Abstract
Multiple myeloma (MM) is a lethal malignancy with an unknown etiology and no prevention strategy. Aspirin inhibits several pathways mediated by nuclear factor (NF)-κB, cyclooxygenase (COX)-2, or their targets that are important in MM pathogenesis. We conducted prospective analyses in the Health Professionals Follow-up Study and Nurses’ Health Study cohorts to examine whether regular aspirin use influences MM risk. We used biennially updated data to characterize aspirin use from baseline through a cancer diagnosis, death, or 2008. We applied a four-year lag in exposure classification to diminish the influence of preclinical MM on aspirin use habits. We obtained hazard ratios (HR) and 95% confidence intervals (CI) from multivariable proportional hazard models to assess the association of aspirin use with MM risk. We tested for trend across increasing quantity and duration of use. During 2,395,458 person-years, we confirmed 328 incident MM diagnoses, including 265 with prospective information on typical aspirin dose and frequency. Participants with a cumulative average of ≥5 adult strength (325-mg) tablets/week had a 39% lower MM risk than non-users (HR, 95% CI: 0.61, 0.39–0.94; tablets/week, P-trend=0.06). Persons with ≥11 years of continuous regular aspirin use also had a lower MM risk (HR, 95% CI: 0.63, 0.41–0.95; duration, P-trend=0.17). The associations appeared stronger in men than in women, possibly reflecting gender differences in aspirin use patterns. This prospective study of aspirin use and MM supports an etiologic role for aspirin-inhibited (i.e., NF-κB- or COX-2-mediated) pathways. The utility of aspirin for MM chemoprevention warrants further evaluation.
Keywords: multiple myeloma, aspirin, epidemiology, prospective, risk factors
Introduction
Multiple myeloma (MM) is a B cell neoplasm that is expected to account for 22,350 new cancer diagnoses and 10,710 cancer deaths in the United States (US) in 2013 (1). A premalignant condition called monoclonal gammopathy of undetermined significance (MGUS) precedes the development of all diagnoses of MM (2,3), but little is known regarding the etiology of MGUS or MM, or about predictors of progression to malignancy in MGUS patients (4). MM incidence rises sharply in older adulthood and is higher in men, African-Americans, persons with a family history of hematologic malignancy, farmers, people with pesticide and solvent exposure, and persons with a higher body mass index (BMI) (5,6). While MM survival has improved recently with the development of more effective therapies (7), 5-year relative survival is still lower than 45% for patients diagnosed between 2003 and 2009 (5), and current knowledge of MM etiology remains inadequate to develop prevention strategies.
In contrast, advances in knowledge of MM pathogenesis have identified numerous signaling pathways with important roles, several of which are inhibited by aspirin. Of particular interest, aspirin suppresses nuclear factor(NF)- κB, a family of transcription factors that mediate normal B cell development and are up-regulated in MM cell lines (8,9). Aspirin can also inhibit cyclooxygenase (COX)-2, which metabolizes arachadonic acid to numerous pro-inflammatory and potentially tumorigenic molecules (10). COX-2 is highly expressed in MM cells, predicts poor outcome in MM patients (11), and is a molecular target of NF- κB. Aspirin may also suppress other targets of NF-κB or COX-2 involved in MM pathogenesis, including interleukin (IL)-6, a pleiotropic pro-inflammatory cytokine and an important growth factor for MM (12), and cyclin D1, which influences normal and malignant cell proliferation and is dysregulated in MM (13,14).
Regular aspirin users may have a reduced risk of Hodgkin lymphoma (HL) (15,16), non-Hodgkin lymphoma (NHL) (17,18), and several solid tumors (19), as well as a reduced risk of mortality from hematologic malignancies with five or more years of regular use (20). An etiologic association of aspirin use with MM is also plausible and has been examined to date in four studies: one hospital-based (117 cases, 483 matched controls) (21) and one population-based case-control study (179 cases, 691 frequency-matched controls) (22), and two prospective studies: the Vitamins and Lifestyle (VITAL) cohort (6–8 years follow-up, 66 cases of plasma cell disorders, cohort N=64,839) (23) and the American Cancer Society (ACS) Cancer Prevention Study (CPS)-II Nutrition cohort (15 years follow-up, 310 cases, cohort N=184,188) (24). Neither case-control study reported an association of aspirin use with MM; duration of regular use was not associated with MM risk in the hospital-based study (21) and was not reported in the population-based study (22). The VITAL study reported a significant inverse association of regular use of 81 mg aspirin with risk of plasma cell disorders (P-trend=0.02) but no association of plasma cell disorder risk with use of regular strength aspirin (23). In the CPS-II study, neither quantity nor duration of aspirin use was associated with MM (24). We undertook the present prospective study in the Nurses’ Health Study and Health Professionals Follow-up Study cohorts to further examine the association of regular aspirin use with risk of MM, taking advantage of biennially updated, detailed information on aspirin use and relevant covariables.
