Abstract
Objective
To determine precisely the role of parathyroid hormone (PTH) and of phosphatonins in the genesis of posthepatectomy hypophosphatemia.
Background
Posthepatectomy hypophosphatemia has recently been related to increased renal fractional excretion of phosphate (FE P). To address the cause of hypophosphatemia, we measured serum concentrations of PTH, various phosphatonins, and the number of removed hepatic segment in patients with this disorder.
Methods
Serum phosphate (PO4), ionized calcium (Ca++), , pH and FE P, intact PTH (I-PTH), carboxyl-terminal fibroblast growth factor 23 (C-FGF-23) and intact fibroblast growth factor 23 (I-FGF-23), FGF-7, and secreted frizzled related-protein-4 (sFRP-4) were measured before and on postoperative (po) days 1, 2, 3, 5, and 7, in 18 patients undergoing liver resection. The number of removed hepatic segments was also assessed.
Results
Serum PO4 concentrations decreased within 24 hours, were lowest (0.66 ± 0.03 mmol/L; P < 0.001) at 48 hours, and returned to normal within 5 days of the procedure. FE P peaked at 25.07% ± 2.26% on po day 1 (P < 0.05). Decreased ionized calcium concentrations (1.10 ± 0.01 mmol/L; P < 0.01) were observed on po day 1 and were negatively correlated with increased I-PTH concentrations (8.8 ± 0.9 pmol/L; P < 0.01; correlation: r = −0.062, P = 0.016). FE P was positively related to I-PTH levels on po day 1 (r = 0.52, P = 0.047) and negatively related to PO4 concentrations (r = −0.56, P = 0.024). Severe hypophosphatemia and increased urinary phosphate excretion persisted for 72 hours even when I-PTH concentrations had returned to normal. I-FGF-23 decreased to its nadir of 7.8 ± 6.9 pg/mL (P < 0.001) on po day 3 and was correlated with PO4 levels on po days 0, 3, 5, and 7 (P < 0.001). C-FGF-23, FGF-7 and sFRP-4 levels could not be related to either PO4 concentrations or FE P.
Conclusion
Posthepatectomy hypophosphatemia is associated with increased FE P unrelated to I-FGF-23 or C-FGF-23, FGF-7, or sFRP-4. I-PTH contributes to excessive FE P partially on po day 1 but not thereafter. Other yet defined factors should explain post hepatectomy hypophosphatemia.
Hypophosphatemia frequently occurs after liver resection and has also been reported after major colorectal and aortic surgeries.1–4 Because of the important role that phosphates play in normal bone mineralization, signal transduction, nucleotide metabolism, intermediary energy metabolism, and enzyme regulation,5–9 hypophosphatemia can result in serious clinical consequences. Manifestations of hypophosphatemia depend on the severity and rapidity of its occurrence and include confusion, convulsion, coma, cardiac failure, and diaphragmatic asthenia.10,11 After liver resection, pulmonary complications are serious, especially, after right hepatectomy.13
For a long time, increased metabolic demands by the regenerating liver were viewed as the underlying pathologic mechanism of hypophosphatemia. The magnitude of phosphate uptake by the recovering liver cannot explain the severity of hypophosphatemia.5,14 Therefore, other factors must play a role in the pathogenesis of hypophosphatemia. We, as well as Salem and Tray, observed an early postoperative (po) increase in the fractional excretion of phosphate (FE P) posthepatectomy.1,2 To determine the role of known phosphaturic agents such as parathyroid hormone (PTH) in the pathogenesis of renal phosphate wasting following hepatectomy, we and others measured PTH concentrations in such patients. Salem and Tray found normal PTH levels in 2 patients after hepatectomy.2 We reviewed our published data on calcium metabolism in surgical patients1,15 and found that 9 hepatectomized patients not included in the actual study1,15 showed significant increases in circulating intact PTH that were associated with hypocalcemia observed following the procedure. Changes in serum calcium and PTH concentrations, however, preceded hypophosphatemia and hyperphosphaturia by more than 24 hours, and therefore were not responsible for the pathogenesis of the hypophosphatemia, which persisted on the second and third po day.1,15
To further investigate the pathogenesis of hypophosphatemia seen following hepatectomy, we measured concentrations of “phosphatonins” (circulating phosphaturic peptide factors), known to play a role in the pathogenesis of disorders such as tumor induced osteomalacia, X-linked hypophosphatemic rickets, autosomal dominant hypophosphatemic rickets, and autosomal recessive hypophosphatemic rickets.14 Among phosphatonins, “fibroblast growth factor-23” (FGF-23) is best characterized.16 FGF-23 inhibits renal phosphate-handling and represses 1,25(OH)2D3 synthesis by renal 25-hydroxyvitamin d-1 alpha-hydroxylase, resulting in hypophosphatemia.17 FGF-7, matrix extracellular phosphoglycoprotein (MEPE) and secreted frizzled-related protein (sFRP)-4 also have phosphatonin activity and suppress renal phosphate-handling.17 Their role after hepatectomy has not yet been explored. We designed this prospective study to more precisely explore the role of intact PTH, FGF-23, FGF-7, and sFRP-4 in hypophosphatemia and hyperphosphaturia observed after liver resection.
