Abstract
Context:
Muscle injuries are extremely common in athletes and often produce pain, dysfunction, and the inability to return to practice or competition. Appropriate diagnosis and management can optimize recovery and minimize time to return to play.
Evidence Acquisition:
Contemporary papers, both basic science and clinical medicine, that investigate muscle healing were reviewed. A Medline/PubMed search inclusive of years 1948 to 2012 was performed.
Results:
Diagnosis can usually be made according to history and physical examination for most injuries. Although data are limited, initial conservative management emphasizing the RICE principles and immobilization of the extremity for several days for higher grade injuries are typically all that is required. Injection of corticosteroids may clinically enhance function after an acute muscle strain. Additional adjunctive treatments (nonsteroidal anti-inflammatory drugs, platelet-rich plasma, and others) to enhance muscle healing and limit scar formation show promise but need additional data to better define their roles.
Conclusion:
Conservative treatment recommendations will typically lead to successful outcomes after a muscle injury. There is limited evidence to support most adjunctive treatments.
Keywords: muscle, contusion, strain, treatment, injury
Muscle lesions are the most common category of injuries in athletes and comprise approximately 10% to 55% of all injuries.5,18,28,35 The majority of muscle injuries (>90%) are contusions or strains, while lacerations are much less common.35 The most severe types can produce chronic pain, dysfunction, recurrence, and even compartment syndrome. A thorough understanding of these types of injuries is needed, since appropriate injury management may determine the difference between an early return to sport and a delayed return.
Basic Anatomy and Physiology of Skeletal Muscle
Skeletal muscle is a composite of multiple muscle fibers (myofibers) arranged in bundles within a connective tissue network (Figure 1). At the basic level, each myofiber contains a contractile element called the myofibril. Actin and myosin protein filaments are arranged in repeating units within the myofibril to form the sarcomere, which extends from Z-line to Z-line within the myofibril.19 The sarcomere is the basic unit of the myofibril and gives skeletal muscle its distinctive striated appearance.
Figure 1.
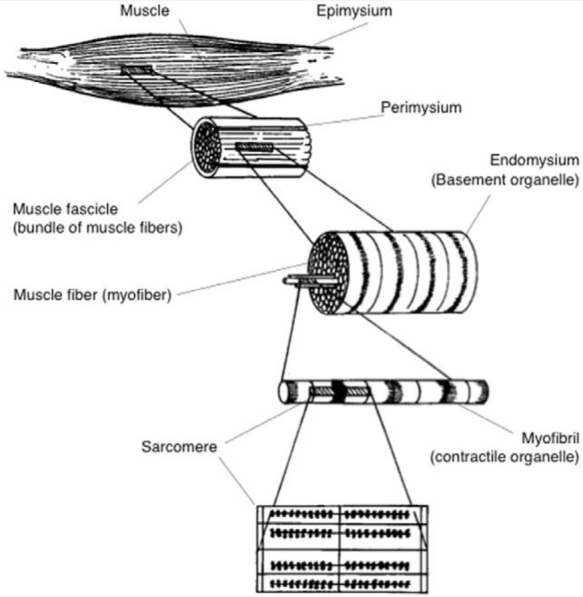
Skeletal muscle anatomy from the gross to the microscopic level. Reprinted with permission from Clanton and Coupe.13
Each myofiber is surrounded by a sarcolemma (plasma membrane) and is further enclosed by a basement membrane that forms the endomysium. The endomysium is contiguous with the perimysium, which surrounds the muscle bundles. Ensheathing the muscle in its entirety is the tough epimysium, which is made up of multiple fascicles.13
Myofibers are attached at both ends of the muscle to tendons and tendon-like fascia, forming what are known as myotendinous junctions (MTJs).62 These MTJs are necessarily durable, with the ability to resist forces of up to 1000 kg,5,19,62 which can be experienced during activity and locomotion.
