Abstract
Purpose
As tyrosine kinase inhibitors have been associated with cardiotoxicity, we evaluated the effect of pazopanib, an inhibitor of vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and c-Kit, on electrocardiographic parameters in patients with cancer.
Methods
This double-blind, placebo-controlled, parallel-group study randomized patients (N = 96) to moxifloxacin (positive control) or placebo on Day 1 followed by pazopanib or placebo 800 mg/day (fasted) on Days 2–8 and 1,600 mg (with food) on Day 9. Treatment effects were evaluated by baseline-adjusted, time-matched, serial Holter electrocardiograms.
Results
Sixty-five patients were evaluable for preplanned analyses. On Day 1, the maximum mean difference in baseline-adjusted, time-matched Fridericia-corrected QT (QTcF) interval in moxifloxacin-treated patients versus placebo was 10.6 ms (90 % confidence interval [CI]: 4.2, 17.0). The administration scheme increased plasma pazopanib concentrations approximately 1.3- to 1.4-fold versus the recommended 800 mg once-daily dose. Pazopanib caused clinically significant increases from baseline in blood pressure, an anticipated class effect, and an unexpected reduction in heart rate from baseline that correlated with pazopanib exposure. On Day 9, the maximum mean difference in baseline-adjusted, time-matched QTcF interval in pazopanib-treated patients versus placebo was 4.4 ms (90 % CI: −2.4, 11.2). Mixed-effects modeling indicated no significant concentration-dependent effect of pazopanib or its metabolites on QTcF interval.
Conclusions
Pazopanib as administered in this study achieved supratherapeutic concentrations, produced a concentration-dependent decrease in heart rate, and caused a small, concentration-independent prolongation of the QTcF interval.
Keywords: Moxifloxacin, Pazopanib, Pharmacokinetics, QTc, Safety, Tyrosine kinase inhibitor
Introduction
Pazopanib (Votrient™, GlaxoSmithKline) is an oral, multitargeted inhibitor of vascular endothelial growth factor (VEGF) receptor tyrosine kinases, platelet-derived growth factor receptor tyrosine kinases, and c-Kit [1–3]. Based primarily on data from the pivotal phase III trial wherein pazopanib monotherapy significantly improved median progression-free survival by 5 months versus placebo (P < 0.001) [3], pazopanib was approved in several countries for the treatment of advanced renal cell carcinoma [4–8]. The safety profile of pazopanib was generally acceptable and tolerable, and the most common adverse events (AEs) reported in the pivotal phase III trial included diarrhea, hypertension, hair-color changes, nausea, anorexia, and vomiting. Although cardiac safety issues, including QTc prolongation and the risk of torsades de pointes, are uncommon in pazopanib-treated patients, they have been reported with other VEGF inhibitors [9].
Preclinical data in monkeys or rats did not indicate an association of prolonged QTc or other changes in electrocardiographic (ECG) activity with pazopanib treatment. However, in clinical studies of patients with renal cell carcinoma, QT prolongation >500 ms was observed in 10/558 pazopanib-treated patients (1.8 %). Two cases of torsade de pointes were identified during clinical development of pazopanib. A retrospective evaluation of QTc intervals from 2 Phase I studies suggested no apparent relationship between plasma pazopanib concentrations and QTc intervals. To fully evaluate the effects of pazopanib on QT interval and other ECG parameters, we conducted a randomized, double-blind, placebo-controlled, parallel-group, repeated-dose, multicenter study (NCT00861029).
Materials and methods
Patients
Eligible patients were ≥18 years of age, with a histologically or cytologically confirmed advanced solid tumor, Eastern Cooperative Oncology Group performance status of 0 or 1, adequate organ and hematologic function, normal concentrations of serum potassium, calcium, and magnesium, and ability to swallow and retain oral medication. All patients provided written informed consent before study entry. The study was approved by the respective institutional ethics committees.
Key exclusion criteria included baseline Fridericia-corrected QT (QTcF) interval >470 ms, PR interval >240 ms or ≤110 ms, QRS duration >120 ms, bradycardia (sinus rate <50 beats per minute [bpm]), abnormal cardiac function, poorly controlled hypertension (systolic blood pressure >140 mmHg or diastolic >90 mmHg), or clinically significant gastrointestinal abnormalities that would adversely affect drug absorption.
Study objectives
The primary objective of this study was to estimate the effect of pazopanib on the QTcF interval compared with placebo. .
Additional QT estimations included Bazett correction (QTcB) and an individualized correction (QTci) where βi is the individual correction term, which is the estimated slope of log-transformed QT versus log-transformed RR for the ith patient [10, 11].
Secondary objectives included analyses of associations between plasma concentrations of pazopanib, its metabolites, and moxifloxacin and ECG parameters; description of the short-term safety of repeated daily doses of 800-mg pazopanib versus placebo; and the acute safety of a single 1,600-mg pazopanib dose.