MATERIALS AND METHODS
Patients
Eighteen patients scheduled for partial liver resection accepted to participate in the study, which was approved by the local human ethics committee. All patients signed informed consent forms. Exclusion criteria were: known hypophosphatemia of any cause, chronic renal disease (creatinine >150 mmol/L), hyperparathyroidism, therapy with oral antacids (aluminum-, calcium- or magnesium-based), and use of diuretics in the week preceding surgery or during hospital stay. Patients contracting significant respiratory (pCO2 <30 mm Hg) or metabolic ( >35 mmol/L) alkalosis (pH >7.45) during the study were also excluded. Serum samples were collected on the morning of the surgery after overnight fasting at 7 am and every morning on po days 1, 2, 3, 5, and 7 at fixed time at 7 am.
Postsurgical complications were defined as follows: pulmonary infection treated with antibiotics, wound infection, and biliary leak. For phosphate replacement, 250 to 500 mL of potassium phosphate (concentration of 60 mmol/L) was administered intravenously over a 4-hour period to maintain a serum phosphate concentration >0.7 mmol/L.
Routine Biochemistry Measurements
Serum sodium, potassium, ionized calcium, , and pH were measured within 30 minutes of sampling with a direct ion-selective electrode (ISE, Bayer RapidLab 865 Blood Gas System, Siemens Medical Solutions Diagnostics, Tarrytown, NY). Serum creatinine, total calcium, serum phosphate, albumin, and magnesium concentrations were quantified by automated colorimetry (Synchron LX20, Beckman-Coulter Inc., Fullerton, CA). Urine was collected for 12 hours before surgery and for 24 hours on po days 1, 2, 3, 5, and 7. FE P was estimated according to the following formula; (100 × 24 hours [or 12 hours] urinary phosphate [mmol/L]/serum phosphate [mmol/L])/(24 hours [or 12 hours] urinary creatinine [mmol/L]/ serum creatinine [mmol/L]). In FE P estimation, serum phosphate and creatinine values used were the mean of the measurements obtained at the beginning and end of the urinary collection. FE P of 10% to 15% was considered normal. Serum aliquots were kept at −70°C and thawed just before I-PTH (Elecsys, Roche Diagnostics, Laval, Quebec, Canada; normal = 1.4 and 6.8 pmol/L) and phosphatonins assays. FGF-23 levels were estimated using 2 different 2-site enzyme-linked immunosorbent assays (ELISAs): (1) a carboxyl-terminal human FGF-23 ELISA (Alpco Diagnostics, Salem, NH) and (2) an intact human FGF-23 ELISA (Kainos Laboratories, Tokyo, Japan). The carboxyl-terminal assay uses polyclonal antiserum and recognizes 2 epitopes on the carboxyl-terminal side of the proteolytic cleavage site of the full molecule (between aminoacids 176 and 180) and thus recognizes both full-length and carboxyl-terminal cleavage fragments of FGF-23. The Alpco Diagnostics carboxyl–terminal FGF-23 (C-FGF-23) assay has an intra-assay coefficient of variation of 5.0%, an interassay coefficient of variation of 5.0% to 7.3%, and a lower limit of detection of 3.0 relative units (RU)/mL. Values bellow 100 RU/mL were considered normal. The Kainos intact FGF-23 (I-FGF-23) assay