Methods
Study Population
The Health Professionals Follow-up Study began in 1986 when 51,529 male US health professionals ages 40 to 75 years completed the enrollment questionnaire. The Nurses’ Health Study began in 1976 when 121,701 female US registered nurses ages 30 to 55 years returned the initial questionnaire (25). The design and methods of the two cohorts are similar. Biennial follow-up questionnaires update cohort members’ information on lifestyle and disease history. The study was approved by the Human Subjects Research Committees at the Harvard School of Public Health and Brigham and Women’s Hospital and was performed in accord with an assurance filed with and approved by the U.S. Department of Health and Human Services. Informed consent was implied by participants’ completion and return of the enrollment questionnaire.
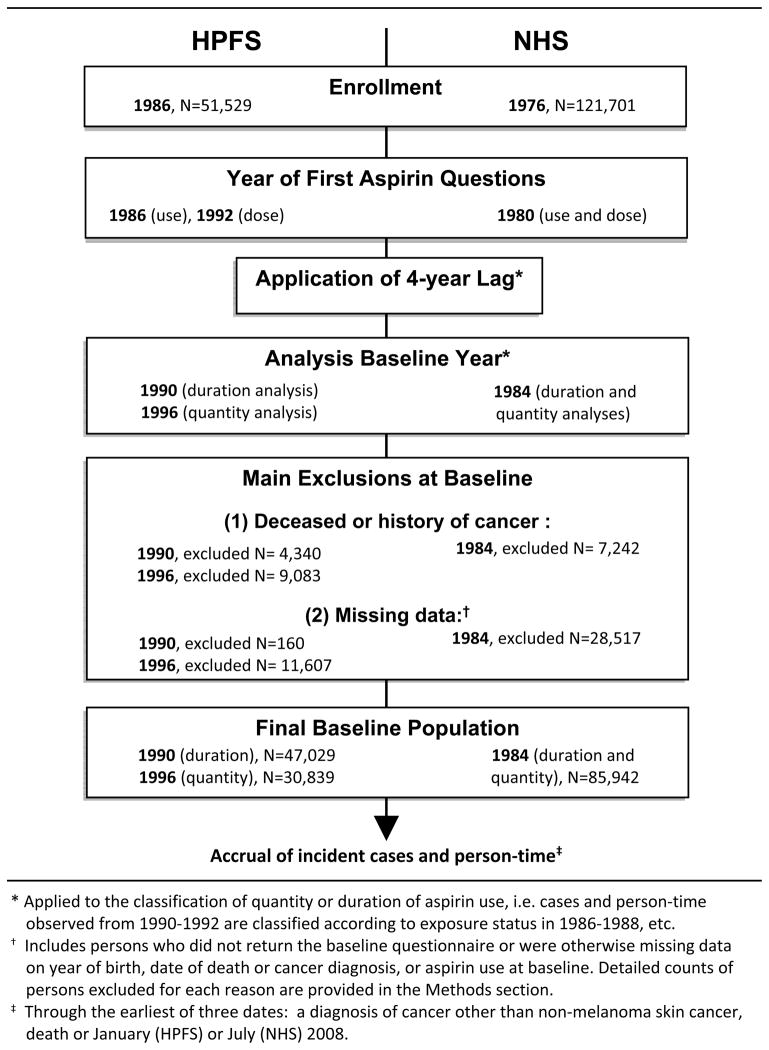
Baseline populations were defined according to the year of first query of the relevant aspirin use details (Figure 1). To diminish the potential influence of preclinical MM on aspirin use habits, we applied a four-year (two follow-up cycle) lag when classifying aspirin use. For example, person-time and cases occurring from 1990–1992 were classified according to aspirin use reported in 1986, those observed from 1992–1994 were classified by aspirin use status as of 1988, etc. We excluded participants who were deceased, had a history of cancer other than non-melanoma skin cancer, or had missing exposure data at baseline (Figure 1). Specifically, for the analysis of duration of aspirin use in HPFS (baseline year=1990 as explained below), we excluded 3,265 men with a baseline history of cancer, 1,075 who were deceased, 149 men with a missing year of birth and 11 with an unknown date of death. For the analysis of quantity of aspirin use in HPFS (baseline=1996), we excluded 4,984 men with a history of cancer, 4,099 who were deceased, 6,632 with missing baseline questionnaires and 4,815 with missing data on aspirin quantity in addition to the aforementioned 160 men with missing dates of birth or death. For both types of analysis in NHS (baseline year=1984), we excluded 5,369 women with a baseline history of cancer, 1,873 who were deceased, 21,787 with missing baseline questionnaires, 6,606 with missing data on aspirin use and 124 with missing year of birth. Thus, the baseline population comprised 30,839 and 47,029 men for analyses of quantity and duration of regular aspirin use, respectively, and 85,942 women for both analyses.
Figure 1.
Definition of the baseline populations for studies of quantity and duration of regular aspirin use among Health Professionals Follow-up Study (HPFS) and Nurses’ Health Study (NHS) cohort members.