Muscle Fiber Type
Skeletal muscle is often characterized as either fast-twitch (type II) or slow-twitch (type I). This distinction is a function of the length of time for the motor unit to reach peak tension and has important clinical significance.18,20 Type I fibers rely predominantly on aerobic metabolism (long-distance running), whereas type II fibers are dependent on anaerobic metabolism (sprinting). Type II muscle fibers can generate greater muscular contraction but fatigue more rapidly than type I fibers.20 Type II muscle fibers are also more prone to injury, since they play a larger role during high-speed and power activities, such as sprinting, football, basketball, soccer, and weight lifting.48
Pathophysiology of Muscle Injury
Muscle injury tends to occur through 2 main mechanisms: (1) the muscle is subjected to a sudden large direct, compressive force, resulting in a contusion or (2) the muscle is subjected to an excessive tensile force, resulting in injury to the myofibers and possible rupture, commonly near the MTJ.35 For a strain injury to occur, the muscle must be stretched beyond its resting length.21
Muscle contusions can occur in any muscle group subjected to a direct blow. Strains, however, tend to occur in muscles that cross 2 joints, such as the rectus femoris, the hamstrings, and the gastrocnemius muscles.18 Muscles that cross 2 joints can generate higher levels of tension by passive joint positioning, as compared with muscles that only cross a single joint.48 Once muscle injury has occurred, healing progresses through 3 distinct phases, regardless of the etiology (contusion, strain, or laceration).32,35 These phases are: (1) destruction, (2) repair, and (3) remodeling (Figure 2).
Figure 2.
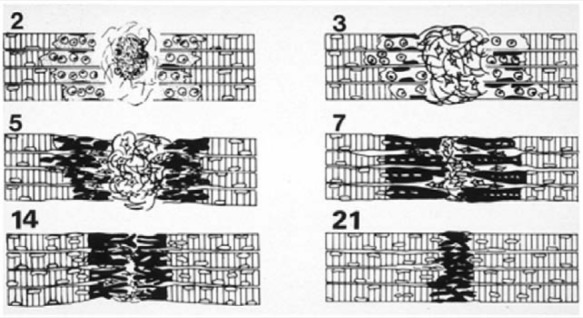
Schematic of skeletal muscle healing. Day 2: Necrotic muscle tissue is removed by macrophages while fibroblasts form scar tissue in the central zone (CZ). Day 3: Satellite cells are activated within the basal lamina cylinders in the regeneration zone (RZ). Day 5: Myoblasts fuse into myotubes in the RZ and scar tissue in the CZ is now denser. Day 7: Regenerating muscle cells migrate into the CZ and begin to pierce through the scar. Day 14: The scar of the CZ is reduced in size, and the regenerating myofibers close the CZ gap. Day 21: The interlacing myofibers are virtually fused with little intervening connective tissue (scar) in between. Reprinted with permission from Järvinen et al.35
Destruction (Inflammatory) Phase (First Week After Injury)
This is characterized by rupture of the myofibers, hematoma formation, and inflammation.35 During muscle injury, the sarcoplasm is disrupted, leading to local necrosis; this process is limited from spreading along the length of the myofiber by the contraction band. This band restricts access to the plasma membrane defect, which, in conjunction with lysosomal vesicles, allows for plasma membrane repair (ie, resealing of the sarcolemma).13,26 Muscle injury also results in local blood vessel injury and subsequent hematoma formation between the myofiber stumps. This sequence initiates an inflammatory cascade in which macrophages play an early, primary role.36,62
Repair (Weeks 2-6 After Injury) and Remodeling Phase (Week 7-Several Months After Injury)
These stages aid in myofiber regeneration as well as connective tissue scar formation. The degree of motor recovery will be determined by the balance struck between muscle healing and fibrosis.35
Muscle Regeneration
Satellite cells are undifferentiated cells located between the sarcolemma and the basal lamina of each myofiber that play an integral role in the regeneration of muscle.35 During the adult stages of development, these cells lay quiescent until the time of injury when they re-enter the cell cycle.34 Satellite cells can proliferate and mature into myoblasts, which can form multinucleated myotubes and ultimately myofibers. The ends of the ruptured myofibers are typically prevented from reuniting completely by the scar tissue that forms during healing. In this scenario, the ends of the repaired fibers attach to the extracellular matrix of the scar by adhesion molecules at the MTJs.29,35,36
Scar Formation
The process of scar formation begins almost immediately following injury. Inflammatory cells degrade the blood clot while fibrin/fibronectin cross-links form an initial extracellular matrix (ECM) that functions as an initial scaffold to support a reparative response.29,35 Immature scar tissue is predominantly composed of type III collagen, which is susceptible to reinjury.37,56 With time, the addition of type I collagen significantly increases the tensile strength of the connective tissue scar.37,41,42 Neoangiogenesis and regeneration of intramuscular neural units are also critical steps that occur during the repair phase.30,31,53,63 In cases of excessive fibroblast proliferation, exuberant scar tissue can form; this may be seen in rerupture or major trauma.18,35
Classification of Muscle Injuries
A consensus classification system for muscle injury currently does not exist. A simple system classifies muscle injuries (strains or contusions) as mild, moderate, or severe based on clinical criteria (Figures 3-5).12,48 Mild muscle injuries (first degree/grade I) present with minor swelling and discomfort with little or no loss of strength or range of motion, which represents minimal tearing of muscle fibers. Moderate muscle injuries (second degree/grade II) are associated with loss of motor function (ie, inability to fully contract the muscle group and limited range of motion). Severe (third degree/grade III) muscle injuries have complete loss of motor function, indicating complete rupture of the muscle.12,32,35
Figure 3.