uses 2 monoclonal antibodies to epitopes on either side of the cleavage site. The capture antibody recognizes an epitope on the aminoterminal side of the cleavage site, whereas the detection antibody recognizes an epitope on the carboxyterminal side; thus, the assay recognizes only the intact molecule. The Kainos I-FGF-23 assay has a lower limit of detection of 3 pg/mL and intra-assay and interassay coefficients of variation of less than 5%. Values between 8 and 52 pg/mL are considered normal. FGF-7 levels were measured using a human FGF-7 ELISA assay (R and D Systems, Minneapolis, MN). This assay uses monoclonal antibodies prepared against E. coli-expressed recombinant human keratinocyte growth factor. The assay was performed based on the manufacturer’s procedure. The FGF-7 ELISA assay has an intra-assay coefficient of variation <5.0%, an interassay coefficient of variation of 5.2% to 7.7%, and a lower limit of detection of 15 pg/mL. Values between 21 and 53 pg/mL are considered normal. sFRP-4 levels were estimated with an sFRP-4 ELISA, based on the use of a monoclonal mouse anti-FRP4 antibody clone bound to well plates.18 sFRP-4 ELISA assay has an intra-assay coefficient of variation of 10%, an interassay coefficient of variation of 18%, and a lower limit of detection of 5 ng/mL. Values bellow 1750 ng/mL are normal.
Statistical Analyses
Statistical analyses were performed using Sigma (GraphPad Software Inc., San Diego, CA) and SAS 9.0 (SAS Institute Inc, Cary, NC). Data were tested for normality and the results are expressed as mean ± SEM. Analysis of variance for repeated measures followed by Tukey’s test for multiple comparisons was used to evaluate significant relations. To identify determining factors of serum phosphate and FE P, Pearson correlation was performed with the number of removed hepatic segments and FGF-23, FGF-7, sFRP-4, and PTH levels for each po day. General correlations were grouped with a Generalized Least Squares mixed procedure. Differences were considered significant when the P value was <0.05.
RESULTS
Eighteen patients (10 women and 8 men) with a mean age of 58 ± 2 (range: 29–73) years, underwent hepatectomy for colorectal cancer metastases (n = 13), biliary tree trauma (n = 2), duodenal adenocarcinoma metastases (n = 1), hepatic cystadenoma (n = 1), or gallbladder adenocarcinoma (n = 1). The mean number of resected hepatic segments was 3.2 ± 0.2, clamping duration was 5.4 ± 1.9 minutes in the 5 patients who had this procedure, and intraoperative bleeding was 636 ± 126 mL. All patients were extubated in the recovery room and only half of them were admitted to intensive care unit for 24 hours except for 2 patients who stayed for 2 days. Six patients required phosphate replacement to maintain serum phosphate above 0.7 mmol/L. Apart from the lower serum phosphate, these patients behave similarly to the others. Three patients (16%) required an average of 1.3 U of red blood cells transfusion. The complication rate was 38 ± 11%, including spontaneously resolved biliary leak (n = 2), respiratory infection (n = 2), and wound infection (n = 2).
