Abstract
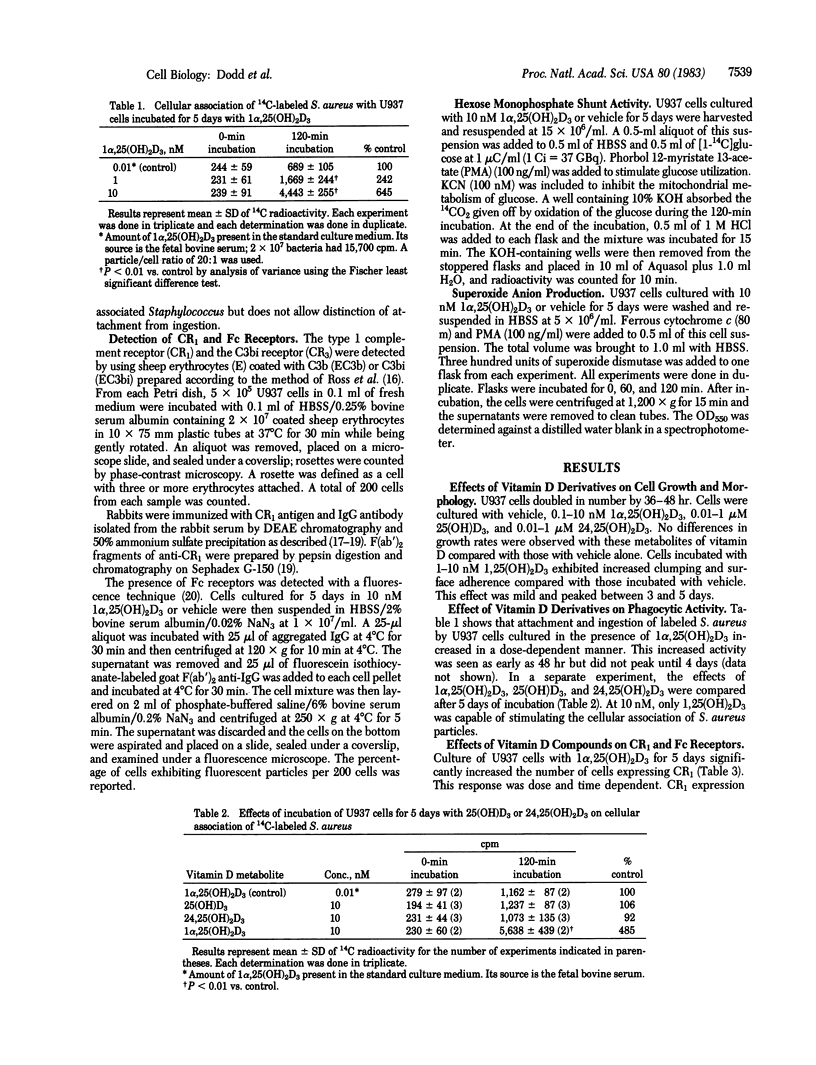
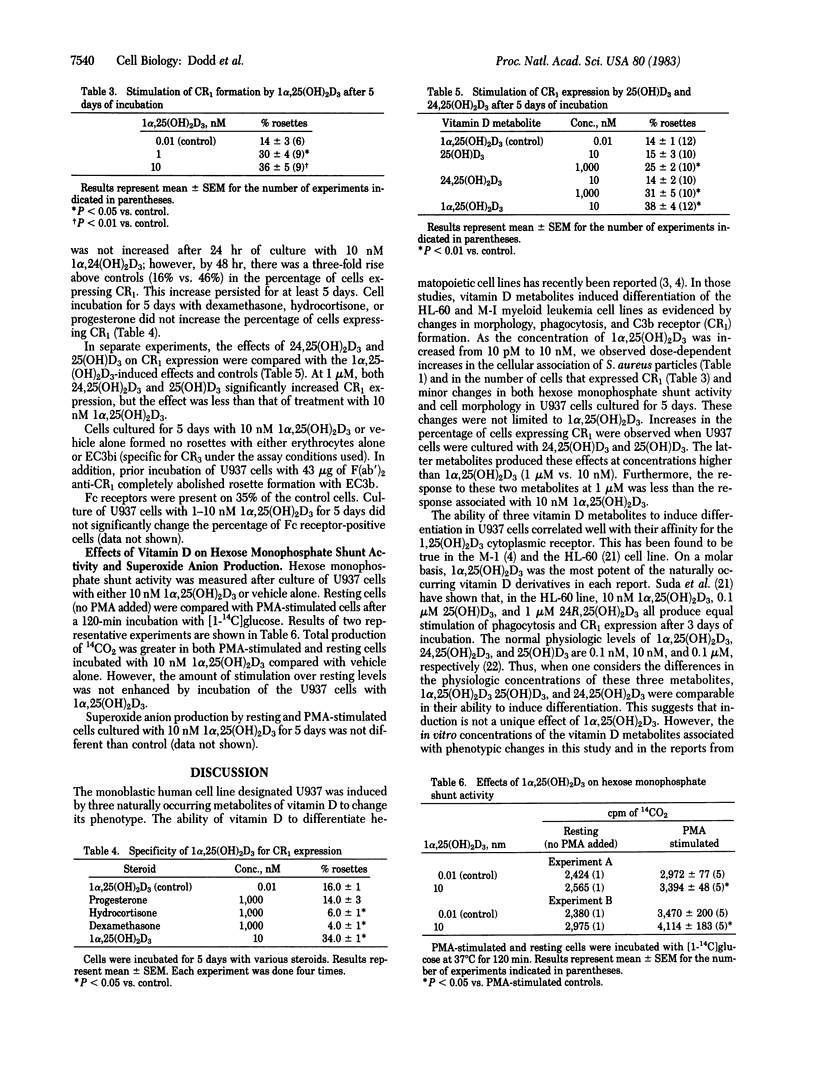
U937 is a human-derived lymphoma cell line that has monoblastic properties and high-affinity receptors for 1 alpha,-dihydroxyvitamin D3. Incubation of these cells with the vitamin D metabolite at 10 nM for 5 days produced marked stimulation in adherence and ingestion of Staphylococcus aureus (645% of control) and of C3b receptor (CR1) expression (292% of control) and a slight increase in hexose monophosphate shunt activity without changing cell growth rates or Fc fragment receptor expression. The changes in cellular association of S. aureus and the CR1 were detected as early as 48 hr of incubation and peaked between 3 and 5 days. Similar changes in the CR1 were induced by 25-hydroxy- and 24,25-dihydroxyvitamin D3 at micromolar concentrations. Dexamethasone, hydrocortisone, and progesterone had no effect on CR1 expression. U937 cells incubated in the presence of vitamin D metabolites exhibited a change in their phenotype. These results suggest that vitamin D metabolites may contribute to monocyte/macrophage differentiation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges K., Levenson R., Housman D., Cantley L. Calcium regulates the commitment of murine erythroleukemia cells to terminal erythroid differentiation. J Cell Biol. 1981 Aug;90(2):542–544. doi: 10.1083/jcb.90.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. II. Temperature-dependent transfer of the hormone to chromatin via a specific cytosol receptor. J Biol Chem. 1974 Feb 25;249(4):1258–1262. [PubMed] [Google Scholar]
- Clement L. T., Lehmeyer J. E. Regulation of the growth and differentiation of a human monocytic cell line by lymphokines. I. Induction of superoxide anion production and chemiluminescence. J Immunol. 1983 Jun;130(6):2763–2766. [PubMed] [Google Scholar]
- Dobson N. J., Lambris J. D., Ross G. D. Characteristics of isolated erythrocyte complement receptor type one (CR1, C4b-C3b receptor) and CR1-specific antibodies. J Immunol. 1981 Feb;126(2):693–698. [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine O., Matsumoto T., Goodman D. B., Rasmussen H. Liponomic control of Ca2+ transport: relationship to mechanism of action of 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1751–1754. doi: 10.1073/pnas.78.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C., Nilsson K. Surface glycoproteins of human non-T, non-B acute lymphocytic leukemia cell lines. Leuk Res. 1980;4(3):279–286. doi: 10.1016/0145-2126(80)90035-1. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Pattengale P. K., Jansson M., Wigzell H., Nilsson K. Natural killer cells kill tumour cells at a given stage of differentiation. Nature. 1981 Aug 27;292(5826):848–850. doi: 10.1038/292848a0. [DOI] [PubMed] [Google Scholar]