Assessment of Regular Aspirin Use
The timing and nature of the aspirin use questions have been described previously in detail (26,27), and the questionnaires are publicly available (28,29). Men were asked about regular aspirin use from 1986 onward but were not queried about dose (81-mg “baby” v. 325-mg “adult” strength) or frequency until 1992. Women were asked detailed aspirin use questions from 1980 onward, except in 1986. In 1994, 1996, and 1998, participants in both cohorts converted baby aspirin intake to adult strength equivalents (4 baby tablets=1 adult strength tablet). To identify the primary indications for aspirin use by cohort members, we conducted surveys among randomly selected self-reported regular aspirin users (Table 1) (27,30).
Table 1.
Primary indications for regular aspirin use among randomly selected subsets of self-reported aspirin users in the HPFS and NHS cohorts.
| Cohort | HPFSa | NHSb | |
|---|---|---|---|
|
| |||
| N surveyed | Quantity not specified | 1–6 tablets/week | 7+ tablets/week |
|
| |||
| 211 | 100 | 100 | |
| Aspirin use indication (%)c | |||
| Cardiovascular disease | |||
|
| |||
| Treatment/management | 25 | NR | NR |
|
| |||
| Prevention | 58 | 9 | 8 |
|
| |||
| Musculoskeletal paind | 33 | 30 | 50 |
|
| |||
| Headache | 25 | 32 | 18 |
|
| |||
| Headache and musculoskeletal pain | NR | 16 | 15 |
|
| |||
| Other | 7 | 13 | 9 |
The HPFS survey was conducted among randomly selected men who reported regular use of aspirin on surveys from 1986–1990 and was first reported in: Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med 1994;121(4):241–246 [reference 27].
The NHS survey was conducted among randomly selected women who reported the indicated level of regular aspirin use on surveys from 1980–1984 and was first reported in: Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Speizer FE, et al. A prospective study of aspirin use and primary prevention of cardiovascular disease in women. JAMA 1991;266(4):521–527 [reference 30].
Men were allowed to report more than one indication; women selected only one.
The musculoskeletal pain category included arthritis on the NHS survey.
Abbreviations: HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; NR, not reported
Covariables
Adult height and current weight were self-reported at enrollment in both cohorts; current weight was updated biennially. Validation studies in these cohorts showed high correlations of the self-reported data with measured height and weight (31). We computed current BMI (current weight divided by adult height squared, or kg/m2) and age at the start of each follow-up interval. We characterized cohort members as regular users of acetaminophen (no/yes/missing) and ibuprofen (no/yes/missing) if they reported use at least twice per week. In the HPFS, separate questions on the use of these other analgesics were included in the enrollment questionnaire and biennially thereafter. In the NHS, a question on the regular use of other non-steroidal analgesics was asked in 1980; the separate classification of acetaminophen and ibuprofen use was possible from 1990 onward.
Identification of Cases
We identified most new diagnoses of cancer through the biennial questionnaires. Deaths were identified using the National Death Index, which is highly sensitive and specific in these cohorts (32,33). For each potential case of MM, we sought permission to obtain the medical records, which we reviewed to confirm the diagnosis according to criteria specified by the International Myeloma Working Group (34) and the diagnosis date. The present analysis included all confirmed incident diagnoses of MM that occurred during follow-up through the mailing date of the 2008 follow-up questionnaires (January for men, June for women).
Statistical Analysis
Accrual of person-time began in 1996 (aspirin quantity analysis) or 1990 (duration of use analysis) for men and in 1984 for women (both analyses) due to the four-year lag (Figure 1). We followed participants through the earliest of three dates: diagnosis of cancer other than non-melanoma skin cancer, death, or the aforementioned 2008 cut-off dates. We computed participants’ cumulative average weekly 325-mg aspirin intake (“quantity”) and years (“duration”) of continuous regular aspirin use as previously described (35,36). Briefly, we determined the number of adult strength tablets taken weekly at baseline and computed an updated cumulative average at the start of each follow-up interval. We also summed the consecutive years in which a participant reported regular aspirin use. If aspirin use information was missing on a given questionnaire, the data from the previous follow-up interval were carried forward for one interval and the exposure variables set to missing thereafter.
We computed hazard ratios (HR) and 95% confidence intervals (CI) using Cox proportional hazard regression to estimate the relative risk of MM associated with regular aspirin use quantity (non-use, <2, 2– <5, ≥5 tablets/week) and duration (non-use, ≤5, 6–10, ≥11 years). We analyzed pooled data from both cohorts and conducted gender- (cohort-) specific analyses. All statistical models were stratified by age (months) and adjusted for BMI (kg/m2) to control for potential confounding. Analyses performed in the combined data set also adjusted for gender (cohort). To obtain a P-value for trend, we entered ordinal variables created from the medians of the quantity and duration variable categories into additional multivariable Cox models.