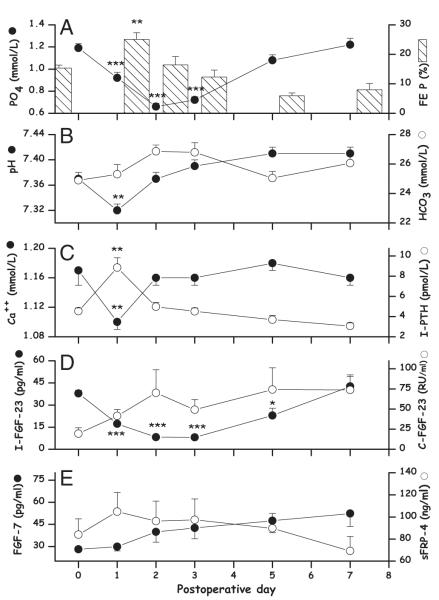
Serum phosphate was significantly lower on po day 1, 2, and 3. It decreased from 1.18 ± 0.03 to a minimum value of 0.66 ± 0.03 mmol/L (P < 0.001) on po day 2 (Fig. 1A). Serum phosphate at the nadir was nearly correlated with the number of resected liver segments (P = 0.08). FE P increased significantly from 15.3 ± 0.9 to 25 ± 2.2% (P < 0.01) on po day 1 (Fig. 1A), and was not correlated with the number of resected segments (P = 0.54). Phosphate replacement was required to maintain serum phosphate >0.70 mmol/L in 6 patients. and pH monitoring showed slight acidemia (pH: 7.32 ± 0.01) on po day 1, which spontaneously resolved on po day 2 without alkalemia (Fig. 1B). Serum ionized calcium fell from 1.16 ± 0.02 to 1.10 ± 0.01 mmol/L (P < 0.01) and I-PTH increased from 4.5 ± 0.3 to 8.8 ± 0.9 pmol/L (P < 0.01) on po day 1 (Fig. 1C). Serum calcium concentrations were inversely correlated to concentrations of I-PTH (r = −0.62, P = 0.016). FE P correlated positively with I-PTH (r = 0.52: P = 0.047) and negatively with phosphate levels (r = −0.56: P = 0.024) on po day 1 (Fig. 2).
FIGURE 1.
Evolution of measured biochemical parameters after partial hepatectomy in 18 patients. Results are means ± SEM. The evolution of serum phosphate (PO4) and FE P (A), of pH and (B), of Ca++ and I-PTH (C), of C-FGF-23 and I-FGF-23 (D), and of FGF-7 and sFRP-4 (E) is illustrated. Changes with time are analyzed with a repeated measurement analysis of variance followed by a Tukey test using day 0 for comparison; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIGURE 2.

Relationship between FE P, I-PTH, and PO4 levels on po day 1.
Levels of I-FGF-23 decreased significantly on po day 1, 2, 3, and 5. It fell from 37.9 ± 9 to 7.8 ± 6.9 pg/mL (nadir) on po day 3 (P < 0.001) (Fig. 1D). I-FGF-23 decrease (P = 0.03) was associated with serum phosphate decrease. Carboxyl-terminal FGF-23 increased from 19.4 ± 7.4 to 70.1 ± 28.8 RU/mL on po day 2, without reaching significance because of a large standard error (Fig. 1D). I- and C-FGF-23 varied inversely but remained nonsignificantly correlated for all po days. FGF-7 increased continually from 28.1 ± 1.11 to 52.4 ± 8.91 pg/mL (not significant) on po day 7. Secreted FRP-4 increased from 83.9 ± 14.2 to 104.8 ± 17.4 ng/mL (not significant) on po day 1 then decreased gradually to presurgical levels on po day 7 (Fig. 1E).
Correlations of serum phosphate and FE P with the potential phosphaturic factors were calculated for each po day. Serum phosphate determining factors in all po days estimated with the Generalized Least Square mixed procedure showed significant association (P < 0.001) with I-FGF-23 decrease (Fig. 1D) but not with C-FGF-23, FGF-7 or sFRP-4.
DISCUSSION
Hypophosphatemia is a frequent disorder after partial hepatectomy. Several hypotheses have been postulated to account for the pathogenesis of hypophosphatemia. It is now believed that increased metabolic demands by regenerating liver, the original hypothesis do not account for the occurrence of hypophosphatemia. We and others have shown that posthepatectomy hypophosphatemia is associated with increased urinary phosphate losses due to increased FE P by the kidney.1,2 The pathogenesis of increased FE P is not clear, and to ascertain factor(s) responsible, we measured the concentration of several phosphaturic peptides.
pH plays a central role in the control of the phosphate level. Alkalosis induces hypophosphatemia by an acute intracellular shift of serum phosphate with resulting intracellular replenishment.5 In our experience, pH increased immediately after the onset of anesthesia and before surgery start; however, the mean pH remains within the normal range (7.36–7.45).15 We did not find alkalosis in the present study but transient acidosis on posthepatectomy day 1. Acute acidosis mobilize intracellular phosphate (depletion) to restore its plasma concentration despite absence of replacement and continuing urinary loss.19 Posthepatectomy hypophosphatemia was not related to alkalosis in our patients and was not corrected by transient acidosis.