- Gray T. K., McAdoo T., Pool D., Lester G. E., Williams M. E., Jones G. A modified radioimmunoassay for 1,25-dihydroxycholecalciferol. Clin Chem. 1981 Mar;27(3):458–463. [PubMed] [Google Scholar]
- Koren H. S., Anderson S. J., Larrick J. W. In vitro activation of a human macrophage-like cell line. Nature. 1979 May 24;279(5711):328–331. doi: 10.1038/279328a0. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Fischer D. G., Anderson S. J., Koren H. S. Characterization of a human macrophage-like cell line stimulated in vitro: a model of macrophage functions. J Immunol. 1980 Jul;125(1):6–12. [PubMed] [Google Scholar]
- Levenson R., Housman D., Cantley L. Amiloride inhibits murine erythroleukemia cell differentiation: evidence for a Ca2+ requirement for commitment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5948–5952. doi: 10.1073/pnas.77.10.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Induction of specific changes in the surface membrane of myeloid leukemic cells by steroid hormones. Int J Cancer. 1975 May 15;15(5):731–740. doi: 10.1002/ijc.2910150504. [DOI] [PubMed] [Google Scholar]
- Miyaura C., Abe E., Kuribayashi T., Tanaka H., Konno K., Nishii Y., Suda T. 1 alpha,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1981 Oct 15;102(3):937–943. doi: 10.1016/0006-291x(81)91628-4. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Roth J., Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982 Fall;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Olsson I. L., Breitman T. R., Gallo R. C. Priming of human myeloid leukemic cell lines HL-60 and U-937 with retinoic acid for differentiation effects of cyclic adenosine 3':5'-monophosphate-inducing agents and a T-lymphocyte-derived differentiation factor. Cancer Res. 1982 Oct;42(10):3928–3933. [PubMed] [Google Scholar]
- Olsson I. L., Breitman T. R. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by retinoic acid and cyclic adenosine 3':5'-monophosphate-inducing agents. Cancer Res. 1982 Oct;42(10):3924–3927. [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Waldrep J. C., Mohanakumar T., Kaplan A. M. Human mononuclear phagocyte-associated antigens. II. Lymphokine-inducible antigens on the macrophage cell line, U937. Cell Immunol. 1981 Jul 15;62(1):140–148. doi: 10.1016/0008-8749(81)90307-5. [DOI] [PubMed] [Google Scholar]
- Winfield J. B., Lobo P. I., Hamilton M. E. Fc receptor heterogeneity: immunofluorescent studies of B, T, and "third population" lymphocytes in human blood with rabbit IgG b4/anti-b4 complexes. J Immunol. 1977 Nov;119(5):1778–1784. [PubMed] [Google Scholar]