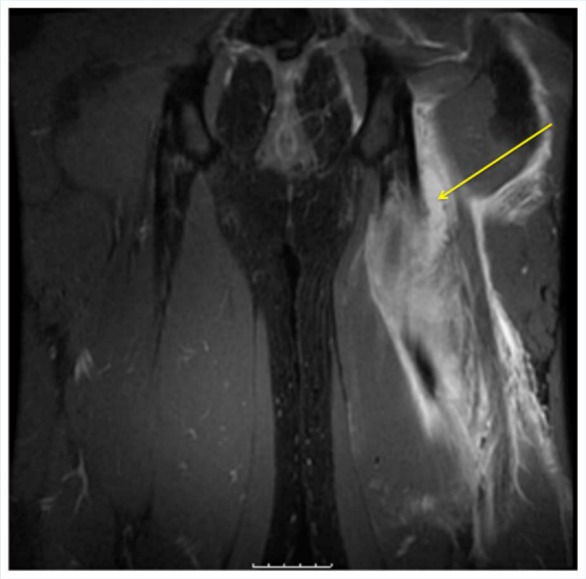
Coronal MRI T2 fat-suppressed image demonstrating high-grade acute muscle strain of the left proximal hamstring.
Figure 5.
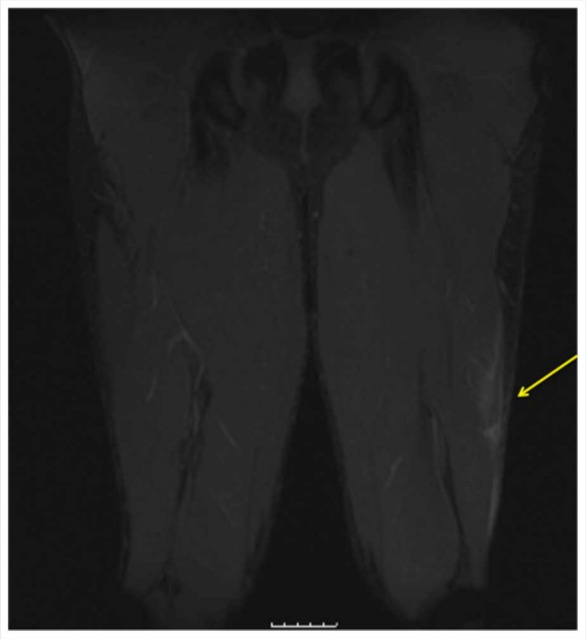
Coronal MRI T2 fat-suppressed image demonstrating low-grade muscle strain of the left biceps femoris.
Figure 4.
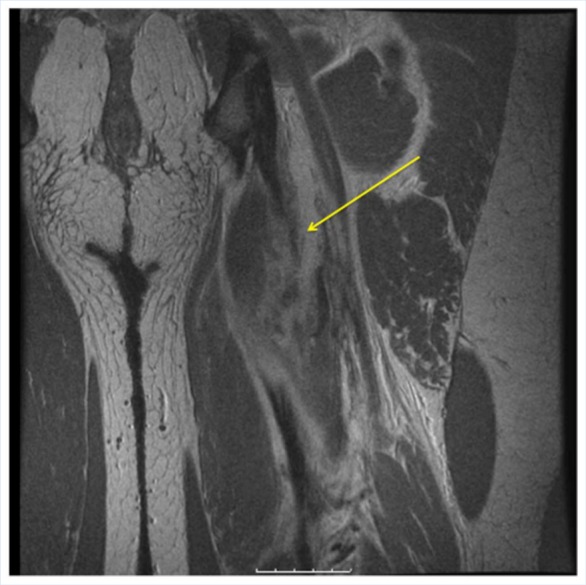
Coronal MRI T1-weighted image of the same injury.