Study design and treatment
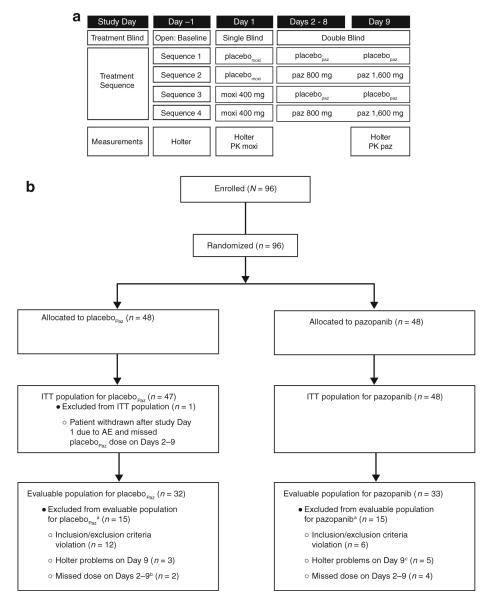
This double-blind, placebo-controlled, parallel-group, multicenter study randomized eligible patients to 1 of 4 treatment sequences (Fig. 1a). Moxifloxacin, a drug known to prolong QT interval, served as a positive control. Patients received either moxifloxacin or equivalent placebo (placebomoxi) on Day 1 followed by 800-mg pazopanib or equivalent placebo (placebopaz) on Days 2 through 8, and 1,600-mg pazopanib or placebopaz on Day 9.
Fig. 1.
Study design. a For Day 1, treatment sequences 1 and 2 were grouped together and labeled as the placebomoxi group, while treatment sequences 3 and 4 were grouped together and labeled as the moxifloxacin (moxi) group. For Days 2 through 9, treatment sequences 1 and 3 were grouped together and labeled as the placebopaz group, while treatment sequences 2 and 4 were grouped together and labeled as the pazopanib (paz) group. b CONSORT diagram describing patient populations. aPatients may be included in >1 category. bOne additional patient who was excluded is captured in the intent-to-treat (ITT) population. cAn additional 2 patients who had Holter problems on Day 1 were excluded from the evaluable population and are not captured here. AE adverse event PK pharmacokinetics
Study drug was administered in a fasted state on Days 1 through 8, and immediately after a standardized meal on Day 9 to enhance pazopanib absorption and increase plasma concentrations [12].
Patients eligible to continue pazopanib treatment were enrolled into a continued-access study (NCT00387205). Patients who discontinued pazopanib were evaluated at a post-treatment follow-up visit within 28 days of the last pazopanib dose.
Measurements and analysis
Continuous digital Holter ECG data were acquired and stored electronically, and manually over-read by an external, central, validated ECG laboratory. At each designated time point, the average value from 3 consecutive 10-s ECG recordings approximately 2 min apart was used to estimate the study-defined ECG parameters.
Serial blood samples for pharmacokinetic analysis of moxifloxacin, pazopanib, and pazopanib metabolites were obtained at the same time points as ECG measurements on Days 1 and 9, respectively. Concentrations of moxifloxacin in plasma samples were evaluated using a validated analytical method based on protein precipitation, followed by high-performance liquid chromatography/mass spectrometry/mass spectrometry analysis. Concentrations of pazopanib and its metabolites (GSK1268992, GSK1268997, and GSK1071306) in plasma samples were evaluated using a validated analytical method as previously described [13].
Statistical methods
No formal hypothesis was tested. The study required a minimum of 60 evaluable patients to complete the study (~15 patients per treatment sequence). Sample size calculations assumed that an estimation approach was followed to evaluate the effect of repeated daily dosing of pazopanib on the QTcF interval versus placebo. Assuming that there would be standard deviation of 20 ms between patients, a sample size of 60 patients was estimated to provide a half-width 90 % confidence interval (CI) of 8.6 ms for the mean QTcF difference between treatments at a particular time point.
Patients who received ≥1 dose of study drug were included in the intent-to-treat (ITT) population. In the preplanned (8-h) analysis, patients were included in the evaluable population if they met eligibility criteria; received full-dose study drug on Days 1, 8, and 9, and ≥5 study drug doses during Days 2 through 7; maintained double-blind treatment allocation through Day 9; and had Holter ECG acquisitions completed through the 8-h time point on Days −1, 1, and 9. To further evaluate the effects of pazopanib on ECG parameters, a post hoc analysis was also conducted using data extracted up to 24 h after pazopanib dosing. For this post hoc (24-h) analysis, the definition of the evaluable population was identical to the original with the exception that Holter ECG acquisitions had to be completed through the 24-h time point on Days −1 and 9.
