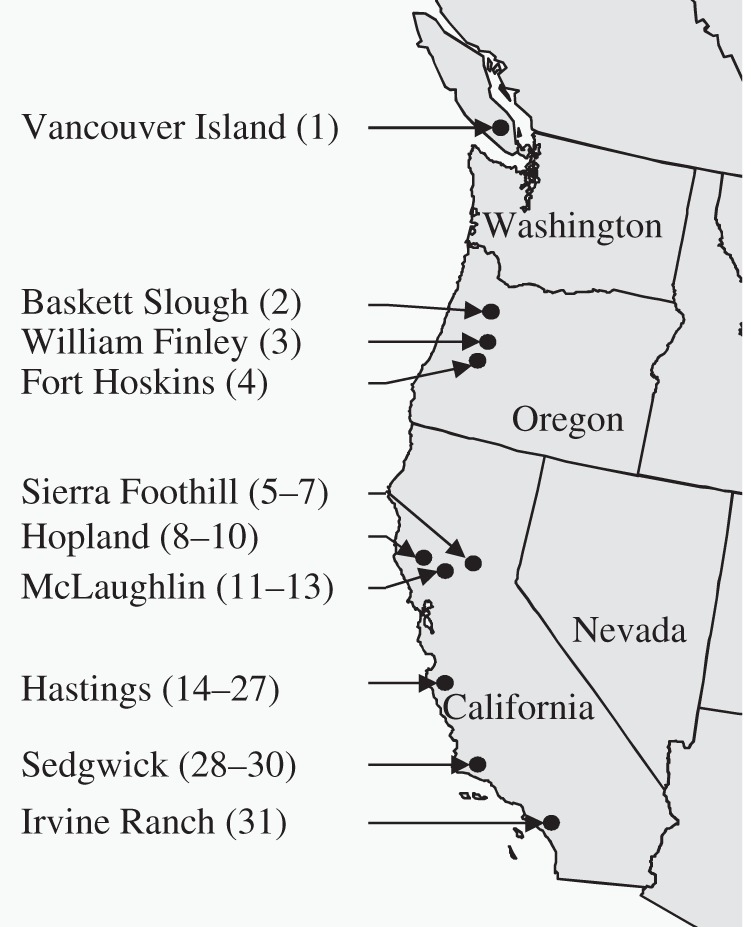
Study sites (solid circles) included in the survey of occurrence and abundance of plant and barley and cereal yellow dwarf virus species. Surveyed plant communities (numerically coded from 1 to 31) were located in 10 reserves and in three states (USA and Canada) along the Pacific Coast of North America.
At each site, we collected leaf tissue from up to 20 individuals of B. hordeaceus. The exotic annual grass B. hordeaceus is one of the most ubiquitous species on the west coast of North America [57], making it an ideal sentinel host species for comparing B/CYDV prevalence at multiple locations. In addition, B. hordeaceus is an effective sentinel, because it is annual, which means that all infection occurred in the current growing season, and it does not experience strong disease-induced mortality [53]. Thus, infection prevalence in this species represents a good estimate of incidence, the probability of being infected during a single growing season.
Whole plants were collected as late as possible in the season when green plant tissue could be collected and well after the seasonal peak in aphid abundance (E. T. Borer 2008, unpublished data). The 609 collected plant leaf samples were shipped overnight from the field and then tested for the presence of four virus species (BYDV-PAV, BYDV-MAV, BYDV-SGV and CYDV-RPV) using double-antibody sandwich enzyme-linked immunosorbent assays (DAS-ELISA; [50]) and appropriate specific antisera (Stewart Gray, Cornell University, NY, and Agdia, Elkhart, IN, USA). Infection prevalence was calculated as the proportion of hosts infected with B/CYDVs across B. hordeaceus plants sampled at each site.
We also estimated the percentage of the local area covered by each plant species present in between 1 and 70 0.5 × 1 m quadrats per site. Quadrats were placed adjacent to B. hordeaceus collection locations at each site in order to characterize local host composition close to hosts collected for virus analyses. Cover was estimated independently for each species, such that total cover can sum up to more than 100% in communities with multi-layered canopies. Plant species local abundance and richness was calculated as the mean cover and the mean number of species present per 0.5 m2 at each site. For each plant species, we used the number of surveyed sites where it was encountered as a measure of its distribution among sites (i.e. ubiquity).
2.3. Host susceptibility to virus infection and transmission rate of secondary inoculations
In greenhouse studies, we measured the susceptibility to virus inoculation and the ability to transmit the infection to susceptible hosts for 20 grass species, corresponding to nine annual and 11 perennial hosts in six different polygenetic tribes (see electronic supplementary material, table S2) that occur in the North American west coast flora [57]. Plants were started in laboratory conditions from seeds which were collected in a subset of our study sites. Ten individually grown plants per species were inoculated at the two-leaf stage (10 days after germination) with the BYDV-PAV BV113 isolate [35]. Test plants received five individuals of the aphid vector R. padi that originated from disease-free laboratory colonies and then fed for 48 h on virus-infected detached plant leaves, immediately prior to challenging the test plants. Aphids on test plants were killed after a 5 day inoculation access period, and leaf tissue was collected from each individual plant 14 days after inoculation to allow time for virus replication. The presence of BYDV-PAV was then assessed using DAS-ELISA [50] with monoclonal antibodies specific to BYDV-PAV (Agdia). At 40 days after primary inoculation, leaf tissue was collected from each infected host for a secondary inoculation, except in a few cases where plants died right after having been sampled for DAS-ELISA. Following the same inoculation protocol, aphids were allowed to feed for 48 h on infected leaves and were then transferred onto healthy barley hosts for a 5 day inoculation period. For each source plant, eight individual barley hosts were inoculated. We assessed each inoculated plant for virus infection 14 days after the secondary inoculation as mentioned above. For each plant species, we used the proportion of hosts infected with BYDV-PAV after the primary and secondary inoculations, respectively, as a measure of host susceptibility and ability to transmit the infection to susceptible hosts.
2.4. Aphid fecundity and host plant preference
We used data from two previously published experiments [38] to assess aphid (R. padi) fecundity and host preference for a subset of host species included in four different phylogenetic tribes (see electronic supplementary material, table S2). Although R. padi is one of many aphid vector species of B/CYDVs, it is an efficient agent in spreading BYDV-PAV [47]. In these studies, R. padi fecundity was assayed on naturally occurring populations of eight focal species in 2006 at one of our survey sites in an open oak savannah in Oregon (Baskett Slough National Wildlife Reserve, 44°58′ N, 123°15′ W). We placed a single mature apterous aphid, originating from disease-free laboratory colonies, in sleeve cages (8 × 2 cm) of 118 µm polyester mesh (Sefar America Inc., Kansas City, MO, USA) affixed to individual grass blades. Sleeve cages were deployed on 10 individuals per host species for each of two temporal blocks between 11–14 June and 20–23 June 2006, except for Taeniatherum caput-medusae, which had senesced by the time we performed the second temporal block (150 sleeve cages total). Test hosts were selected randomly for both temporal blocks. After 4 days, aphid fecundity was assessed by recording adult survival and counting young aphids in each cage.