PTH is a powerful phosphaturic agent that reduces renal proximal tubular phosphate uptake resulting in an increased FE P and hypophosphatemia.5 In our study, I-PTH values were normal except on po day 1 where the level was increased 2-fold and was correlated with FE P. FE P and I-PTH levels were not correlated on other po days. Experimental models have shown that FE P increases very rapidly (<20 minutes) in response to PTH with a simultaneous down-regulation of NPt2a cotransporters.20,21 Surgical monitoring of I-PTH values in our previous study1,15 showed an early 9-fold increase during the surgical procedure, which was associated with hypocalcemia. PTH changes preceded the nadir of hypophosphatemia by 24 hours.1,15 Results of the present study are similar except for the fact that I-PTH values were the only determining factor of FE P on po day 1. It is important to note that changes in serum calcium and I-PTH had resolved when hypophosphatemia was maximal on po days 2 and 3. Therefore, the duration of posthepatectomy hypophosphatemia has to be explained by other phosphaturic factor than PTH.
Phosphatonins are phosphaturic peptides that decrease renal sodium-dependent cotransport of phosphate.17 They have been isolated from tumors inducing renal phosphate loss and osteomalacia. FGF-23, an archetypal phosphatonin is detected in normal plasma and at elevated concentrations in tumor-induced osteomalacia, X-linked hypophosphatemia, and autosomic dominant hypophosphatemic rickets.17,22 FGF-23 represses 1,25-dihydroxy vitamin D synthesis and decreases intestinal phosphate absorption.23 FGF-23 is not associated, however, with urinary phosphate wasting in tumoral calcinosis and other malignancies.17,24 Elevated FGF-23 levels may be associated with hypophosphatemia in some fibrous dysphasia and, controversially, with phosphate retention in end-stage renal failure. FGF-23 is not associated with serum phosphate in advanced-stage ovarian cancer patients.17,25 The role of FGF-23 in phosphate homeostasis under normal and surgical circumstances is unknown and the absence of consensual phosphatonins assays does not facilitate physiological investigations.17 Published data remain unclear whether they report I- or C-FGF-23.17 According to a recent report, dietary phosphate was a key regulator of circulating I-FGF-23 that was also associated with serum phosphate.23 Interestingly, in our study, serum phosphate was associated with I-FGF-23, but not C-FGF-23 (Fig. 1). Serum phosphate seemed to act as a negative feedback regulator of I-FGF-23, therefore eliminating the contribution of this major phosphaturic factor to the already inappropriately high phosphate excretion. I-FGF-23 and C-FGF-23 varied inversely (Fig. 1), while the opposite changes, however, did not reaching statistical significance. Carboxyl-terminal fragments of FGF-23 could be produced from I-FGF-23 degradation. Both I-FGF-23 and C-FGF-23 levels were not determining factors for FE P after hepatectomy.
FGF-7 or keratinocyte growth factor, a member of the FGF family, is involved in tissue repair26 and other physiological roles in embryonic development and in adulthood.27 As it is over-expressed in tumor-induced osteomalacia with renal phosphate wasting, FGF-7 has become a candidate phosphatonin.28 In renal epithelial cells in culture, FGF-7 inhibits the sodium-dependant cotransport of phosphate. Our investigation shows that FGF-7 is a circulating agent without significant po augmentation after partial hepatectomy and does not contribute to renal phosphate loss.