Treatment
Rest, Ice, Compression, Elevation
Rest, ice, compression, and elevation (RICE) is the initial line of therapy despite no randomized, controlled trial demonstrating its effectiveness to treat soft tissue injuries.
Immobilization Versus Mobilization
Early mobilization versus immobilization of an injured muscle has been controversial. In a recently published animal model,39 contusions of the gastrocnemii of rats were produced using a drop-ball technique. Half of the animals were immobilized in a plaster cast, while the other half were immediately mobilized on a running wheel. Nerve fiber density and myotubule formation were significantly higher and lymphocyte density was decreased in the early mobilization group, thereby suggesting that mobilization promoted an improved healing response. Other data, however, have suggested that immobilization may prevent further retraction of the ruptured muscle stumps, thereby reducing gaps between the tissue and the size of the scar formed. Immobilization may allow for the formation of a collagen scaffold through which satellite cells can migrate.33,35
Prolonged immobilization can lead to excessive scarring,56 resulting in decreased load to failure.48 Järvinen and Lehto34 recommend initial immobilization followed by early mobilization as a means of orienting regenerating muscle fibers through connective tissue that forms concomitantly, while limiting adhesions.
The position of immobilization has also been debated. A recent study of immobilization of the knee in 120° of flexion following a quadriceps contusion utilized a select population (midshipmen in the United States Naval Academy) and did not have a control group. The degree of injury was not graded. The mean time to return to unrestricted athletic activity was 3.5 days, which was an improvement over historical results.2
Ice
Limited evidence in humans suggests cryotherapy may improve pain and function in muscle strains.14 In animal models, cryotherapy decreases inflammation, tissue necrosis, and hematoma size after muscle injury.16,31,58 Prolonged cryotherapy attenuates microcirculatory impairment after injury in rats.58
Compression
The application of a maximum compression bandage within 5 minutes of a muscle trauma did not significantly reduce the size of the hematoma or shorten the time to complete subjective recovery.61
Elevation
Elevation of soft tissue injury has not been rigorously tested; the rationale is that it will decrease hydrostatic pressure and local edema.36
Pharmacotherapy
Nonsteroidal Anti-Inflammatory Drugs
The inflammatory stage of repair is characterized by a cytokine cascade that mobilizes cells and bioactive factors to the site of injury. Although the inflammation phase is critical to healing, it is associated with pain and swelling that exacerbates injury and limits function.
The mechanism of action of nonsteroidal anti-inflammatory drugs (NSAIDs) is cyclo-oxygenase inhibition, which limits prostaglandin production from arachidonic acid. NSAID use prevents an exaggerated inflammatory response after injury and provides for analgesic benefit as well. NSAID use can limit prostaglandin levels and edema after injury.65 Recent translational data, however, demonstrate that initial benefits from NSAID use may ultimately result in diminished outcomes.47,50,55 Piroxicam did not adversely affect strength recovery at 1 week in a rabbit muscle strain injury model but did lead to delays in repair, delayed degradation of damaged tissue, and attenuated muscle regeneration.50 In a rabbit strain injury model, flurbiprofen positively affected contractile properties and histology in 7 days but led to functional losses in 28 days.47 A double-blind, randomized trial of acute hamstring strains treated with meclofenamate and diclofenac demonstrated no benefit in pain (visual analog scale), swelling (thigh circumference), or isokinetic performance.