Rhopalosiphum padi preferential feeding was assessed by placing 30 adult aphids in the centre of each of 17 pots (3.78 l), equidistant from each individual (one per host species) that had been planted in a randomized radial pattern five weeks before. Pots were then covered with a 118 µm polyester mesh hood and placed in a growth chamber at 21.1°C. After 24 h, we counted the number of adult aphids on each individual plant. Because plants of the same age differed in size, we removed, dried and weighed the above-ground plant tissue of each plant. Aphid counts were then calculated per gram of dried host tissue.
2.5. Statistical analyses
All statistical analyses were performed using R version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria).
First, we used generalized linear models [58] to assess whether plant species richness and local abundance (mean number of species and mean cover per 0.5 m2 at each site) affected B/CYDV prevalence in natural grassland communities using a logistic regression. In order to test the relative importance of plant species diversity and of local abundance on disease prevalence, we tested the robustness of the disease–host diversity relationship using a linear model after having controlled for the variation in the cover of the focal host B. hordeaceus and in the total cover of hosts. In addition, we used a linear model to examine the relationship between plant species richness and host local abundance.
Second, we assessed the variation in plant community composition in assemblages of various host diversity with the nestedness temperature calculator [59] using one randomly selected 0.5 m2 quadrat per site. The calculator tests to what extent the species in species-poor samples are nested subsets of species present in richer assemblages.
Third, we assessed the relationship between host competence for disease spread and the species loss order using a measure of host ubiquity (the number of sites where each species was present). We used generalized linear models with a logistic regression to analyse host competence data for virus infection and transmission and a Poisson regression for aphid vector reproduction and preference data.
3. Results
3.1. Plant and virus species occurrence and abundance
The mean overall prevalence of B/CYDVs was 28.7% and ranged from 0% to 85% of tested samples at each site. The mean virus species prevalence was 21.7% for BYDV-PAV, 11.3% for BYDV-MAV, 7% for CYDV-RPV and 3.4% for BYDV-SGV. The mean cover per 0.5 m2 of the sentinel host B. hordeaceus and of all hosts (i.e. all grass species including B. hordeaceus) was 15.2% (ranging from 0.25% to 60%) and 58.8% (ranging from 27.5% to 130%), respectively. On average, 4.05 host species occurred per 0.5 m2 (ranging from two to seven species).
3.2. Host species richness and virus prevalence
The mean overall prevalence of B/CYDVs in the sentinel host B. hordeaceus was negatively associated with host richness (p < 0.01, slope = −0.33 ± 0.12; figure 2). The prevalence of the virus species BYDV-PAV also significantly decreased with host richness (p < 0.001, slope = −0.64 ± 0.15). By contrast, host species richness did not significantly affect the prevalence of BYDV-MAV (p = 0.69, slope = −0.06 ± 0.15), CYDV-RPV (p = 0.44, slope = −0.16 ± 0.20) and BYDV-SGV (p = 0.19, slope = 0.39 ± 0.3).
Figure 2.
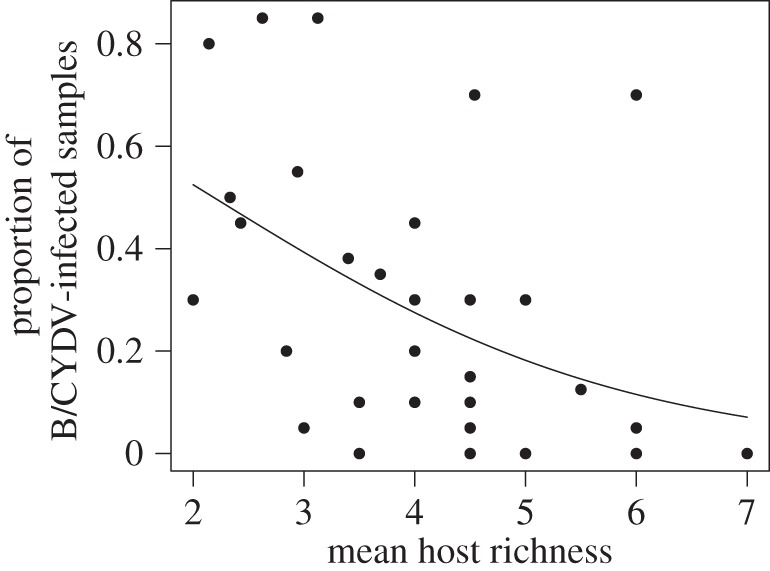
Within-site mean overall prevalence of B/CYDVs in collected Bromus hordeaceus hosts as a function of host species diversity. The line shows the negative significant relationship between virus prevalence and host richness.
Both the mean overall virus prevalence (p < 0.001, slope = 0.04 ± 0.007; figure 3a) and the prevalence of BYDV-PAV (p < 0.001, slope = 0.04 ± 0.079) increased with local abundance (mean cover) of the focal host B. hordeaceus. However, the variation in the cover of B. hordeaceus was not associated with host species richness (p = 0.29, adjusted R2 = 0.004; figure 3b) and the negative relationship between host species richness with mean overall prevalence (p = 0.01, slope = −0.92 ± 0.35; figure 3c) and prevalence of BYDV-PAV (p < 0.01, slope = −1.08 ± 0.36) remained significant after controlling for variation in B. hordeaceus abundance.
Figure 3.
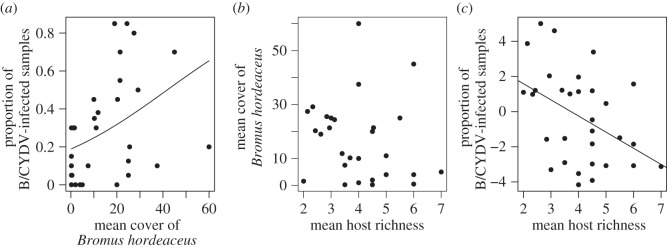
(a) Mean overall prevalence of B/CYDVs was increased with high local abundance (mean cover per 0.5 m2) of the focal host Bromus hordeaceus. (b) The mean cover of the latter was not significantly affected by host species richness. (c) After having controlled for variability in local abundance of B. hordeaceus, the residual variation in B/CYDV prevalence remained negatively affected by host species diversity. The lines show the slope of significant relationships.