To assess potential confounding by concurrent use of other analgesics, we performed additional analyses in which the age-stratified, BMI-adjusted Cox models were further adjusted for regular use of acetaminophen and/or ibuprofen. In women, those analyses were restricted to the follow-up intervals in which acetaminophen and ibuprofen use could be classified separately, i.e., 1994–2008 after incorporation of the exposure lag, and the findings were compared to results from age-stratified, BMI-adjusted models run within the same restricted follow-up interval (i.e., without control for other analgesic use). To examine effect modification by BMI (<25 v. ≥25 kg/m2), we conducted stratified analyses of the aspirin use variables and MM risk in the combined population. We also calculated cohort-specific (i.e., sex-specific) age-adjusted standardized incidence rates (SIR) of MM and corresponding 95% CIs (37–39) to compare the incidence of MM in the analyzed HPFS and NHS population samples with those reported in the Surveillance, Epidemiology and End Results (SEER) program. Because the HPFS and NHS populations are predominantly Caucasian we used SEER age-incidence rates for Whites as the standard (5). In HPFS, we based the SIR calculations on the 30,839 men in the aspirin quantity analysis (1996 baseline). All P-values were two-tailed.
Results
At baseline for the aspirin quantity analysis, regular aspirin use was generally more common in men, although regular use of five or more 325-mg tablets per week was reported more frequently in women than in men (Table 2). Mean age and BMI did not vary notably by baseline aspirin use status among men or women. In men, the use of other analgesics was rare at baseline; fewer than 5% (1,468) reported use of acetaminophen at least twice per week, and fewer than 10% (2,905) reported regular use of ibuprofen. In women in 1994—the earliest follow-up cycle for which the two other analgesics could be classified separately after incorporating the exposure lag—the prevalence of regular use of acetaminophen (42.7%) and ibuprofen (36.5%) was lower than that of aspirin (46.5%); use of both aspirin and another analgesic was infrequent (aspirin and acetaminophen, 15%; aspirin and ibuprofen, 14%).
Table 2.
Selected characteristics at baselinea in men and women by regular quantity of aspirin use.
| Adult strength tablets/weekb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Men (HPFS; N=30,839)
|
Women (NHS; N=85,942)
|
|||||||
| Non-user | <2 | 2–<5 | ≥5 | Non-user | <2 | 2–<5 | ≥5 | |
| N (%) | 10,111 (32.8) | 13,594 (44.1) | 5,049 (16.4) | 2,085 (6.8) | 49,402 (57.5) | 12,334 (14.4) | 8,711 (10.1) | 15,495 (18.0) |
|
| ||||||||
| Mean age, years | 62.1 | 62.4 | 65.0 | 62.9 | 50.6 | 50.5 | 50.3 | 51.8 |
|
| ||||||||
| Mean BMI, kg/m2 c | 25.1 | 25.5 | 25.7 | 25.7 | 24.9 | 24.9 | 25.1 | 25.5 |
Due to the use of a four-year lag, baseline for the analysis of cumulative average tablets/week of aspirin use is defined as 1996 in men and 1984 in women.
Calculated by converting reported usual dose and weekly quantity of tablets to 325-mg equivalents per week.
Standardized to the baseline age distribution of the specified cohort.
Abbreviations: HPFS, Health Professionals Follow-up Study; MM, multiple myeloma; NHS, Nurses’ Health Study; BMI, body mass index
We observed 328 incident diagnoses of MM (132 male, 196 female), during 2,395,458 person-years of observation (Table 3). Of those, 265 cases (72 male, 193 female) that occurred over 1,997,506 person-years could be classified with regard to regular aspirin quantity. The sex-specific age-incidence rates for MM in the analyzed HPFS and NHS populations were not significantly different from expected; the SIR (95% CI) was 0.90 (0.71–1.14) in men in the aspirin quantity analysis (i.e., the smallest population we analyzed from HPFS) and 1.00 (0.87–1.16) in women when compared to sex-specific SEER incidence rates for Whites (5).
Table 3.
Hazard ratios and 95% confidence intervals for risk of multiple myeloma according to quantity and duration of regular aspirin use in men and women.
| Aspirin use | Men (HPFS) | Women (NHS) | Combined | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cases | Person-Years | HR (95% CI)a | Cases | Person-Years | HR (95% CI)a | HR (95% CI)b | |
| Cumulative average 325-mg tablets per week | |||||||
| N | 72 | 319,474 | 193 | 1,678,032 | |||
|
| |||||||
| Non-users | 19 | 73,701 | 1.00 (ref) | 30 | 369,337 | 1.00 (ref) | 1.00 (ref) |
|
| |||||||
| <2 | 29 | 133,637 | 0.79 (0.44–1.42) | 75 | 609,143 | 0.99 (0.63–1.54) | 0.92 (0.66–1.30) |
|
| |||||||
| 2–<5 | 21 | 77,289 | 0.88 (0.47–1.65) | 56 | 351,847 | 1.13 (0.71–1.79) | 1.04 (0.72–1.50) |
|
| |||||||
| ≥5 | 3 | 34,847 | 0.28 (0.08–0.95) | 32 | 347,705 | 0.71 (0.43–1.18) | 0.61 (0.39–0.94) |
|
| |||||||
| P-trend=0.11c | P-trend=0.22c | P-trend=0.06c | |||||
| Continuous duration of regular use, years | |||||||
| N | 132 | 664,264 | 196 | 1,731,194 | |||
|
| |||||||
| Non-users | 52 | 256,203 | 1.00 (ref) | 30 | 369,337 | 1.00 (ref) | 1.00 (ref) |
|
| |||||||
| ≤5 | 55 | 269,879 | 0.77 (0.53–1.14) | 94 | 867,731 | 0.92 (0.60–1.39) | 0.80 (0.61–1.06) |
|
| |||||||
| 6–10 | 19 | 99,134 | 0.65 (0.38–1.10) | 44 | 293,653 | 1.32 (0.83–2.11) | 0.97 (0.69–1.35) |
|
| |||||||
| ≥11 | 6 | 39,047 | 0.42 (0.18–0.98) | 28 | 200,472 | 0.81 (0.48–1.37) | 0.63 (0.41–0.95) |
|
| |||||||
| P-trend=0.02c | P-trend=0.96c | P-trend=0.17c | |||||
HRs and 95% CIs were computed in Cox proportional hazard models that stratified on age in months and adjusted for current BMI in kg/m2.