The possible role of sFRP-4 after hepatic surgery was explored for the first time. sFRP-4 inhibits sodium-dependent phosphate cotransport in renal epithelial cells in culture.29 sFRP-4 activity was dose-dependent and PTH-unrelated in an animal model.18 Also, sFRP-4 decreased serum phosphate and increased FE P without variation of 25-hydroxyvitamine D 1 α-hydroxylase mRNA renal expression.18 In this study, sFRP-4 slightly increased by 20% on po day 1 but did not show significant variation after hepatectomy or correlations with serum phosphate or FE P. sFRP-4 was not a phosphaturic factor after liver resection.
The possible role of MEPE and of its biologic active fragment30 acidic serine-aspartate-rich MEPE-associated motif was not investigated during our study. Acidic serine-aspartate-rich MEPE-associated motif increases NaPi2 expression and phosphate loss. It remains a possible candidate phosphatonin in posthepatectomy hypophosphatemia.
Phosphate renal loss was shown to be a more credible cause of hypophosphatemia than demands from the regenerating liver after hepatectomy.1 The 3 phosphatonins measured in this study were not responsible for the classic posthepatectomy hypophosphatemia. The resulting hypophosphatemia contributed to down-regulation of I-FGF-23, possibly by favoring its degradation into C-FGF-23.
ACKNOWLEDGMENTS
The authors thank Louise Rousseau, Research Assistant, and Ovid Da Silva, Research Support Office, Research Centre, CHUM, for editing this article.
REFERENCES
- 1.Nafidi O, Lepage R, Lapointe RW, et al. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2007;245:1000–1002. doi: 10.1097/SLA.0b013e31805d0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2005;241:343–348. doi: 10.1097/01.sla.0000152093.43468.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovannini I, Chiarla C, Giuliante F, et al. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2006;243:429. doi: 10.1097/01.sla.0000202002.17260.c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J, Kogan A, Sahar G, et al. Hypophosphatemia following open heart surgery: incidence and consequences. Eur J Cardiothorac Surg. 2004;26:306–310. doi: 10.1016/j.ejcts.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Datta HK, Malik M, Neely RD. Hepatic surgery-related hypophosphatemia. Clin Chim Acta. 2007;380:13–23. doi: 10.1016/j.cca.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Hettleman BD, Sabina RL, Drezner MK, et al. Defective adenosine triphosphate synthesis. An explanation for skeletal muscle dysfunction in phosphate-deficient mice. J Clin Invest. 1983;72:582–589. doi: 10.1172/JCI111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preston CJ, Noorwali A, Challa A, et al. Intracellular inorganic phosphate and ATP levels in human blood erythrocytes, leucocytes and platelets in normal subjects and in diseases associated with altered phosphate metabolism. Adv Exp Med Biol. 1982;151:147–155. doi: 10.1007/978-1-4684-4259-5_20. [DOI] [PubMed] [Google Scholar]
- 8.Young JA, Lichtman MA, Cohen J. Reduced red cell 2,3-diphosphoglycerate and adenosine triphosphate, hypophosphatemia, and increased hemoglobin-oxygen affinity after cardiac surgery. Circulation. 1973;47:1313–1318. doi: 10.1161/01.cir.47.6.1313. [DOI] [PubMed] [Google Scholar]
- 9.Ambuhl PM, Meier D, Wolf B, et al. Metabolic aspects of phosphate replacement therapy for hypophosphatemia after renal transplantation: impact on muscular phosphate content, mineral metabolism, and acid/base homeostasis. Am J Kidney Dis. 1999;34:875–883. doi: 10.1016/S0272-6386(99)70045-4. [DOI] [PubMed] [Google Scholar]
- 10.Amanzadeh J, Reilly RF., Jr. Hypophosphatemia: an evidence-based approach to its clinical consequences and management [review] Nat Clin Pract Nephrol. 2006;2:136–148. doi: 10.1038/ncpneph0124. [DOI] [PubMed] [Google Scholar]
- 11.Miller DW, Slovis CM. Hypophosphatemia in the emergency department therapeutics. Am J Emerg Med. 2000;18:457–461. doi: 10.1053/ajem.2000.7347. [DOI] [PubMed] [Google Scholar]