Increased mean cover of all hosts was negatively related to both the mean overall virus prevalence (p < 0.001, slope = −0.02 ± 0.005) and BYDV-PAV prevalence (p < 0.001, slope = −0.03 ± 0.006). The relationship of either overall prevalence or BYDV-PAV prevalence to host species richness was not robust to controlling for variation in mean host cover (overall prevalence, p = 0.181; BYDV-PAV prevalence, p = 0.134). Mean host cover was positively associated with host species richness (p < 0.01, adjusted R2 = 0.203).
3.3. Grassland community structure
Twenty-seven host species were found across all 31 study sites (table 1). The nestedness analysis, performed on a matrix of presence/absence of host (grass) species at each study site, indicated that host plant composition was nested among sites (p = 0.01, nestedness temperature value = 19.2). Thus, grass species composition was predictable across the observed gradient in species diversity. Grass communities at species-poor sites included ubiquitous species and constituted subsets of the assemblages found at richer sites, which included both ubiquitous hosts and species with a restricted distribution among sites (table 1). Half of the perennial species in the host community were each encountered at a single site, whereas the other perennial hosts were observed in 2–11 of the 31 study sites. The two most ubiquitous species, B. hordeaceus and Bromus diandrus (annual exotic grasses), were present in more than half of the study sites in both species-rich and -poor communities (table 1).
Table 1.
Characteristics of grass species observed across all 31 study sites and composition of communities along the gradient of host diversity. Species are listed by decreasing order of ubiquity (i.e. distribution among sites). Values correspond to the mean percentage area covered by each species per host diversity level.
| plant taxon |
host plant community |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| species | tribe | life history | origin | mean host richness | 2 | 3 | 4 | 5 | 6 | 7 | no. sitesa |
| site code | 14, 19, 21, 28 | 5, 10, 15, 16, 17 | 4, 8, 18, 20, 22, 24, 26, 27, 29, 30 | 1, 6, 7, 13, 25 | 3, 9, 11, 12, 31 | 2 | |||||
| Bromus hordeaceus | Bromeae | annual | exotic | 8.8 | 20.0 | 14.2 | 12.3 | 22.8 | 5.0 | 25 | |
| Bromus diandrus | Bromeae | annual | exotic | 22.5 | 28.4 | 5.7 | 7.0 | 6.0 | 19 | ||
| Avena fatua | Aveneae | annual | exotic | 10.6 | 2.1 | 3.8 | 3.6 | 11 | |||
| Elymus glaucus | Triticeae | perennial | native | 8.8 | 0.3 | 6.0 | 0.5 | 11 | |||
| Vulpia myuros | Poeae | annual | exotic | 1.9 | 1.0 | 7.8 | 15.0 | 8 | |||
| Aira caryophyllea | Aveneae | annual | exotic | 0.4 | 9.5 | 3.0 | 5.0 | 10.0 | 7 | ||
| Bromus carinatus | Bromeae | perennial | native | 5.6 | 5 | ||||||
| Lolium multiflorum | Poeae | annual | exotic | 2.0 | 22.0 | 1.2 | 5 | ||||
| Bromus madritensis | Bromeae | annual | exotic | 0.1 | 2.0 | 0.4 | 4 | ||||
| Nassella pulchra | Stipeae | perennial | native | 5.0 | 2.9 | 4 | |||||
| Avena barbata | Aveneae | annual | exotic | 0.4 | 0.2 | 10.0 | 3 | ||||
| Hordeum murinum | Triticeae | annual | exotic | 0.3 | 1.0 | 0.4 | 3 | ||||
| Polypogon species | Aveneae | n.a. | n.a. | 6.0 | 1.0 | 17.0 | 3 | ||||
| Vulpia microstachys | Poeae | annual | native | 0.5 | 6.0 | 0.6 | 3 | ||||
| Arrhenatherum elatius | Aveneae | perennial | exotic | 2.0 | 10.0 | 2 | |||||
| Briza maxima | Poeae | annual | exotic | 8.2 | 15.0 | 2 | |||||
| Cynosurus echinatus | Poeae | annual | exotic | 1.0 | 2.0 | 2 | |||||
| Lolium temulentum | Poeae | annual | exotic | 2.5 | 0.9 | 2 | |||||
| Vulpia bromoides | Poeae | annual | exotic | 0.3 | 0.4 | 2 | |||||
| Aegilops triuncialis | Triticeae | annual | exotic | 2.0 | 1 | ||||||
| Anthoxanthum odoratum | Aveneae | perennial | exotic | 0.2 | 1 | ||||||
| Bromus sterilis | Bromeae | annual | exotic | 9.6 | 1 | ||||||
| Festuca arundinacea | Poeae | perennial | exotic | 3.0 | 1 | ||||||
| Holcus lanatus | Aveneae | perennial | exotic | 4.1 | 1 | ||||||
| Poa pratensis | Poeae | perennial | exotic | 0.4 | 1 | ||||||
| Poa species | Poeae | n.a. | n.a. | 0.5 | 1 | ||||||
| Taeniatherum caput-medusae | Triticeae | annual | exotic | 35.0 | 1 | ||||||
aNumber of study sites where each plant species has been encountered.
3.4. Host competence and ubiquity
Host competence for virus spread varied widely across all host species. The susceptibility of plants to infection and the transmission rate from plants to healthy barley hosts ranged from 0% to 90% and from 18% to 82% of infected samples, respectively (see electronic supplementary material, table S2). Aphid fecundity and aphid preference ranged from 0.1 to 1.05 R. padi nymphs produced per adult per day and from 2.6 to 20 R. padi individuals on each host per above-ground dry plant mass, respectively (see electronic supplementary material, table S2). Bromus hordeaceus was one of the most competent hosts for virus transmission (82.05% of infected plants), aphid fecundity (1.04 R. padi nymphs per adult per day) and preference (17.86 R. padi individuals per above-ground host mass), but not for host susceptibility (50% of infected plants).
Annual species were superior hosts compared with perennials in terms of the susceptibility to inoculation (p < 0.001), the ability to transmit the infection to healthy barley hosts via vectors (p < 0.01) as well as for aphid reproduction (p < 0.001) and preference (p < 0.001). Plant origin (native versus exotic) did not significantly affect host susceptibility (p = 0.13), host ability to transmit virus infection to healthy barley hosts (p = 0.96), or host ability to support vector reproduction (p = 0.54). Nevertheless, exotic species were more attractive to R. padi aphids than native species (p = 0.03). Host susceptibility was not significantly different across plant phylogenetic tribe (p = 0.13). By contrast, virus transmission to healthy barley hosts (p < 0.001) and vector preference (p < 0.001) were significantly affected by phylogenetic group, with higher scores for host species included in the Bromeae tribe. Aphid fecundity also differed across phylogenetic groups (p < 0.01), mainly because fecundity on Triticeae hosts was lower than on other hosts.