The utility of NSAIDs may be more pronounced for eccentrically induced muscle soreness. Naproxen improved recovery after eccentric exercise by blunting the inflammatory response and may improve early management of contusion injuries as well.17,52
Regular use of intramuscular NSAIDs is not recommended because of potentially elevated drug levels and the risk of infection and muscle necrosis (Nicolau syndrome).24,51 Three days of oral NSAIDs for higher grade muscle injury has demonstrated benefits after injury in animal studies.47,50
Steroids (Glucocorticoids)
Glucocorticoid use for muscle injuries is controversial. Animal models showed delays in removal of hematoma and necrotic tissue with retardation of muscle regeneration.7 Corticosteroid treatment of muscle contusions in a rat model demonstrated early improvement in motor strength but irreversible damage to the healing muscle group in the long term, including disordered fiber structure and strength diminution.7 A single injection of dexamethasone after muscle strain injury in rats led to reductions in interleukin-1ß and transforming growth factor-ß1 (TGF-ß1) with improved contractility in the initial stages of recovery; no adverse effects were seen in the long term.23
In a case series of 3 professional baseball pitchers with internal oblique muscle injuries (but without controls), ultrasound-guided injection of steroid and local anesthetic led to significant pain relief within days, the ability to pitch at full speed within 3 weeks, and return to play by 5 weeks.59 Positive results were also reported with intramuscular corticosteroid injection for hamstring injuries in professional football players.43 Eighty-four percent of players with severe hamstring injuries with palpable defects were able to return to play without missing any games and with no strength deficits at final examination.
Topical Anesthetic Use
A single, 8-hour application of methyl salicylate and 1 menthol patch can provide significant pain relief.27
Experimental Pharmacologic Agents
Platelet-Rich Plasma
High-quality clinical studies of platelet-rich plasma (PRP) for muscle injuries do not yet exist. In an abstract presented in 2005, ultrasound-guided injections of PRP in 22 muscle injuries produced no complications and full functional recovery in half of the expected recovery time.57 This study, however, had no control group and provided no details regarding methodology or outcome measures. In a rat model, faster recovery with PRP for tibialis anterior strain injury has been reported.25
Curcumin
Oral curcumin (an element in the Indian spice tumeric; NF-kappa B inhibitor) improved recovery of running performance, blunting of the inflammatory cytokine, and creatine kinase levels after extended activity in an animal model.15 Positive effects of curcumin on myogenesis were also seen after trauma.60
Angiotensin II Receptor Blockers
Angiotensin receptor blockers inhibit TGF-ß1, a key cytokine in skeletal muscle fibrosis.44 Angiotensin receptor blocker (losartan)–treated mice exhibit dose-dependent muscle regeneration and diminished fibrous tissue formation.4
Suramin
Suramin is an antiparasitic and antineoplastic agent that can inhibit TGF-ß1 activity. This agent can improve motor recovery and decrease fibrous scar formation after both contusion and strain injuries in animals.11,49 In vitro trials demonstrate enhanced myoblast and muscle-derived stem cell (MDSC) differentiation while inhibiting the negative effects of myostatin on myogenic differentiation.49
Alternative Modalities
Therapeutic Ultrasound
Ultrasound has been promoted as a potential treatment modality for muscle injury, for both pain relief and muscle regeneration.5,19,64 However, little data exist to support its use. In a recent study using a rat contusion model, daily therapeutic ultrasound had no effect on muscle healing or regeneration.65 Earlier translational work demonstrated that pulsed ultrasound could promote satellite cell proliferation, although this modality did not have a significant effect on the overall morphological manifestations of muscle regeneration.54 Finally, the combination of ultrasound and exercise showed no benefit to skeletal muscle regeneration after contusion injury.46 Thus, despite its widespread use, the benefits of therapeutic ultrasound have yet to be confirmed in practice.
Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy has received a great deal of attention in recent years. Early reports cited accelerated muscle recovery after stretch injury in rabbits and enhanced strength recovery from delayed-onset muscle soreness (DOMS) in humans.9 However, a recent Cochrane Database meta-analysis concluded that there was insufficient evidence to support its use for muscle injuries and that it may increase pain in DOMS.8
Complications of Muscle Injuries
Myositis Ossificans (Posttraumatic Calcific Metaplasia)
Myositis ossificans can occur in any muscle group but is most common in the quadriceps and the brachialis.3,22 The incidence is highest in contact sports where protection is limited. Individuals with bleeding disorders may also be at increased risk.6 The cause of myositis ossificans is not known; myoblasts may be the cause response to bone morphogenetic protein (BMP) signaling.38 Additionally, endothelial precursor cells contribute to all stages of heterotopic ossification in animals.45 Clinical factors including early vigorous massage and excessive mobilization may also contribute.40 However, full motion and return to normal activity may occur despite heterotic bone exostosis.2
Optimal treatment and prevention are unknown. No data exist to support indomethacin for myositis ossificans prevention. Surgical excision may be necessary for symptomatic myositis ossificans.5 Surgical timing is critical—a year-long wait from the time of injury may be necessary to ensure full maturation of the lesion and thereby minimize recurrence.