Cohort-combined Cox proportional hazard models were stratified on age in months and controlled for BMI (kg/m2) and cohort (gender).
For the trend tests, cumulative average tablets/week or continuous duration of regular aspirin use was modeled as an ordinal variable using the mid-point of each category of the respective variable in Cox proportional hazard models stratified by age in months and adjusted for the co-variables indicated.
Abbreviations: HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; HR, hazard ratio; CI, confidence interval; ref, reference category
In analyses performed in the combined HPFS and NHS population, we observed a 39% lower MM risk in persons who used an average of five or more adult-strength tablets per week compared to individuals who did not use aspirin regularly (HR, 95% CI: 0.61, 0.39–0.94; P-trend=0.06). Weekly use of fewer than five tablets did not appear to predict MM risk (Table 3). An inverse association was also suggested for quantity of aspirin use among men. Compared to male non-users, MM risk was 72% lower in men who used a cumulative average of five or more tablets per week, based on three exposed cases (HR, 95% CI: 0.28, 0.08–0.95; P-trend=0.11). A significant association of regular aspirin quantity with MM was not apparent in women, although those who reported regular use of five or more tablets per week had a non-significant 29% reduction in MM risk (Table 3). An inverse association with MM was apparent for duration of continuous regular aspirin use, but the P-trend was statistically significant only among men (Table 3); the suggested benefit in men was most notable in those with eleven or more continuous years of regular aspirin use (HR, 95% CI: 0.42, 0.18–0.98; P-trend=0.02).
Adjustment for regular use of acetaminophen and ibuprofen (individually or simultaneously) did not change the BMI-adjusted associations of aspirin use with MM risk in men or women. In men who used a cumulative average of fewer than two, two to fewer than five, or five or more adult strength aspirin tablets per week, compared to non-users, with simultaneous adjustment for BMI, acetaminophen and ibuprofen use, the HRs (95% CIs) for MM risk were 0.79 (0.44–1.42), 0.87 (0.46–1.64) and 0.27 (0.08–0.94), respectively (P-trend=0.10). In those multivariable models, the corresponding HRs (95% CIs) for MM risk with regular use of acetaminophen and ibuprofen compared to non-use were 1.12 (0.48–2.63) and 1.03 (0.53–1.99), respectively, based on six and 11 exposed cases, respectively. In women, the BMI-adjusted, other analgesic use-unadjusted findings for cumulative average tablets/week of aspirin and MM risk were very similar when examined in the restricted follow-up interval to those reported in Table 3 for the complete follow-up period. In the interval from 1994–2008, the HRs (95% CI) in women who reported a cumulative average of fewer than two, two to fewer than five or five or more tablets per week of aspirin compared to non-users were 1.11 (0.63–1.95), 1.22 (0.68–2.20) and 0.71 (0.36–1.39), respectively (P-trend=0.24), with stratification by age and control for BMI. With further adjustment for acetaminophen and ibuprofen use, the findings were virtually unchanged (v. non-use, HR, 95% CI for <2 tablets/week=1.09, 0.62–1.92; 2– <5 tablets/week=1.19, 0.66–2.14; ≥5 tablets/week=0.69, 0.35–1.35; P-trend=0.20). In those mutually adjusted models, the HR (95% CI) for regular use of acetaminophen and ibuprofen and MM risk compared to non-use were 0.68 (0.45–1.03) and 1.33 (0.92–1.92), respectively, based on 35 and 47 exposed cases, respectively. We had insufficient statistical power to examine their associations with MM in greater detail.
We evaluated whether the association of regular aspirin use with MM varied by category of BMI (<25 kg/m2, ≥25 kg/m2) and did not observe evidence of heterogeneity (data not shown). The findings in the BMI-defined strata were similar to those observed in the main models (data not shown).
Discussion
We report results from our large (for MM) prospective study on the association of regular aspirin use with MM risk. Our findings suggest that individuals who regularly take five or more adult-strength aspirin tablets per week have a nearly 40% lower MM risk than persons who do not use aspirin regularly, and that this association is independent of use of other analgesics. Individuals with longer continuous regular aspirin use may also have a lower MM risk, although the duration findings were only statistically significant among men.