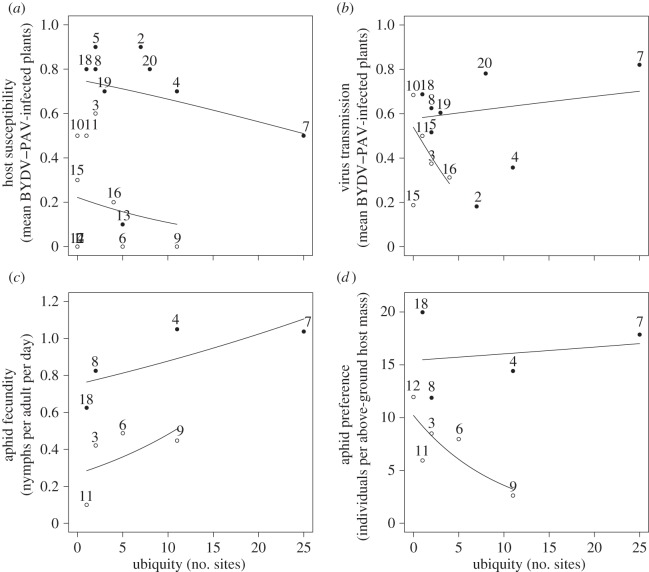
Plant life history and host ubiquity (figure 4) had a significant interactive effect on host susceptibility to infection (p < 0.01), virus transmission to healthy barley hosts (p < 0.01), and vector fecundity (p < 0.01) and preference (p < 0.001). Host susceptibility was negatively associated with host ubiquity for both annual and perennial species (figure 4a). A positive relationship was found between ubiquity and both annual and perennial host ability to support vector reproduction (figure 4c). Host competence for virus transmission to vectors and vector preference increased with ubiquity of annual species, whereas the converse was found for perennials (figure 4b,d).
Figure 4.
Relationship between host ubiquity (number of sites where found present) and the proportion of host infected after the primary inoculations (host susceptibility (a) and secondary infections (virus transmission (b)), the mean number of offspring produced per adult aphid per day (vector fecundity (c)) and the number of adult aphids per gram of dried plant tissue found on each host (vector preference (d)). The lines shows the slope of significant relationships for annual (filled circles) and perennial (open circles) host species. 1, Agrostis capillaris; 2, Aira caryophyllea; 3, Arrhenatherum elatius; 4, Avena fatua; 5, Briza maxima; 6, Bromus carinatus; 7, Bromus hordeaceus; 8, Cynosurus echinatus; 9, Elymus glaucus; 10, Elymus multisetus; 11, Festuca arundinacea; 12, Koeleria macrantha; 13, Lolium multiflorum; 14, Melica californica; 15, Nassella lepida; 16, Nassella pulchra; 17, Poa secunda; 18, Taeniatherum caput-medusae; 19, Vulpia microstachys; 20, Vulpia myuros.
4. Discussion
By concurrently examining grass community composition along gradients of diversity and host competence for disease spread, this study demonstrates the importance of processes of community disassembly for disease dilution to occur in a focal host.
4.1. Host species diversity and pathogen prevalence
The decline in prevalence in our sentinel species with increasing host species richness is consistent with ‘dilution’ [2], the negative biodiversity–disease relationship described in several animal [18,21,60] and plant [19,20,22,61,62] parasite systems. Potential drivers of disease prevalence in both vectored and directly transmitted parasite systems include host abundance [22,34]; thus, the amplification or dilution of disease can be driven by the trajectory of host abundance with declining diversity. In this study, we found that the infection prevalence in our focal, vectored, generalist phytoviruses B/CYDVs increased with declining host diversity but was not explained by variation in abundance of the sentinel host B. hordeaceus. By contrast, overall host density increased with total plant diversity, but experienced reduced virus prevalence. These results suggest that virus incidence in the focal host species is affected by the composition of the entire host community rather than a simple dependence on species diversity, consistent with previous work on several generalist pathogens [18,19,22,43,61].
Variability in host functional traits is particularly important for generalist pathogens with frequency-dependent transmission (e.g. insect vectored), because transmission in communities depends primarily on the competence of multiple host species [32]. For many pathogens, host competence for pathogen transmission varies according to host physiological phenotype, and transmission can be increased through a spillover effect in the presence of a single highly suitable host [35,63], whereas the addition of non- or poorly competent species in communities can lead to a dilution effect [23,32]. B/CYDV prevalence in previous surveys of both natural and experimentally manipulated plant communities was not associated with host species richness [51,61,63,64], but previous studies [43,65] have demonstrated a strong impact of host identity and host community composition on virus prevalence.
4.2. Composition of host communities in species-rich and species-poor assemblages
The current analyses reveal that host communities at species-poor sites represent nested subsets of plant assemblages in more species-rich environments, indicating a non-random pattern of biodiversity loss with important ramifications for ecosystem invasions [39,41]. Our results provide insights into the role of this community disassembly pattern in disease dynamics. Under this scenario of disproportionate loss of particular species, ecosystem functions, such as the regulation of disease prevalence, will depend on the traits of species left in low-richness communities [26,41,66]. Furthermore, the effects of declining diversity on vectored disease risk will also rely on whether host competence for parasite and vector population growth covaries among hosts. In our study system, there is strong evidence that disease is amplified by loss of diversity because of the association between host competence and loss order.
Ecosystems, including grasslands along the North American west coast, have undergone major changes in community composition and species diversity caused by environmental stressors such as climate change, modifications of nutrient cycling and introduction of exotic species [67,68]. Grasslands in California have been invaded by exotic annual grasses from the Mediterranean region that eventually became dominant over native perennial species [44,45]. Hence, our results suggest that disease amplification in this system could have arisen from the introduction of a suite of highly competent and ubiquitous hosts into communities that were initially dominated by less competent hosts, and from the subsequent decline of species to the highly competent subset of invaders.
4.3. Host ubiquity and competence for pathogen spread
We found that competence for each of the four parameters related to virus epidemiology differed widely across tested species. Our focal ubiquitous host species B. hordeaceus (annual, exotic, Bromeae phylogenetic tribe) was characterized by an average susceptibility to virus infection across all species tested. However, B. hordeaceus was one of the two most efficient species to transmit virus infection, to attract aphid vectors and to support vector reproduction. Annual species were superior hosts compared with perennials for each of these parameters, which is consistent with previous findings [35,38], and the most ubiquitous annual hosts were also the most competent for aphid reproduction, preferential feeding and virus transmission.