Changes from baseline in time-matched ECG parameters (QTcF, QTcB, QTci, and RR) at each time point on Day 9 were analyzed by linear mixed-effects modeling (PROC MIXED in SAS) for the comparison between pazopanib and placebo. Treatment (pazopanib or placebo), time point, treatment/time point interaction term, and baseline values were included in the model as fixed effects. Patient was included as random effect. Least-squares means and corresponding 2-sided 90 % CIs were constructed for the mean difference between pazopanib and placebo. Similar analyses were conducted for ECG parameters on Day 1 for the comparison between moxifloxacin and placebo. Mixed-effects model was applied to examine the relationship of QTc with RR interval (from Holter ECG data) at baseline with patient as random effect in the model.
The relationship between plasma concentrations of moxifloxacin, pazopanib, or pazopanib metabolites and changes in QTcF was explored graphically and by linear mixed-effects modeling performed using NONMEM VI (ICON, Ellicott City, Maryland). Data from patients who received placebo were used to account for diurnal effects and for the effect of the morning meal consumed on Day 9. The change from baseline in QTcF for each patient who received moxifloxacin or pazopanib was corrected by subtracting the time-matched mean change from baseline in QTcF in patients who received the corresponding placebo.
A term for the slope of the effect of plasma moxifloxacin, pazopanib, or pazopanib metabolite concentrations on the placebo-corrected change from baseline in QTcF was added to the initial model. The slope term was considered significant if addition of the term to the base model resulted in a decrease in the objective function of >3.84 (P < 0.05), and the 95 % CI parameter value did not contain 0.
Results
Patient enrollment and demographics
Of the 96 patients randomized, 95 patients (99 %) completed the study and 65 (68 %) met the prespecified definition of evaluability (evaluable population) for the preplanned analyses (Fig. 1b). One patient (1 %), randomized to moxifloxacin on Day 1 followed by placebo on Days 2 through 9, was withdrawn after Day 1 treatment due to an AE. Thirty-one patients (32 %) were deemed nonevaluable, most of whom (18 patients) were not evaluable due to reasons that occurred before randomization (violation of enrollment criteria) or due to technical problems related to Holter recording (12 patients). A minority of patients were not evaluable due to missed dose(s) during the study (6 patients, 6 %). Decisions regarding patient evaluability based on prespecified criteria were made while the treatment allocation blind was maintained. Table 1 summarizes the baseline demographic data by treatment group on Day 1 and Days 2 through 9. Baseline QTcF and QTcB values by treatment group are summarized in Online Tables S1 and S2. All patients had advanced cancer and had received prior anticancer treatment.
Table 1.
Characteristics and demographics of intent-to-treat and evaluable populations
| Intent-to-treat population (N = 96) |
Evaluable population (N = 65) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebomoxi (n = 48) |
Moxi (n = 48) |
Placebopaz (n = 47) |
Paz (n = 48) |
Placebomoxi (n = 31) |
Moxi (n = 34) |
Placebopaz (n = 32) |
Paz (n = 33) |
|
| Age, median years (min, max) | 61 (33, 78) | 58 (22, 79) | 56 (27, 78) | 61 (22, 79) | 58 (39, 72) | 56 (22, 79) | 57 (27, 77) | 58 (22, 79) |
| Female, n (%) | 18 (38) | 28 (58) | 23 (49) | 23 (48) | 12 (39) | 16 (47) | 14 (44) | 14 (42) |
| Male, n (%) | 30 (63) | 20 (42) | 24 (51) | 25 (52) | 19 (61) | 18 (53) | 18 (56) | 19 (58) |
| Height, median cm (min, max) | 173 (152, 200) | 167 (152, 186) | 167 (152, 188) | 173 (154, 200) | 175 (158, 200) | 170 (152, 186) | 169 (152, 199) | 174 (154, 200) |
| Weight, median kg (min, max) | 78.8 (47, 115) | 74.7 (46, 109) | 80.5 (46, 115) | 74.7 (51, 104) | 81.0 (55, 115) | 73.9 (47, 109) | 79.9 (47, 115) | 74.7 (51, 104) |
| Ethnicity | ||||||||
| Hispanic/Latino, n (%) | 3 (6) | 2 (4) | 3 (6) | 2 (4) | 2 (6) | 2 (6) | 2 (6) | 2 (6) |
| Not Hispanic/Latino, n (%) | 45 (94) | 46 (96) | 44 (94) | 46 (96) | 29 (94) | 32 (94) | 30 (94) | 31 (94) |
| White, n (%) | 44 (92) | 43 (90) | 43 (91) | 43 (90) | 29 (94) | 31 (91) | 31 (97) | 29 (88) |
| African American/African heritage, n (%) | 3 (6) | 4 (8) | 3 (6) | 4 (8) | 2 (6) | 3 (9) | 1 (3) | 4 (12) |
| Asian/Japanese/East Asian heritage, n (%) | 1 (2) | 1 (2) | 1 (2) | 1 (2) | 0 | 0 | 0 | 0 |
Moxi moxifloxacin, Paz pazopanib
Effect of moxifloxacin
On Day 1, the maximum mean difference in the baseline-adjusted, time-matched QTcF for moxifloxacin-treated patients compared with placebo was 10.6 ms (90 % CI: 4.2, 17.0) at 3 h post-dose in the evaluable population. No heart rate changes were observed on Day 1 for patients receiving moxifloxacin or placebo. The results were similar for the ITT population (Online Table S3).