Conclusion
Most muscle injuries will respond to conservative management.3,5,13,48 Although commonly recommended, there is little evidence to support the RICE principles.10 Early mobilization for lower grade injuries and brief (1-3 days) immobilization of the extremity for higher grade injuries appear to be beneficial.33-35,39,48,56 A single corticosteroid injection may play a role in the recovery from acute muscle injuires.7,43,59 Other adjunctive therapies hold promise for improving muscle healing and limiting scar formation, but further research is needed to define their roles.9,11,44,49,50,57,65
Footnotes
The authors report no potential conflicts of interest in the development and publication of this manuscript.
References
- 1. Almekinders LC. Muscles injuries and antiinflammatory treatment. Med Sci Sports Exerc. 1991;23(suppl):110S [Google Scholar]
- 2. Aronen JG, Garrick JG, Chronister RD, McDevitt ER. Quadriceps contusions: clinical results of immediate immobilization in 120 degrees of knee flexion. Clin J Sport Med. 2006;16(5):383-387 [DOI] [PubMed] [Google Scholar]
- 3. Arrington ED, Miller MD. Skeletal muscle injuries. Orthop Clin North Am. 1995;26(3):411-422 [PubMed] [Google Scholar]
- 4. Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med. 2008;36(8):1548-1554 [DOI] [PubMed] [Google Scholar]
- 5. Beiner JM, Jokl P. Muscle contusion injuries: current treatment options. J Am Acad Ortho Surg. 2001;9(4):227-237 [DOI] [PubMed] [Google Scholar]
- 6. Beiner JM, Jokl P. Muscle contusion injury and myositis ossificans traumatica. Clin Orthop Relat Res. 2002;(403 suppl):S110-S119 [DOI] [PubMed] [Google Scholar]
- 7. Beiner JM, Jokl P, Cholewicki J, Panjabi MM. The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury. Am J Sports Med. 1999;27(1):2-9 [DOI] [PubMed] [Google Scholar]
- 8. Bennett M, Best TM, Babul S, Taunton J, Lepawsky M. Hyperbaric oxygen therapy for delayed onset muscle soreness and closed soft tissue injury. Cochrane Database Syst Rev. 2005;(4):CD004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Best TM, Loitz-Ramage B, Corr DT, Vanderby R. Hyperbaric oxygen in the treatment of acute muscle stretch injuries. Results in an animal model. Am J Sports Med. 1998;26(3):367-372 [DOI] [PubMed] [Google Scholar]
- 10. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261 [DOI] [PubMed] [Google Scholar]
- 11. Chan Y-S, Li Y, Foster W, Fu FH, Huard J. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med. 2005;33(1):43-51 [DOI] [PubMed] [Google Scholar]
- 12. Ciullo JV, Zarins B. Biomechanics of the musculotendinous unit: relation to athletic performance and injury. Clin Sports Med. 1983;2(1):71-86 [PubMed] [Google Scholar]
- 13. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248 [DOI] [PubMed] [Google Scholar]
- 14. Collins NC. Is ice right? Does cryotherapy improve outcome for acute soft tissue injury? Emerg Med J. 2008;25(2):65-68 [DOI] [PubMed] [Google Scholar]
- 15. Davis JM, Murphy EA, Carmichael MD, et al. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2007;292(6):R2168-R2173 [DOI] [PubMed] [Google Scholar]
- 16. Deal DN, Tipton J, Rosencrance E, Curl WW, Smith TL. Ice reduces edema. A study of microvascular permeability in rats. J Bone Joint Surg Am. 2002;84-A(9):1573-1578 [PubMed] [Google Scholar]
- 17. Dudley GA, Czerkawski J, Meinrod A, Gillis G, Baldwin A, Scarpone M. Efficacy of naproxen sodium for exercise-induced dysfunction muscle injury and soreness. Clin J Sport Med. 1997;7(1):3-10 [DOI] [PubMed] [Google Scholar]
- 18. Garrett WE. Muscle strain injuries. Am J Sports Med. 1996;24(6 suppl):S2-S8 [PubMed] [Google Scholar]
- 19. Garrett WE, Jr, Best TM. Anatomy, Physiology and Mechanics of Skeletal Muscle. In: Orthopaedic Basic Science (pp. 89-127). Edited by Simon S.R. Rosemont: American Academy of Orthopaedic Surgeons, 1994 [Google Scholar]