Interestingly, the ubiquity–competence relationship that underlies the disease dilution effect in this study is more related to annual host ability for transmission to other hosts than to susceptibility to virus inoculations. While host susceptibility to primary infections is critical for initiating disease dynamics in communities, variability in host ability for pathogen transmission can drive disease dynamics [69,70]. Host ability to become infected (susceptibility) and to host ability support disease transmission are not always coupled [71–73], suggesting that these two epidemiological parameters might have evolved under different ecological and/or evolutionary pressures. While the drivers of interspecific variability of host susceptibility to parasite infection remain conjectural [74], the disease transmission rate is often related to within-host virus accumulation [75,76].
Disease dilution might have been driven in this system through the presence and abundance of perennial hosts that are less competent for further community-wide disease amplification by supporting low aphid fecundity and virus transmission rate. Specifically, the effect of highly competent hosts for disease accumulation and transmission can be diluted if less competent hosts are present in more diverse communities, relative to low-diversity communities in which hosts are all highly competent for disease transmission. This effect could thus increase the probabilities of pathogen transmission even in less diverse communities dominated by low- to -intermediate competent species for susceptibility, as illustrated in our system by the robustness of the negative disease–host diversity relationship to the variation in the abundance of the sentinel host.
Identifying the mechanisms leading to inter- and intra-specific variability in host competence for disease spread remains a central area of investigation in disease epidemiology and ecology [36,37,77]. Species are characterized by different fitness-related traits and strategies that were shaped by trade-offs that constrain investment of resources in certain functions at the expense of others in the context of resource limitation [36,78–80]. For example, investment in resistance to infection by parasites can be associated with a fitness cost and has been shown to vary according to host lifespan [36,37,81]. Across kingdoms, short-lived species (e.g. annual plants, white-footed mice) display relatively high reproductive output, population growth, metabolic rates and resilience to disturbance, but invest less in defences against pathogens compared with closely related but longer-lived species [35,42,82,83]. Thus, a relationship between host ubiquity and competence probably results from evolutionary trade-offs among host growth, resilience and parasite resistance.
Furthermore, the effects of declining diversity on vectored disease risk will also depend on whether vector abundance, diversity and foraging behaviour (for long-distance vectors) respond to host diversity. We found in this study that the most ubiquitous hosts were also the most competent for the proliferation of aphid vectors of our focal virus species, which contributes to disease dilution in this system. Nevertheless, assessing how vector abundance actually varies with host species diversity would clarify the relative importance of host competence for disease transmission and vector reproduction on disease dilution. Positive bottom-up effects of plant richness on insect diversity [20,61] could promote parasite diversity at the scale of all host species present in species-rich communities, which could counteract the effect of heterogeneity in competence of host reservoirs on disease dilution [26,32]. In addition, as pointed out by Randolph & Dobson [23], if vectors preferentially feed and reproduce on the most competent hosts, then the addition of low-competence species would not be expected to decrease infection prevalence in species-rich communities, unless vector behaviour is altered [2,33]. Under conditions of high aphid abundance in genetically diverse plant populations, aphid movement rates between plants were higher, and single host tenure time was lower [84]. Given that transmission of persistently transmitted viruses such as B/CYDV requires several hours of feeding on both source and sink plants, virus prevalence could be reduced in species-rich communities compared with species-poor communities because of both reduced host tenure time of aphids and low competence for pathogen spread of other grasses compared with the most competent host.
Taken together, the results of this study highlight the interconnectedness of host species composition, trait variability and the non-random loss order rather than highlight simply the number of species in a community, as critical components of the mechanism underlying observed biodiversity–disease relationships [23,26,29]. Our results indicate that disease dilution of B/CYDVs arises from a suite of traits displayed by our focal host species including co-occurrence with poor- to non-reservoirs in species-rich environments, persistence in the local species pool as biodiversity declines, and high competence for transmission of infectious agents and proliferation of their aphid vectors. Our study, focusing on generalist parasites transmitted by vectors that disperse over long distances, is one of the few studies indicating the ubiquity–competence relationship as a mechanism for promoting disease dilution in real communities (also see [18,43,85]), and demonstrates that the ubiquity–competence mechanism also applies to the plant kingdom. Interestingly, declining host community diversity also has been shown to control specialist pathogens with density-dependent transmission via increased contact among hosts [22] and can also increase the incidence of generalist pathogens via reduced variance and increased mean host competence [32], as seen in this study. Thus, in the context of the growing corpus of work examining the dilution effect in a wide variety of hosts, this study provides insights into the most general influences of host community composition on disease. Specifically, we find the emerging generality that the abundance trajectory of the most competent hosts with declining diversity, and the relative competence of the focal host in this context, will allow us to predict amplification or dilution of disease in focal host species, in particular in the context of environmental changes such as the invasion by exotic species. In doing so, an empirically parametrized and synthetic model incorporating disease prevalence, host species characteristics and various spatial scales would constitute a fruitful avenue for further research and would provide interesting insights on the relative contribution of each species’ abundance as well as each host's competence for pathogen transmission, vector proliferation and diversity on community-wide disease incidence in assemblages varying in diversity [28,65].
The current work demonstrates the importance of the larger ecosystem context governing host–pathogen interactions. In natural systems, human activities have led to increased system eutrophication, species extinctions, and the spread of invasive species sharing a distinct suite of demographic traits [9,86]. Our results demonstrate that alterations to the physical or biotic environment that cause non-random species losses and changes in the distribution of species traits can also alter infectious disease [34,55,64] dynamics. Thus, our results suggest that insights from ecological research can motivate further studies at the interface of disease ecology and statistical physics and mathematics in order to model interaction networks in complex host–pathogen systems and can provide general insights into the dynamics of infectious diseases in the face of increasing human impacts on global ecosystems [1,9].
Acknowledgements
We thank the Borer, Power, Mitchell and Seabloom technicians, undergraduates and graduate students, and especially Jasmine S. Peters, Emily E. Puckett, Scot M. Waring, and Miranda E. Welsh, Emily Orling, Stan Harpole, Andrew McDougall and Vincent Adams for help in the field and laboratory. All authors contributed to conceiving and designing this project. E.T.B, C.E.M., A.G.P., E.W.S. and many assistants collected the data. C.L. and E.W.S. analysed the data. C.L. wrote the paper and E.T.B, C.E.M., A.G.P., E.W.S. and A.J. contributed substantially to the writing process.