Effect of pazopanib
Blood pressure and heart rate
On Day 9, pazopanib caused clinically significant increases in systolic [+17.7 mmHg (standard deviation 14.2)] and diastolic [+11.9 mmHg (standard deviation 6.3)] blood pressure at 8 h post-dose that returned to near baseline by 24 h, consistent with the known pharmacodynamic effect of this class of agents. There was, however, an unexpected reduction in heart rate observed for pazopanib-treated patients (Table 2). From the linear mixed-effect model of heart rate change from baseline based on Holter ECG measurements, heart rate decreased on Day 9 in pazopanib-treated patients compared with placebo-treated patients; the maximum difference was noted at 8 h, with -8.2 bpm in the evaluable population (90 % CI: −12.7, −3.7) and −9.9 bpm in the ITT population (90 % CI: −13.9, −5.8).
Table 2.
Least-squares mean differences in heart rate changes from baseline for patients receiving pazopanib compared with placebo
| Day 9 time point, h |
Heart rate mean difference in bpm (90 % CI) evaluable population, N = 65 |
|---|---|
| −0.5 | −0.68 (−5.27, 3.92) |
| 1 | −5.48 (−9.99, −0.97) |
| 2 | −4.11 (−8.63, 0.41) |
| 3 | −4.30 (−8.81, 0.21) |
| 4 | −4.33 (−8.85, 0.18) |
| 6 | −7.75 (−12.26, −3.23) |
| 8 | −8.21 (−12.74, −3.68) |
|
| |
| ITT population, N = 96 | |
|
| |
| −0.5 | −1.50 (−5.62, 2.62) |
| 1 | −5.29 (−9.32, −1.26) |
| 2 | −4.19 (−8.25, −0.13) |
| 3 | −5.10 (−9.16, −1.05) |
| 4 | −4.70 (−8.75, −0.65) |
| 6 | −8.29 (−12.34, −4.24) |
| 8 | −9.86 (−13.91, −5.80) |
bpm beats per minute, CI confidence interval, ITT intent-to-treat
QT interval
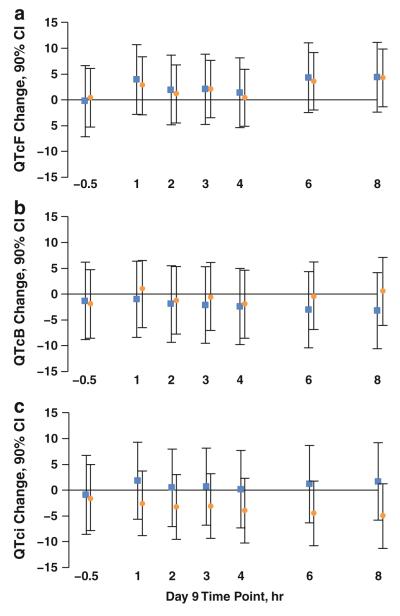
On Day 9, results from the mixed-effects model indicated that the maximum mean increase in the baseline-adjusted, time-matched QTcF for pazopanib-treated patients compared with placebo was 4.4 ms (90 % CI: −2.4, 11.2) at 8 h post-dose. (Fig. 2; Online Table S4).
Fig. 2.
Least-squares mean difference in QTc change from baseline for pazopanib-treated patients versus placebo. a Fridericia-corrected QT (QTcF); b Bazett-corrected QT (QTcB); c individualized correction (QTci). Data points and 90 % confidence intervals (CI) are shown in ms. Squares indicate the mean difference in QTc for the evaluable population (n = 65); circles indicate the mean difference in QTc for the intent-to-treat population (n = 96)
In light of the unexpected and variable effect of pazopanib on heart rate and the known dependence of QTc duration on RR interval, in addition to QTcF, QTci was calculated as an exploratory endpoint. The maximum mean increase in the baseline-adjusted, time-matched QTci for pazopanib-treated patients compared with placebo was 1.9 ms (90 % CI: −5.7, 9.4) at 1 h post-dose in the evaluable population; results were similar in the ITT population (Fig. 2; Online Table S4). Based on the magnitude of the regression coefficient of QTc versus RR interval in a mixed-effects model, QTci appeared to be least dependent on RR interval (Online Table S4).