- 12.Dondero F, Taillé C, Mal H, et al. Respiratory complications: a major concern after right hepatectomy in living liver donors. Transplantation. 2006;81:181–186. doi: 10.1097/01.tp.0000191624.70135.35. [DOI] [PubMed] [Google Scholar]
- 13.Burak KW, Rosen CB, Fidler JL, et al. Hypophosphataemia after right hepatectomy for living donor liver transplantation. Can J Gastroenterol. 2004;18:729–733. doi: 10.1155/2004/328027. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh A, Berndt T, Kumar R. Regulation of phosphate homeostasis by the phosphatonins and other novel mediators. Pediatr Nephrol. 2008;23:1203–1210. doi: 10.1007/s00467-008-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepage R, Légaré G, Racicot C, et al. Hypocalcemia induced during major and minor abdominal surgery in humans. J Clin Endocrinol Metab. 1999;84:2654–2658. doi: 10.1210/jcem.84.8.5889. [DOI] [PubMed] [Google Scholar]
- 16.Schiavi SC, Kumar R. The phosphatonin pathway: new insights in phosphate homeostasis [review] Kidney Int. 2004;65:1–14. doi: 10.1111/j.1523-1755.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- 17.Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis [review] Annu Rev Physiol. 2007;69:341–359. doi: 10.1146/annurev.physiol.69.040705.141729. [DOI] [PubMed] [Google Scholar]
- 18.Berndt T, Craig TA, Bowe AE, et al. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmese S, Pezza M, De Robertis E. Hypophosphatemia and metabolic acidosis. Minerva Anestesiol. 2005;71:237–242. [PubMed] [Google Scholar]
- 20.Lötscher M, Scarpetta Y, Levi M, et al. Rapid downregulation of rat renal Na/P(i) cotransporter in response to parathyroid hormone involves microtubule rearrangement. J Clin Invest. 1999;104:483–494. doi: 10.1172/JCI3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedlaender MM, Wald H, Dranitzky-Elhalel M, et al. Recovery of renal tubule phosphate reabsorption despite reduced levels of sodium-phosphate transporter. Eur J Endocrinol. 2004;151:797–801. doi: 10.1530/eje.0.1510797. [DOI] [PubMed] [Google Scholar]
- 22.Sommer S, Berndt T, Craig T, et al. The phosphatonins and the regulation of phosphate transport and vitamin D metabolism. J Steroid Biochem Mol Biol. 2007;103:497–503. doi: 10.1016/j.jsbmb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Perwad F, Zhang MY, Tenenhouse HS, et al. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 24.Frishberg Y, Topaz O, Bergman R, et al. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med. 2005;83:33–38. doi: 10.1007/s00109-004-0610-8. [DOI] [PubMed] [Google Scholar]
- 25.Tebben P, Kalli KR, Cliby WA, et al. Elevated fibroblast growth factor 23 concentrations in women with malignant ovarian tumors. Mayo Clin Proc. 2005;80:745–751. doi: 10.1016/S0025-6196(11)61528-0. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter TO, Ellis BK, Insogna KL, et al. Fibroblast growth factor 7: an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab. 2004;90:1012–1020. doi: 10.1210/jc.2004-0357. [DOI] [PubMed] [Google Scholar]
- 27.Danilenko DM. Preclinical and early clinical development of keratinocyte growth factor, an epithelial-specific tissue growth factor. Toxicol Pathol. 1999;27:64–71. doi: 10.1177/019262339902700113. [DOI] [PubMed] [Google Scholar]
- 28.Cancilla B, Davies A, Cauchi JA, et al. Fibroblast growth factor receptors and their ligands in the adult rat kidney. Kidney Int. 2001;60:147–155. doi: 10.1046/j.1523-1755.2001.00781.x. [DOI] [PubMed] [Google Scholar]
- 29.Berndt TJ, Bielesz B, Craig TA, et al. Secreted frizzled-related protein-4 reduces sodium-phosphate co-transporter abundance and activity in proximal tubule cells. Pflugers Arch. 2006;451:579–587. doi: 10.1007/s00424-005-1495-2. [DOI] [PubMed] [Google Scholar]
- 30.Guicciardi ME, Deussing J, Miyoshi H, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]