Funding statement
We received support from Oregon State University and the NSF programme in Ecology and Evolution of Infectious Disease (grants NSF/NIH EID 05-25666 and 10-15805 to E.T.B. and E.W.S., NSF/NIH EID 05-25669 to A.G.P., and NSF/NIH EID 05-25641 and 10-15909 to C.E.M.).
References
- 1.Borer ET, et al. 2011. Bridging taxonomic and disciplinary divides in infectious disease. Ecohealth 8, 261–267. ( 10.1007/s10393-011-0718-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. ( 10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minakawa N, Omukunda E, Zhou G, Githeko A, Yan G. 2006. Malaria vector productivity in relation to the highland environment in Kenya. Am. J. Trop. Med. Hyg. 75, 448–453. [PubMed] [Google Scholar]
- 4.Pope K, Masuoka P, Rejmankova E, Grieco J, Johnson S, Roberts D. 2005. Mosquito habitats, land use, and malaria risk in Belize from satellite imagery. Ecol. Appl. 15, 1223–1232. ( 10.1890/04-0934) [DOI] [Google Scholar]
- 5.Smith V. 2007. Host resource supplies influence the dynamics and outcome of infectious disease. Integr. Comp. Biol. 47, 310–316. ( 10.1093/icb/icm006) [DOI] [PubMed] [Google Scholar]
- 6.Ricotta C. 2000. From theoretical ecology to statistical physics and back: self-similar landscape metrics as a synthesis of ecological diversity and geometrical complexity. Ecol. Model. 125, 245–253. ( 10.1016/s0304-3800(99)00185-4) [DOI] [Google Scholar]
- 7.Estrada E. 2007. Food webs robustness to biodiversity loss: the roles of connectance, expansibility and degree distribution. J. Theor. Biol. 244, 296–307. ( 10.1016/j.jtbi.2006.08.002) [DOI] [PubMed] [Google Scholar]
- 8.Bowler MG, Kelly CK. 2012. On the statistical mechanics of species abundance distributions. Theor. Popul. Biol. 82, 85–91. ( 10.1016/j.tpb.2012.05.006) [DOI] [PubMed] [Google Scholar]
- 9.Rockstrom J, et al. 2009. A safe operating space for humanity. Nature 461, 472–475. ( 10.1038/461472a) [DOI] [PubMed] [Google Scholar]
- 10.Harpole WS, Tilman D. 2007. Grassland species loss resulting from reduced niche dimension. Nature 446, 791–793. ( 10.1038/nature05684) [DOI] [PubMed] [Google Scholar]
- 11.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 12.Naeem S, Duffy JE, Zavaleta E. 2012. The functions of biological diversity in an age of extinction. Science 336, 1401–1406. ( 10.1126/science.1215855) [DOI] [PubMed] [Google Scholar]
- 13.Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. 2004. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 19, 535–544. ( 10.1016/j.tree.2004.07.021) [DOI] [PubMed] [Google Scholar]
- 14.Dobson A, Foufopoulos J. 2001. Emerging infectious pathogens of wildlife. Phil. Trans. R. Soc. Lond. B 356, 1001–1012. ( 10.1098/rstb.2001.0900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor LH, Latham SM, Woolhouse MEJ. 2001. Risk factors for human disease emergence. Phil. Trans. R. Soc. Lond. B 356, 983–989. ( 10.1098/rstb.2001.0888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostfeld R, Keesing F. 2000. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 78, 2061–2078. ( 10.1139/cjz-78-12-2061) [DOI] [Google Scholar]
- 17.Vourc'h G, Plantard O, Morand S. 2012. How does biodiversity influence the ecology of infectious disease? New Front. Mol. Epidemiol. Infect. Dis 78, 291–309. ( 10.1007/978-94-007-2114-2_13) [DOI] [Google Scholar]
- 18.Allan BF, et al. 2009. Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia 158, 699–708. ( 10.1007/s00442-008-1169-9) [DOI] [PubMed] [Google Scholar]
- 19.Haas SE, Hooten MB, Rizzo DM, Meentemeyer RK. 2011. Forest species diversity reduces disease risk in a generalist plant pathogen invasion. Ecol. Lett. 14, 1108–1116. ( 10.1111/j.1461-0248.2011.01679.x) [DOI] [PubMed] [Google Scholar]
- 20.Knops JMH, et al. 1999. Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol. Lett. 2, 286–293. ( 10.1046/j.1461-0248.1999.00083.x) [DOI] [PubMed] [Google Scholar]
- 21.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. USA 100, 567–571. ( 10.1073/pnas.0233733100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell CE, Tilman D, Groth JV. 2002. Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology 83, 1713–1726. ( 10.1890/0012-9658(2002)083[1713:EOGPSD]2.0.CO;2) [DOI] [Google Scholar]
- 23.Randolph SE, Dobson ADM. 2012. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology 139, 847–863. ( 10.1017/s0031182012000200) [DOI] [PubMed] [Google Scholar]
- 24.Ostfeld RS. 2013. A Candide response to Panglossian accusations by Randolph and Dobson: biodiversity buffers disease. Parasitology 140, 1196–1198. ( 10.1017/s0031182013000541) [DOI] [PubMed] [Google Scholar]
- 25.Randolph SE. 2013. Commentary on ‘A Candide response to Panglossian accusations by Randolph and Dobson: biodiversity buffers disease’ by Dr R. Ostfeld (Parasitology 2013, in press). Parasitology 140, 1199–1200. ( 10.1017/s0031182013000620) [DOI] [PubMed] [Google Scholar]
- 26.Brooks CP, Zhang H. 2010. A null model of community disassembly effects on vector-borne disease risk. J. Theor. Biol. 264, 866–873. ( 10.1016/j.jtbi.2010.03.016) [DOI] [PubMed] [Google Scholar]
- 27.Salkeld DJ, Padgett KA, Jones JH. 2013. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol. Lett. 16, 679–686. ( 10.1111/ele.12101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood CL, Lafferty KD. 2013. Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol. Evol. 28, 239–247. ( 10.1016/j.tree.2012.10.011) [DOI] [PubMed] [Google Scholar]
- 29.Dobson A. 2004. Population dynamics of pathogens with multiple host species. Am. Nat. 164, S64–S78. ( 10.1086/424681) [DOI] [PubMed] [Google Scholar]