Two previous case-control studies examined aspirin use and MM risk with generally null findings. Neither frequency nor duration of regular aspirin use was associated with MM in the hospital-based study (117 MM patients, 483 controls) (21). Participants reported their aspirin use frequency and duration prior to the onset of their illness, but the influence of preclinical MM on aspirin use by cases could not be evaluated (21). In a population-based case-control study of women in Connecticut (179 MM cases, 691 controls), regular aspirin use at least one year prior to diagnosis or interview was not associated with MM risk; duration of aspirin use was not evaluated (22). The null findings in the Connecticut study may be explained in part by the relatively stringent definition of regular aspirin use: daily use for six months or more during the reference period. Only 9 MM patients and 35 controls reported regular use by that definition (22). In contrast to the published case-control studies, the present analysis utilized biennially updated information on aspirin use that was prospectively acquired during up to 24 years of follow-up from health professionals likely to have accurate recall of their analgesic use patterns in any given 2-year period. The utilization of a four-year lag diminished the influence of preclinical MM on aspirin use by cases, although the true latency of MM is unknown and may be longer than four years; we could not consider longer lag intervals well due to the loss of statistical power from the exclusion of more cases from the analysis. By computing an updated cumulative average aspirin tablets per week we reduced the influence of misclassification of the tablets per week reported in a given follow-up interval (40).
Our report of an inverse association of quantity and duration of regular aspirin use with MM is consistent with reported associations of regular aspirin use with risk of several solid tumors (19,20) as well as of HL (15,16) and NHL(17,18). The present findings are also consistent with those from a recent pooled analysis of data from seven randomized clinical trials (20), and with those from the VITAL cohort (23). In the meta-analysis of clinical trial participants followed for at least five years post-randomization, which included 50 hematologic cancer deaths, persons allocated to aspirin use had a non-significant 66% reduction in mortality from hematologic cancers compared to those allocated to placebo arms (20). In the VITAL cohort, regular use of baby aspirin during the ten years prior to study enrollment was inversely associated with risk of plasma cell disorders (P-trend=0.02); “high” users of baby aspirin (i.e., ≥4 days/week for ≥4 years) had a marginally significant 60% lower risk of plasma cell disorders compared to non-users (23). In contrast, Teras and colleagues (24) did not observe an association of regular aspirin quantity or duration with MM risk in the CPS-II cohort in an analysis of comparable sample size to the present study. Differences in the computation of CPS-II participants’ usual aspirin quantity from self-reported baby v. adult strength tablets, and in the choice of exposure lag, may partially explain the discrepant findings. For example, the CPS-II analysis treated tablets per week of baby and adult strength aspirin as equivalent when computing usual pills per month, whereas in the HPFS and NHS, study participants converted baby aspirin use to adult-strength equivalents as previously noted. In addition, the CPS-II analysis incorporated no exposure lag or a lag of only two years in contrast to the four-year lag utilized in the present analysis.
Our findings and the aforementioned published reports of a reduced risk of MM among regular aspirin users are biologically plausible and may be explained in part by suppression of NF-κB and several of its molecular targets such as COX-2, IL-6, and cyclin D1 by aspirin (14,41,42). These molecules regulate normal immune responses and B cell development but are aberrantly expressed or activated in MM (8–11, 43). Our findings are consistent with an etiologic role for one or more of these biologic pathways with known importance to MM pathogenesis, although a role for any candidate molecule or pathway must be further explored with prospective biomarker-based studies. In one such study, we pooled individual level data across eight large prospective cohorts (493 cases of MM, 978 matched controls with pre-diagnosis blood samples) and observed that the peripheral blood concentration of the soluble IL-6 receptor was positively associated with risk of a subsequent diagnosis of MM within six years of blood collection (44). Those findings suggest that inflammation or IL-6 perturbations have an etiologic role in MM and support the biologic plausibility of the aspirin-MM associations we report in the present study.
We noted somewhat stronger associations of aspirin use with MM in men compared to women, although the associations were generally inverse across both genders. The incidence rates for MM in our cohort samples were similar to expected based on SEER age-incidence rates, which suggests that unusual patterns of incidence do not explain the gender discrepancies. Rather, the apparent gender differences may be due to chance or may reflect true differences in aspirin use patterns or the physiologic effects of regular aspirin use. The surveys obtained from randomly selected regular aspirin users in our cohorts indicated gender differences in the primary reasons for aspirin use: cardiovascular disease prevention was cited considerably more frequently in men than women (nearly 60% v. <10%, respectively), whereas headache, arthritis and musculoskeletal pain comprised the primary indications in women (approximately 80% in women v. 25% in men)(27,30). Thus, the typical patterns of aspirin use may also differ by gender in our cohort populations. For example, daily use of baby aspirin may be more common among men, whereas women may achieve a similar weekly dose by taking adult strength aspirin more sporadically. These gender-specific patterns of use may result in different physiologic effects on COX-2, NF-κB or other relevant pathways. Of interest, in the VITAL study risk of plasma cell disorders was associated with baby aspirin but not with regular strength aspirin use (23). We could not directly evaluate MM risk associated with regular use of baby versus adult strength aspirin because baby aspirin use was reported in adult strength equivalents for most of the follow-up period covered in the present analysis.