Categorical changes in QTcF and QTcB occurred in a small number of patients in the ITT population. No pazopanib-treated patient had a QTcF or QTcB >480 ms, whereas 1 (2 %) and 3 (6 %) placebopaz-treated patients had QTcF and QTcB >480 ms, respectively. No patient had QTcF >500 ms at any post-dose time point in either treatment group, whereas 1 patient (2 %) had QTcB interval >500 ms in the placebopaz group. No patients in either treatment group had a maximum change from baseline in QTcF or QTcB >60 ms.
ECG abnormalities
The numbers of patients with, and frequency of, changes from baseline in certain morphological ECG waveform abnormalities were increased for pazopanib-treated patients relative to placebopaz in the ITT population and included the following abnormalities: sinus bradycardia, T wave changes (inversion, flat, and biphasic), ectopic supraventricular beats, first-degree AV block, and ST depression (Online Table S5). Because these changes were detected on Holter ECGs and not identified in real time, direct correlation with any clinical significance is not possible.
Effect of pazopanib dosing schedule on plasma concentrations
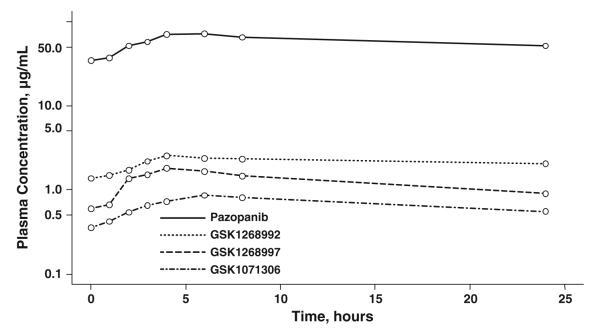
Figure 3 shows the median plasma concentration–time profiles of pazopanib and its metabolites GSK1268992, GSK1268997, and GSK1071306 in the ITT population (n = 46) after 800-mg pazopanib once daily (fasted) on Days 2 through 8 and 1,600-mg (fed) on Day 9. The geometric mean (95 % CI) pazopanib maximum concentration and steady-state concentration values on Day 9 were 78.1 (69.9, 87.4) and 49.2 (42.7, 56.6) μg/mL, respectively.
Fig. 3.
Median plasma concentration–time profiles of pazopanib and its 3 major metabolites (all n = 46) on Day 9, after 800-mg pazopanib once daily on Days 2 through 8 and after 1,600-mg pazopanib with food on Day 9
Relationship between pharmacokinetic and pharmacodynamic parameters
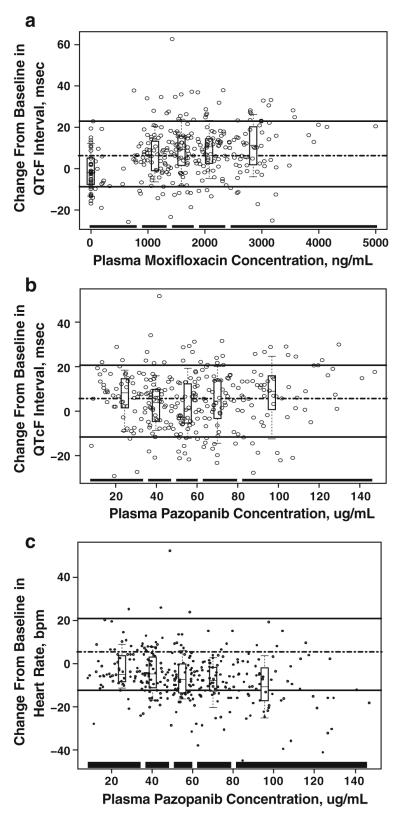
Linear mixed-effects modeling revealed a concentration-dependent relationship between plasma moxifloxacin concentrations and the placebo-corrected change from baseline in QTcF (Fig. 4a). No significant relationship between plasma pazopanib concentrations and the placebo-corrected change from baseline in QTcF was observed (Fig. 4b). A concentration-dependent relationship between plasma pazopanib concentrations and a decrease in time-matched, baseline-adjusted heart rate was observed (Fig. 4c).
Fig. 4.
Relationship between changes from baseline in Fridericia-corrected QT (QTcF) interval and heart rate and plasma concentrations of study drugs. a, b Boxes in each figure panel display the 90th percentile (upper whisker), the 75th percentile (upper border), median (middle line), 25th percentile (lower border), and the 10th percentile (lower whisker) of the placebo-corrected change from baseline in QTcF values within each plasma concentration range. The broken line in the center of the graph indicates the median change in QTcF. The upper solid line represents the 90th percentile QTcF change, and the lower broken line represents the 10th percentile QTcF change across patients. The thick solid lines at the bottom of the graph represent the plasma concentration range of each quintile. c Boxes display the 90th percentile (upper whisker), the 75th percentile (upper border), median (middle line), 25th percentile (lower border), and the 10th percentile (lower whisker) of the change from baseline in heart rate values within each plasma concentration range. The broken line in the center of the graph indicates the median change in heart rate. The upper solid line represents the 90th percentile heart rate change, and the lower broken line represents the 10th percentile heart rate change across patients. The thick solid lines at the bottom of the graph represent the plasma concentration range of each quintile
Safety results
More AEs were reported in the pazopanib group versus the placebopaz group. The most common (>10 %) AEs reported by pazopanib-treated patients included fatigue (27 %), nausea (25 %), decreased appetite (17 %), hypertension (15 %), and weight decrease (10 %) (Online Table S6). Most AEs were grade 1 or 2. No dose reductions due to AEs were reported during the study. In all, 3 patients (3 %) experienced serious AEs during the study: 2 patients while receiving placebopaz (pulmonary hemorrhage, 1 patient; back pain, 1 patient) and 1 patient while receiving pazopanib (upper gastrointestinal hemorrhage, cholangitis, elevated bilirubin/aminotransferase/alkaline phosphatase). No grade 4 or 5 serious AEs were reported during the study.