- 30.Roche B, Dobson AP, Guegan J-F, Rohani P. 2012. Linking community and disease ecology: the impact of biodiversity on pathogen transmission. Phil. Trans. R. Soc. B 367, 2807–2813. ( 10.1098/rstb.2011.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanbuskirk J, Ostfeld RS. 1995. Controlling Lyme-disease by modifying the density and species composition of tick hosts. Ecol. Appl. 5, 1133–1140. ( 10.2307/2269360) [DOI] [Google Scholar]
- 32.Roche B, Rohani P, Dobson AP, Guegan J-F. 2013. The impact of community organization on vector-borne pathogens. Am. Nat. 181, 1–11. ( 10.1086/668591) [DOI] [PubMed] [Google Scholar]
- 33.Allan BF, Keesing F, Ostfeld RS. 2003. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol. 17, 267–272. ( 10.1046/j.1523-1739.2003.01260.x) [DOI] [Google Scholar]
- 34.Borer ET, Mitchell CE, Power AG, Seabloom EW. 2009. Consumers indirectly increase infection risk in grassland food webs. Proc. Natl Acad. Sci. USA 106, 503–506. ( 10.1073/pnas.0808778106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cronin JP, Welsh ME, Dekkers MG, Abercrombie ST, Mitchell CE. 2010. Host physiological phenotype explains pathogen reservoir potential. Ecol. Lett. 13, 1221–1232. ( 10.1111/j.1461-0248.2010.01513.x) [DOI] [PubMed] [Google Scholar]
- 36.Dowling DK, Simmons LW. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 1737–1745. ( 10.1098/rspb.2008.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller MR, White A, Boots M. 2007. Host life span and the evolution of resistance characteristics. Evolution 61, 2–14. ( 10.1111/j.1558-5646.2007.00001.x) [DOI] [PubMed] [Google Scholar]
- 38.Borer ET, Adams VT, Engler GA, Adams AL, Schumann CB, Seabloom EW. 2009. Aphid fecundity and grassland invasion: invader life history is the key. Ecol. Appl. 19, 1187–1196. ( 10.1890/08-1205.1) [DOI] [PubMed] [Google Scholar]
- 39.Bracken MES, Low NHN. 2012. Realistic losses of rare species disproportionately impact higher trophic levels. Ecol. Lett. 15, 461–467. ( 10.1111/j.1461-0248.2012.01758.x) [DOI] [PubMed] [Google Scholar]
- 40.Selmants PC, Zavaleta ES, Pasari JR, Hernandez DL. 2012. Realistic plant species losses reduce invasion resistance in a California serpentine grassland. J. Ecol. 100, 723–731. ( 10.1111/j.1365-2745.2011.01949.x) [DOI] [Google Scholar]
- 41.Zavaleta ES, Hulvey KB. 2004. Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science 306, 1175–1177. ( 10.1126/science.1102643) [DOI] [PubMed] [Google Scholar]
- 42.Lee KA, Wikelski M, Robinson WD, Robinson TR, Klasing KC. 2008. Constitutive immune defences correlate with life-history variables in tropical birds. J. Anim. Ecol. 77, 356–363. ( 10.1111/j.1365-2656.2007.01347.x) [DOI] [PubMed] [Google Scholar]
- 43.Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD. 2013. Biodiversity decreases disease through predictable changes in host community competence. Nature 494, 230–233. ( 10.1038/nature11883) [DOI] [PubMed] [Google Scholar]
- 44.Jackson LE. 1985. Ecological origins of California's mediterranean grasses. J. Biogeogr. 12, 349–361. ( 10.2307/2844866) [DOI] [Google Scholar]
- 45.Mooney HA, Drake JA. 1986. Ecology of biological invasions of North America and Hawaii. New York, NY: Springer. [Google Scholar]
- 46.Gray S, Gildow FE. 2003. Luteovirus–aphid interactions. Annu. Rev. Phytopathol. 41, 539–566. ( 10.1146/annurev.phyto.41.012203.105815) [DOI] [PubMed] [Google Scholar]
- 47.Power AG, Gray SM. 1995. Aphid transmission of barley yellow dwarf viruses: interactions between viruses, vectors and host plants. In Barley yellow dwarf: 40 years of progress (eds D'arcy CJ, Burnett PA.), pp. 259–289. St. Paul, MN: The American Phytopathological Society. [Google Scholar]
- 48.Hall GS, Peters JS, Little DP, Power AG. 2010. Plant community diversity influences vector behaviour and Barley yellow dwarf virus population structure. Plant Pathol. 59, 1152–1158. ( 10.1111/j.1365-3059.2010.02351.x) [DOI] [Google Scholar]
- 49.Leclercq-Le Quillec F, Plantegenest M, Riault G, Dedryver CA. 2000. Analyzing and modeling temporal disease progress of barley yellow dwarf virus serotypes in barley fields. Phytopathology 90, 860–866. ( 10.1094/phyto.2000.90.8.860) [DOI] [PubMed] [Google Scholar]
- 50.Rochow WF, Carmichael LE. 1979. Specificity among barley yellow dwarf viruses in enzyme immunosorbent assays. Virology 95, 415–420. ( 10.1016/0042-6822(79)90496-3) [DOI] [PubMed] [Google Scholar]
- 51.Seabloom EW, Borer ET, Mitchell CE, Power AG. 2010. Viral diversity and prevalence gradients in North American Pacific Coast grasslands. Ecology 91, 721–732. ( 10.1890/08-2170.1) [DOI] [PubMed] [Google Scholar]
- 52.D'arcy CJ. 1995. Symptomatology and host range of barley yellow dwarf. In Barley yellow dwarf, 40 years of progress (eds D'arcy CJ, Burnett PA.), pp. 9–28. St. Paul, MN: The American Phytopathological Society. [Google Scholar]
- 53.Malmstrom CM, Hughes CC, Newton LA, Stoner CJ. 2005. Virus infection in remnant native bunchgrasses from invaded California grasslands. New Phytol. 168, 217–230. ( 10.1111/j.1469-8137.2005.01479.x) [DOI] [PubMed] [Google Scholar]
- 54.Perry KL, Kolb FL, Sammons B, Lawson C, Cisar G, Ohm H. 2000. Yield effects of barley yellow dwarf virus in soft red winter wheat. Phytopathology 90, 1043–1048. ( 10.1094/phyto.2000.90.9.1043) [DOI] [PubMed] [Google Scholar]
- 55.Borer ET, Hosseini PR, Seabloom EW, Dobson AP. 2007. Pathogen-induced reversal of native dominance in a grassland community. Proc. Natl Acad. Sci. USA 104, 5473–5478. ( 10.1073/pnas.0608573104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malmstrom CM, McCullough AJ, Johnson HA, Newton LA, Borer ET. 2005. Invasive annual grasses indirectly increase virus incidence in California native perennial bunchgrasses. Oecologia 145, 153–164. ( 10.1007/s00442-005-0099-z) [DOI] [PubMed] [Google Scholar]
- 57.Jepson WL, Hickman JC. 1993. The Jepson manual: higher plants of California. Berkeley, CA: University of California Press. [Google Scholar]