The inability to explore gender-specific aspirin use patterns or control for primary indication are important limitations of the present analysis. Other limitations include an inability to jointly examine the use of acetaminophen (21) and other non-steroidal anti-inflammatory drugs (45) due to inadequate statistical power. The secondary analyses in which we evaluated potential confounding by acetaminophen and ibuprofen use suggested that the aspirin-MM associations we observed are not strongly confounded by concurrent use of those other medications; however, we were not able to examine use of the other analgesics in detail and cannot rule out the possibility that they have independent associations with MM or may interact with aspirin to influence MM risk. Lastly, we had inadequate statistical power to evaluate exposure lags of more than four years, to separately examine the higher quantities of regular aspirin use (i.e., as high as >14 tablets/week) that predicted a reduced risk of colorectal cancer in the same cohorts (35,46), or to jointly examine quantity and duration of regular aspirin use. The considerable strengths of our study have already been noted in detail and include the prospective design, biennially updated aspirin use data, long follow-up, lagged exposure classification, and accurate reporting of medication use by the health professionals in our cohorts. Because the cohort members are predominantly Caucasian (approximately 95%), residual confounding by race is unlikely.
The aforementioned limitations call for caution in the interpretation of our findings. With no available cure for MM, it is encouraging to consider that persons who regularly use aspirin for other purposes may also have a lower risk of MM. However, confirmation of our findings in other large populations is necessary, as is further exploration of the apparent gender differences we observed. As has been noted in other studies of regular aspirin use and cancer risk, the aspirin dose and frequency that would provide the most favorable risk/benefit ratio is not known (19,41), and neither is the biologic mechanism by which aspirin use may influence MM risk. A clinical trial in patients with MGUS or smoldering MM would be informative to evaluate the influence of regular aspirin use on risk of malignant progression, and whether any confirmed benefit of regular aspirin use varies by progression risk as characterized recently by Kyle and colleagues (4). In conclusion, the prospective evidence from the present study supports the further exploration of aspirin use, and of the biologic pathways affected by regular aspirin use, in the etiology and prevention of MM. An investment in carefully-designed prospective studies of MGUS and MM is warranted to obtain further insights into the etiology and prevention of this lethal malignancy.
Acknowledgments
This work was supported by grants from the National Cancer Institute at the National Institutes of Health: K07 CA115687 (B.M.Birmann), R01 CA127435, P01 CA87969, and UM1 CA167552. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
We thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. We thank Gideon Aweh and Catherine Suppan for assistance with statistical programming, Drs. Andrew Chan and Diane Feskanich for guidance in the characterization of regular aspirin use, and Ms. Tricia Fu for assistance in preparing the manuscript. The authors also gratefully acknowledge the members of the Nurses’ Health Study and Health Professionals Follow-up Study cohorts, without whose faithful dedication the present research would not be possible.
Footnotes
The authors declare no competing financial or other interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss B, Abadie J, Verma P, Howard R, Kuehl W. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113(22):5418–5422. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]
- 6.Birmann B, Chiu B, Muench K, Suppan C, Cozen W. Epidemiology and etiology of multiple myeloma. In: Podar K, Anderson K, editors. Multiple myeloma -- a new era of treatment strategies. Bentham Science Publishers; 2011. pp. 15–57. [Google Scholar]
- 7.Brenner H, Gondos A, Pulte D. Expected long-term survival of patients diagnosed with multiple myeloma in 2006–2010. Haematologica. 2009;94(2):270–275. doi: 10.3324/haematol.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demchenko Y, Glebov O, Zingone A, Keats J, Bergsagel P, Kuehl W. Classical and/or alternative NF-kappaB pathway activation in multiple myeloma. Blood. 2010;115(17):3541–3552. doi: 10.1182/blood-2009-09-243535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein B. Positioning NK-kappaB in multiple myeloma. Blood. 2010;115(17):3422–3424. doi: 10.1182/blood-2010-01-264796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard M, Bancos S, Sime P, Phipps R. Targeting cyclooxygenase-2 in hematological malignancies: rationale and promise. Curr Pharm Des. 2008;14(21):2051–2060. doi: 10.2174/138161208785294654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladetto M, Vallet S, Trojan A, Dell’Aquila M, Monitillo L, Rosato R, et al. Cyclooxygenase-2 (COX-2) is frequently expressed in multiple myeloma and is an independent predictor of poor outcome. Blood. 2005;105(12):4784–4791. doi: 10.1182/blood-2004-11-4201. [DOI] [PubMed] [Google Scholar]
- 12.Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 13.Bergsagel P, Kuehl W. Critical roles for immunoglobulin translocations and cyclin D dysregulation in multiple myeloma. Immunol Rev. 2003;194(1):96–104. doi: 10.1034/j.1600-065x.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 14.Naugler W, Karin M. NF-κB and cancer—identifying the targets and mechanisms. Curr Opin Genet Dev. 2008;18(1):19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang ET, Zheng T, Weir EG, Borowitz M, Mann RB, Spiegelman D, et al. Aspirin and the risk of Hodgkin’s lymphoma in a population-based case-control study. J Natl Cancer Inst. 2004;96:305–315. doi: 10.1093/jnci/djh038. [DOI] [PubMed] [Google Scholar]