- 20. Garrett WE, Califf JC, Bassett FH. Histochemical correlates of hamstring injuries. Am J Sports Med. 1984;12(2):98-103 [DOI] [PubMed] [Google Scholar]
- 21. Garrett WE, Nikolaou PK, Ribbeck BM, Glisson RR, Seaber AV. The effect of muscle architecture on the biomechanical failure properties of skeletal muscle under passive extension. Am J Sports Med. 1988;16(1):7-12 [DOI] [PubMed] [Google Scholar]
- 22. Hait G, Boswick JA, Stone NH. Heterotopic bone formation secondary to trauma (myositis ossificans traumatica). J Trauma. 1970;10(5):405-411 [DOI] [PubMed] [Google Scholar]
- 23. Hakim M, Hage W, Lovering RM, Moorman CT, Curl LA, De Deyne PG. Dexamethasone and recovery of contractile tension after a muscle injury. Clin Orthop Relat Res. 2005;439:235-242 [DOI] [PubMed] [Google Scholar]
- 24. Hamilton B, Fowler P, Galloway H, Popovic N. Nicolau syndrome in an athlete following intra-muscular diclofenac injection. Acta Orthop Belg. 2008;74(6):860-864 [PubMed] [Google Scholar]
- 25. Hammond JW, Hinton RY, Curl LA, Muriel JM, Lovering RM. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med. 2009;37(6):1135-1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hartig DE, Henderson JM. Increasing hamstring flexibility decreases lower extremity overuse injuries in military basic trainees. Am J Sports Med. 1999;27(2):173-176 [DOI] [PubMed] [Google Scholar]
- 27. Higashi Y, Kiuchi T, Furuta K. Efficacy and safety profile of a topical methyl salicylate and menthol patch in adult patients with mild to moderate muscle strain: a randomized, double-blind, parallel-group, placebo-controlled, multicenter study. Clin Ther. 2010;32(1):34-43 [DOI] [PubMed] [Google Scholar]
- 28. Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A(5):822-832 [PubMed] [Google Scholar]
- 29. Hurme T, Kalimo H, Lehto M, Järvinen M. Healing of skeletal muscle injury: an ultrastructural and immunohistochemical study. Med Sci Sports Exerc. 1991;23(7):801-810 [PubMed] [Google Scholar]
- 30. Hurme T, Lehto M, Falck B, Tainio H, Kalimo H. Electromyography and morphology during regeneration of muscle injury in rats. Acta Physiol Scand. 1991;142(4):443-456 [DOI] [PubMed] [Google Scholar]
- 31. Hurme T, Rantanen J. Effects of early cryotherapy in experimental skeletal muscle injury. Scand J Med Sci Sports. 1993;3:46-51 [Google Scholar]
- 32. Jankowski RJ, Deasy BM, Cao B, Gates C, Huard J. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J Cell Sci. 2002;115(Pt 22):4361-4374 [DOI] [PubMed] [Google Scholar]
- 33. Järvinen M. Healing of a crush injury in rat striated muscle. 3. A micro-angiographical study of the effect of early mobilization and immobilization on capillary ingrowth. Acta Pathol Microbiol Scand A. 1976;84(1):85-94 [PubMed] [Google Scholar]
- 34. Järvinen MJ, Lehto MU. The effects of early mobilisation and immobilisation on the healing process following muscle injuries. Sports Med. 1993;15(2):78-89 [DOI] [PubMed] [Google Scholar]
- 35. Järvinen TAH, Järvinen TLN, Kääriäinen M, et al. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764 [DOI] [PubMed] [Google Scholar]
- 36. Järvinen TAH, Järvinen TLN, Kääriäinen M, et al. Muscle injuries: optimising recovery. Best Pract Res Clin Rheumatol. 2007;21(2):317-331 [DOI] [PubMed] [Google Scholar]
- 37. Kääriäinen M, Kääriäinen J, Järvinen TL, Sievänen H, Kalimo H, Järvinen MJ. Correlation between biomechanical and structural changes during the regeneration of skeletal muscle after laceration injury. J Orthop Res. 1998;16(2):197-206 [DOI] [PubMed] [Google Scholar]