- 58.McCullagh P, Nelder J. 1989. Generalized linear models. New York, NY: Chapman and Hall. [Google Scholar]
- 59.Atmar W, Patterson BD. 1993. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96, 373–382. ( 10.1007/bf00317508) [DOI] [PubMed] [Google Scholar]
- 60.Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ, Keesing F. 2006. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 4, 1058–1068. ( 10.1371/journal.pbio.0040145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore SM, Borer ET. 2012. The influence of host diversity and composition on epidemiological patterns at multiple spatial scales. Ecology 93, 1095–1105. ( 10.1890/11-0086.1) [DOI] [PubMed] [Google Scholar]
- 62.Pagan I, Gonzalez-Jara P, Moreno-Letelier A, Rodelo-Urrego M, Fraile A, Pinero D, Garcia-Arenal F. 2012. Effect of biodiversity changes in disease risk: exploring disease emergence in a plant–virus system. PLoS Pathog. 8, e1002796 ( 10.1371/journal.ppat.1002796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Power AG, Mitchell CE. 2004. Pathogen spillover in disease epidemics. Am. Nat. 164, S79–S89. ( 10.1086/424610) [DOI] [PubMed] [Google Scholar]
- 64.Borer ET, Seabloom EW, Mitchell CE, Power AG. 2010. Local context drives infection of grasses by vector-borne generalist viruses. Ecol. Lett. 13, 810–818. ( 10.1111/j.1461-0248.2010.01475.x) [DOI] [PubMed] [Google Scholar]
- 65.Schmidt KA, Ostfeld RS. 2001. Biodiversity and the dilution effect in disease ecology. Ecology 82, 609–619. ( 10.1890/0012-9658(2001)082[0609:batdei]2.0.co;2) [DOI] [Google Scholar]
- 66.Zavaleta E, Pasari J, Moore J, Hernandez D, Suttle KB, Wilmers CC. 2009. Ecosystem responses to community disassembly. In Year in ecology and conservation biology (eds Ostfeld RS, Schlesinger WH.), pp. 311–333. Annals of the New York Academy of Sciences, vol. 1162. Hoboken, NJ: Wiley Blackwell. [DOI] [PubMed] [Google Scholar]
- 67.Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C. 2006. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 989–992. ( 10.1038/nature05202) [DOI] [PubMed] [Google Scholar]
- 68.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 69.Garrett KA, Mundt CC. 1999. Epidemiology in mixed host populations. Phytopathology 89, 984–990. ( 10.1094/PHYTO.1999.89.11.984) [DOI] [PubMed] [Google Scholar]
- 70.Paull SH, Song S, McClure KM, Sackett LC, Kilpatrick AM, Johnson PTJ. 2012. From superspreaders to disease hotspots: linking transmission across hosts and space. Front. Ecol. Environ. 10, 75–82. ( 10.1890/110111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laine AL. 2005. Spatial scale of local adaptation in a plant–pathogen metapopulation. J. Evol. Biol. 18, 930–938. ( 10.1111/j.1420-9101.2005.00933.x) [DOI] [PubMed] [Google Scholar]
- 72.Sicard D, Pennings PS, Grandclement C, Acosta J, Kaltz O, Shykoff JA. 2007. Specialization and local adaptation of a fungal parasite on two host plant species as revealed by two fitness traits. Evolution 61, 27–41. ( 10.1111/j.1558-5646.2007.00003.x) [DOI] [PubMed] [Google Scholar]
- 73.Fargette D, Pinel A, Traore O, Ghesquiere A, Konate G. 2002. Emergence of resistance-breaking isolates of rice yellow mottle virus during serial inoculations. Eur. J. Plant Pathol. 108, 585–591. ( 10.1023/A:1019952907105) [DOI] [Google Scholar]
- 74.Beldomenico PM, Begon M. 2010. ‘Vicious circles’ and disease spread: elements of discussion. Response. Trends Ecol. Evol. 25, 132–132. ( 10.1016/j.tree.2009.10.006) [DOI] [PubMed] [Google Scholar]
- 75.Ng JCK, Falk BW. 2006. Virus–vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 44, 183–212. ( 10.1146/annurev.phyto.44.070505.143325) [DOI] [PubMed] [Google Scholar]
- 76.Rotenberg D, Krishna Kumar NK, Ullman DE, Montero-Astua M, Willis DK, German TL, Whitfield AE. 2009. Variation in tomato spotted wilt virus titer in Frankliniella occidentalis and its association with frequency of transmission. Phytopathology 99, 404–410. ( 10.1094/phyto-99-4-0404) [DOI] [PubMed] [Google Scholar]
- 77.Hawley DM, Altizer SM. 2011. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct. Ecol. 25, 48–60. ( 10.1111/j.1365-2435.2010.01753.x) [DOI] [Google Scholar]
- 78.Lind EM, et al. 2013. Life-history constraints in grassland plant species: a growth-defence trade-off is the norm. Ecol. Lett. 16, 513–521. ( 10.1111/ele.12078) [DOI] [PubMed] [Google Scholar]
- 79.Ricklefs RE, Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. ( 10.1016/s0169-5347(02)02578-8) [DOI] [Google Scholar]
- 80.Sheldon BC, Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321. ( 10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 81.Brown JKM. 2007. Fitness costs of plant disease resistance. In Encyclopedia of life sciences. Chichester, UK: John Wiley & Sons Ltd. See http://www.els.net/WileyCDA/ElsArticle/refId-a0020094.html. [Google Scholar]
- 82.Martin LB, II, Hasselquist D, Wikelski M. 2006. Investment in immune defense is linked to pace of life in house sparrows. Oecologia 147, 565–575. ( 10.1007/s00442-005-0314-y) [DOI] [PubMed] [Google Scholar]
- 83.Ostfeld RS, LoGiudice K. 2003. Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology 84, 1421–1427. ( 10.1890/02-3125) [DOI] [Google Scholar]
- 84.Power AG. 1991. Virus spread and vector dynamics in genetically diverse plant populations. Ecology 72, 232–241. ( 10.2307/1938917) [DOI] [Google Scholar]
- 85.Huang ZYX, De Boer WF, Van Langevelde F, Olson V, Blackburn TM, Prins HHT. 2013. Species’ life-history traits explain interspecific variation in reservoir competence: a possible mechanism underlying the dilution effect. PLoS ONE 8, e54341 ( 10.1371/journal.pone.0054341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Kleunen M, Weber E, Fischer M. 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 13, 235–245. ( 10.1111/j.1461-0248.2009.01418.x) [DOI] [PubMed] [Google Scholar]