- 16.Chang ET, Cronin-Fenton DP, Friis S, Hjalgrim H, Sørensen HT, Pedersen L. Aspirin and other nonsteroidal anti-inflammatory drugs in relation to Hodgkin lymphoma risk in northern Denmark. Cancer Epidemiol Biomark Prev. 2010;19(1):59–64. doi: 10.1158/1055-9965.EPI-09-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flick ED, Chan KA, Bracci PM, Holly EA. Use of nonsteroidal antiinflammatory drugs and non-Hodgkin lymphoma: a population-based case-control study. Am J Epidemiol. 2006;164(5):497–504. doi: 10.1093/aje/kwj223. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Holford TR, Leaderer B, Zahm SH, Boyle P, Morton LM, et al. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control. 2004;15(4):419–428. doi: 10.1023/B:CACO.0000027506.55846.5d. [DOI] [PubMed] [Google Scholar]
- 19.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 21.Moysich KB, Bonner MR, Beehler GP, Marshall JR, Menezes RJ, Baker JA, et al. Regular analgesic use and risk of multiple myeloma. Leuk Res. 2007;31(4):547–551. doi: 10.1016/j.leukres.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Landgren O, Zhang Y, Zahm SH, Inskip P, Zheng T, Baris D. Risk of multiple myeloma following medication use and medical conditions: a case-control study in Connecticut women. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2342–2347. doi: 10.1158/1055-9965.EPI-06-0097. [DOI] [PubMed] [Google Scholar]
- 23.Walter RB, Milano F, Brasky TM, White E. Long-term use of acetaminophen, aspirin, and other nonsteroidal anti-inflammatory drugs and risk of hematologic malignancies: results from the prospective Vitamins and Lifestyle (VITAL) study. J Clin Oncol. 2011;29(17):2424–2431. doi: 10.1200/JCO.2011.34.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teras LR, Gapstur SM, Patel AV, Thun MJ, Diver WR, Zhai Y, et al. Aspirin and other nonsteroidal anti-inflammatory drugs and risk of non-hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2013;22(3):422–428. doi: 10.1158/1055-9965.EPI-12-1158. [DOI] [PubMed] [Google Scholar]
- 25.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5(5):388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, et al. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333(10):609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 27.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med. 1994;121(4):241–246. doi: 10.7326/0003-4819-121-4-199408150-00001. [DOI] [PubMed] [Google Scholar]
- 28.Biennial questionnaires completed by men in the Health Professionals Follow-up Study can be found on-line at http://www.hsph.harvard.edu/hpfs/hpfs_qx.htm
- 29.Biennial questionnaires completed by women in the Nurses’ Health Study can be found on-line at http://www.channing.harvard.edu/nhs/questionnaires/index.shtml
- 30.Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Speizer FE, et al. A prospective study of aspirin use and primary prevention of cardiovascular disease in women. JAMA. 1991;266(4):521–527. [PubMed] [Google Scholar]
- 31.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 33.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 34.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- 35.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134(1):21–28. doi: 10.1053/j.gastro.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feskanich D, Bain C, Chan AT, Pandeya N, Speizer FE, Colditz GA. Aspirin and lung cancer risk in a cohort study of women: dosage, duration and latency. Br J Cancer. 2007;97(9):1295–1299. doi: 10.1038/sj.bjc.6603996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol II, The Design and Analysis of Cohort Studies (IARC Scientific Publication No. 82) Lyon, France: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 38.Sahai H, Khursid A. Confidence intervals for the mean of a Poisson distribution: a review. Biometrical J. 1993;35:857–67. [Google Scholar]
- 39.Sahai H, Khurshid A. Statistics in Epidemiology: Methods, Techniques, and Applications. Boca Raton, FL: CRC Press, Inc; 1996. [Google Scholar]
- 40.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 41.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373(9671):1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 42.Robak P, Smolewski P, Robak T. The role of non-steroidal anti-inflammatory drugs in the risk of development and treatment of hematologic malignancies. Leuk Lymphoma. 2008;49(8):1452–1462. doi: 10.1080/10428190802108854. [DOI] [PubMed] [Google Scholar]
- 43.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, et al. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21(37):5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 44.Birmann BM, Neuhouser ML, Rosner B, Albanes D, Buring JE, Giles G, et al. Prediagnosis biomarkers of insulin-like growth factor-1, insulin, and interleukin-6 dysregulation and multiple myeloma risk in the Multiple Myeloma Cohort Consortium. Blood. 2012;120(25):4929–4937. doi: 10.1182/blood-2012-03-417253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sørensen HT, Friis S, Nørgård B, Mellemkjaer L, Blot WJ, McLaughlin JK, et al. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer. 2003;88(11):1687–1692. doi: 10.1038/sj.bjc.6600945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and non-steroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294(8):914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]