Two patients (2 %) died during the study; both deaths were attributed to underlying disease progression. A third death, not included in this study, involved a 54-year-old patient with metastatic pancreatic cancer who received moxifloxacin followed by placebopaz, subsequently entered the continued-access study (NCT00387205), and received 7 once-daily 800-mg pazopanib doses before experiencing a fatal, potentially drug-related arrhythmia. This patient’s medical records indicated a history of diabetes mellitus type 2, hypercholesterolemia, obesity, and prior history of smoking. A post-mortem examination of this patient identified a previously undocumented idiopathic dilated cardiomyopathy.
Discussion
In general, inhibitors of the VEGF pathway, including oral multitargeted tyrosine kinase inhibitors, have known cardiovascular effects such as hypertension and heart failure. Bello et al. [9] recently reported a dose/concentration-dependent effect of sunitinib on QTc interval in patients with advanced cancer; the mean maximum increase in QTcF was 9.6 ms at therapeutic concentrations and 15.4 ms at supratherapeutic concentrations (n = 48). Tolcher et al. [14] reported a concentration-independent effect of sorafenib on QT interval and heart rate in patients with cancer; the mean maximum increase in QTcB and QTcF was 16.4 and 19.8 ms, respectively (n = 53), while Houk et al. [15] reported that the effect of axitinib on QT interval was small (<10 ms) up to 3 h after a single dose in healthy volunteers (n = 32). As there are limited clinical data on the effect of pazopanib on QT prolongation, it was critical to provide a prospective and rigorous assessment of the potential of pazopanib to affect ECG parameters, including the QT interval.
The International Conference on Harmonisation (ICH) E14 guidelines [16] recommend a “thorough QT/QTc study” in a healthy volunteer population, whenever possible, to control for potential confounding factors (e.g., concomitant medications, comorbidities). However, a healthy volunteer population was not considered appropriate because of previous clinical experiences in elderly healthy volunteers in whom grade 1/2 elevations of aspartate aminotransferase and alanine aminotransferase were reported within a few days of initiating once-daily 100-mg pazopanib (data on file). The current study did, however, incorporate many key ICH E14 study design recommendations, including a randomized, double-blind, placebo- and positive-controlled, and parallel-group design. In addition, data from the moxifloxacin control show that plasma concentrations after 400-mg moxifloxacin were in the anticipated range [17], and the QTc interval was prolonged at several time points, validating the sensitivity of the study design to detect QTc prolongation in this population of patients with advanced solid tumors.
The administration of 800-mg pazopanib (fasted) on Days 2 through 8 and 1,600-mg (fed) on Day 9 achieved plasma concentrations that were approximately 1.3–1.4 times higher than those previously reported for the clinically used and recommended 800-mg once-daily therapeutic dose. Under these conditions, pazopanib caused a clinically significant increase in systolic and diastolic blood pressure at 8 h on Day 9, an effect anticipated for this class of agents [18]. There was an unexpected reduction in heart rate that was not associated with any reported AEs. While the Fridericia method tended to overcorrect (overestimate QTc effect), the Bazett method tended to undercorrect for this change in heart rate (underestimate QTc effect). Our analyses suggested that QTci was the more appropriate correction method demonstrating the least dependence on RR interval (i.e., heart rate).
Previous clinical studies have shown that the peak plasma concentration of pazopanib typically occurs 3–4 h after dosing. Thus, it was hypothesized that in the present study, the peak pharmacodynamic cardiac electrophysiological effects of pazopanib would be captured within the preplanned 8-h time window, and that assessments beyond 8 h would not be necessary. However, since the peak effects of QTcF on heart rate were observed at the 8-h time point, additional Holter data were extracted, and a post hoc analysis including time points at 12, 16, 20, and 24 h was performed. Based on this post hoc analysis, an additional nadir in heart rate was observed 16 h post-dose in both the evaluable population (−10.1 bpm, 90 % CI: −14.9, −5.4) and the ITT population (−13.3 bpm, 90 % CI: −17.2, −9.4) of pazopanib-treated patients versus placebopaz (Online Table S7). Although these changes are numerically larger, they are consistent with the results of the original 8-h analysis (Table 2). In addition, a second peak in the QTcF change from baseline versus placebo was observed at 24-h post-dose among both the evaluable and the ITT populations (Online Table S8), but the values were numerically smaller than the maximum increase in QTcF observed in the original 8-h analysis (Fig. 2; Online Table S4). While these additional data were prospectively collected under double-blind conditions and retrospectively analyzed by blinded ECG readers, several limitations of the post hoc analysis should be highlighted. The post hoc analysis does not include concurrent safety (e.g., vital signs) and pharmacokinetic assessments, and includes time points from 8 to 24 h that were not collected under similar controlled conditions as in the original planned analysis from 0 to 8 h (e.g., food consumption, physical activity, and sleep/wake cycles were not strictly controlled during the 8- to 24-h period).
Overall, these data are important because these changes occurred at pazopanib concentrations higher than those normally achieved in routine clinical dosing. Finally, although a significant proportion of patients (32 %) were deemed nonevaluable, it should be noted that the proportion of nonevaluable patients was nearly equally distributed across the 4 treatment sequences, and the results for the primary endpoint did not differ in any meaningful way based on either the evaluable (n = 65) or the ITT population (n = 96).
The short-term safety of repeated once-daily oral doses of 800-mg pazopanib was concordant with that observed in previous pazopanib monotherapy studies. Furthermore, the administration of a single oral dose of 1,600-mg pazopanib following repeated once-daily dosing with 800-mg pazopanib demonstrated no immediate safety issues and was successful in increasing acute exposure to pazopanib in the context of this controlled clinical trial. Only 1 patient discontinued the study due to an AE (ear hemorrhage) while receiving placebopaz.
In summary, the schedule of pazopanib administration studied over 8 days achieved supratherapeutic concentrations, produced a concentration-dependent decrease in heart rate, and caused a small, concentration-independent QTcF prolongation. As this study was conducted in patients with normal electrolytes who were not taking medications known to prolong QTc interval and who did not have pre-existing ECG interval abnormalities, it remains important to periodically monitor electrolytes and on-treatment ECGs for patients receiving pazopanib. In addition, pazopanib should be used with caution in patients with low heart rate at baseline (<60 bpm), a history of syncope or arrhythmia, sick sinus disease, or congestive heart failure.
Supplementary Material
Acknowledgments
We thank the patients who made this study possible. We also wish to acknowledge the Study Investigators: Lee Rosen, MD, Marilyn Mulay, NP, and Jonathan Goldman, MD (Premiere Oncology, Santa Monica, CA) and Lorrin Yee, MD (Northwest Medical Specialties, Tacoma, WA); and the Study Coordinators: Nancy Gelfand (Premiere Oncology, Santa Monica, CA), Lisa Johnson, RN (Institute of Translational Oncology Research, Greenville, SC), Karen Forman, CCRP (Barbara Ann Karmanos Cancer Institute, Wayne State University, Detroit, MI), Julie Skarsvog, RN (Northwest Medical Specialties, Tacoma, WA), Viktoria McMahan (Sarah Cannon Cancer Center, Nashville, TN), Ian Williams (The Norris Cotton Cancer Center and The Geisel School of Medicine at Dartmouth, Lebanon, NH), Abigail Guinto (City of Hope Comprehensive Cancer Center, Duarte, CA), and Shari Adams (The Cancer Institute of New Jersey, New Brunswick, NJ). This study was supported by GlaxoSmithKline Pharmaceuticals, Philadelphia, Pennsylvania. Medical editorial assistance was provided by Jerome F Sah, PhD, at ProEd Communications, Inc., Beachwood, Ohio, and was supported by GlaxoSmithKline.
Footnotes
Conflict of interest T. Luu has received research support from NCI/CTEP, Bayer/Onyx, Wyeth, Merck, Bristol-Myers Squibb, GlaxoSmithKline, and Pfizer, and has been a consultant/advisor to Novartis and Genomic Health. A. Tan has received research funding from GlaxoSmithKline. Authors Kleha, Ma, Suttle, Ball, and Dar are employees of GlaxoSmithKline. All other authors report no potential conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s00280-012-2030-8) contains supplementary material, which is available to authorized users.
Contributor Information
Elisabeth I. Heath, Division of Hematology/Oncology, Barbara Ann Karmanos Cancer Institute, Wayne State University, 4100 John R, Detroit, MI 48201, USA
Jeffrey Infante, Drug Development Unit, Sarah Cannon Research Institute, Nashville, TN, USA.
Lionel D. Lewis, Department of Clinical Pharmacology, The Norris Cotton Cancer Center, Lebanon, NH, USA; Geisel School of Medicine at Dartmouth, Lebanon, NH, USA
Thehang Luu, Medical Oncology, City of Hope National Medical Center, Duarte, CA, USA.
Joe Stephenson, Institute for Translational Oncology Research, Greenville Hospital University Medical Center, Greenville, SC, USA.
Antoinette R. Tan, Medical Oncology, The Cancer Institute of New Jersey, New Brunswick, NJ, USA
Saifuddin Kasubhai, Department of Hematology/Oncology, Northwest Medical Specialists, Tacoma, WA, USA.
Patricia LoRusso, Division of Hematology/Oncology, Barbara Ann Karmanos Cancer Institute, Wayne State University, 4100 John R, Detroit, MI 48201, USA.
Bo Ma, GlaxoSmithKline, Research Triangle Park, NC, USA.
A. Benjamin Suttle, GlaxoSmithKline, Research Triangle Park, NC, USA.
Joseph F. Kleha, GlaxoSmithKline, Research Triangle Park, NC, USA
Howard A. Ball, GlaxoSmithKline, Upper Providence, PA, USA
Mohammed M. Dar, GlaxoSmithKline, Research Triangle Park, NC, USA
References
- 1.Hutson TE, Davis ID, Machiels JP, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28:475–480. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 2.Sonpavde G, Hutson TE, Sternberg CN. Pazopanib, a potent orally administered small-molecule multitargeted tyrosine kinase inhibitor for renal cell carcinoma. Expert Opin Investig Drugs. 2008;17:253–261. doi: 10.1517/13543784.17.2.253. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 4.Australian Government Department of Health and Ageing. Drugs designated as orphan drugs; Pazopanib (PATORMA): [Accessed 23 July 2012]. 2010. http://www.tga.gov.au/industry/pm-orphan-drugs.htm. [Google Scholar]
- 5.Instituto de Salud Publica de Chile [Accessed 23 July 2012];Votrient coated tablets [product registration] 2010 http://200.68.11.21/RegistrosISP/fiFichaProducto.asp?RegistroISP=F-18018/10.
- 6.Kidney Cancer Canada [Accessed 23 July 2012];Votrient (pazopanib) approved by Health Canada for the treatment of renal cell carcinoma. 2010 http://www.kidneycancercanada.org/main.php?p=600&lan=1.
- 7.U.S. Department of Health and Human Services Food & Drug Administration [Accessed 23 July 2012];Pazopanib [approval notice] 2009 http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm187509.htm.
- 8.European Medicines Agency [Accessed 23 July 2012];Votrient (pazopanib) authorisation details. 2010 http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001141/human_med_001337.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124&jsenabled=true.
- 9.Bello CL, Mulay M, Huang X, et al. Electrocardiographic characterization of the QTc interval in patients with advanced solid tumors: pharmacokinetic–pharmacodynamic evaluation of sunitinib. Clin Cancer Res. 2009;15:7045–7052. doi: 10.1158/1078-0432.CCR-09-1521. [DOI] [PubMed] [Google Scholar]
- 10.Desai M, Li L, Desta Z, Malik M, Flockhart D. Variability of heart rate correction methods for the QT interval. Br J Clin Pharmacol. 2003;55:511–517. doi: 10.1046/j.1365-2125.2003.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:411–420. doi: 10.1046/j.1540-8167.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 12.Heath EI, Chiorean EG, Sweeney CJ, et al. A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88:818–823. doi: 10.1038/clpt.2010.199. [DOI] [PubMed] [Google Scholar]
- 13.Goh BC, Reddy NJ, Dandamudi UB, et al. An evaluation of the drug interaction potential of pazopanib, an oral vascular endothelial growth factor receptor tyrosine kinase inhibitor, using a modified Cooperstown 5 + 1 cocktail in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88:652–659. doi: 10.1038/clpt.2010.158. [DOI] [PubMed] [Google Scholar]
- 14.Tolcher AW, Appleman LJ, Shapiro GI, Mita AC, Cihon F, Mazzu A, Sundaresan PR. A phase I open-label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;67:751–764. doi: 10.1007/s00280-010-1372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houk BE, Sarapa N, Pithavala YK. Effect of axitinib (AG-013736) concentration on QT interval after administration alone and in combination with ketoconazole in healthy volunteers (abstract PII-36). Presented at the 109th annual meeting of the American society for clinical pharmacology and therapeutics.2008. [Google Scholar]
- 16.U. S. Food and Drug Administration [updated 05/26/2009]; [Accessed 23 July 2012];E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005 Oct; http://www.fda.gov/RegulatoryInformation/Guidances/ucm129335.htm.
- 17.Yan LK, Zhang J, Ng MJ, Dang Q. Statistical characteristics of moxifloxacin-induced QTc effect. J Biopharm Stat. 2010;20:497–507. doi: 10.1080/10543400903581945. [DOI] [PubMed] [Google Scholar]
- 18.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.