- 38. Katagiri T, Yamaguchi A, Komaki M, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127(6 Pt 1):1755-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khattak MJ, Ahmad T, Rehman R, Umer M, Hasan SH, Ahmed M. Muscle healing and nerve regeneration in a muscle contusion model in the rat. J Bone Joint Surg Br. 2010;92(6):894-899 [DOI] [PubMed] [Google Scholar]
- 40. Kujala UM, Orava S, Järvinen MJ. Hamstring injuries. Current trends in treatment and prevention. Sports Med. 1997;23(6):397-404 [DOI] [PubMed] [Google Scholar]
- 41. Lehto M, Duance VC, Restall D. Collagen and fibronectin in a healing skeletal muscle injury. An immunohistological study of the effects of physical activity on the repair of injured gastrocnemius muscle in the rat. J Bone Joint Surg Br. 1985;67(5):820-828 [DOI] [PubMed] [Google Scholar]
- 42. Lehto M, Sims TJ, Bailey AJ. Skeletal muscle injury—molecular changes in the collagen during healing. Res Exp Med (Berl). 1985;185(2):95-106 [DOI] [PubMed] [Google Scholar]
- 43. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300 [DOI] [PubMed] [Google Scholar]
- 44. Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161(3):895-907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lounev VY, Ramachandran R, Wosczyna MN, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91(3):652-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Markert CD, Merrick MA, Kirby TE, Devor ST. Nonthermal ultrasound and exercise in skeletal muscle regeneration. Arch Phys Med Rehabil. 2005;86(7):1304-1310 [DOI] [PubMed] [Google Scholar]
- 47. Mishra DK, Fridén J, Schmitz MC, Lieber RL. Anti-inflammatory medication after muscle injury. A treatment resulting in short-term improvement but subsequent loss of muscle function. J Bone Joint Surg Am. 1995;77(10):1510-1519 [DOI] [PubMed] [Google Scholar]
- 48. Noonan TJ, Garrett WJ. Muscle strain injury: diagnosis and treatment. J Am Acad Ortho Surg. 1999;7(4):262-269 [DOI] [PubMed] [Google Scholar]
- 49. Nozaki M, Li Y, Zhu J, et al. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. Am J Sports Med. 2008;36(12):2354-2362 [DOI] [PubMed] [Google Scholar]
- 50. Obremsky WT, Seaber AV, Ribbeck BM, Garrett WE. Biomechanical and histologic assessment of a controlled muscle strain injury treated with piroxicam. Am J Sports Med. 1994;22(4):558-561 [DOI] [PubMed] [Google Scholar]
- 51. Paoloni JA, Milne C, Orchard JW, Hamilton B. Non-steroidal anti-inflammatory drugs in sports medicine: guidelines for practical but sensible use. Br J Sports Med. 2009;43(11):863-865 [DOI] [PubMed] [Google Scholar]
- 52. Predel HG. Diclofenac patch for topical treatment of acute impact injuries: a randomised, double blind, placebo controlled, multicentre study. Br J Sports Med. 2004;38(3):318-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rantanen J, Ranne J, Hurme T, Kalimo H. Denervated segments of injured skeletal muscle fibers are reinnervated by newly formed neuromuscular junctions. J Neuropathol Exp Neurol. 1995;54(2):188-194 [DOI] [PubMed] [Google Scholar]
- 54. Rantanen J, Thorsson O, Wollmer P, Hurme T, Kalimo H. Effects of therapeutic ultrasound on the regeneration of skeletal myofibers after experimental muscle injury. Am J Sports Med. 1999;27(1):54-59 [DOI] [PubMed] [Google Scholar]
- 55. Reynolds JF, Noakes TD, Schwellnus MP, Windt A, Bowerbank P. Non-steroidal anti-inflammatory drugs fail to enhance healing of acute hamstring injuries treated with physiotherapy. S Afr Med J. 1995;85:517-522 [PubMed] [Google Scholar]
- 56. Saartok T. Muscle injuries associated with soccer. Clin Sports Med. 1998;17(4):811-817, viii. [DOI] [PubMed] [Google Scholar]
- 57. Sanchez M, Anitua E. Application of autologous growth factors on skeletal muscle healing. Paper presented at: 2nd World Congress on Regenerative Medicine; May 2005; Leipzig, Germany [Google Scholar]
- 58. Schaser K-D, Disch AC, Stover JF, Lauffer A, Bail HJ, Mittlmeier T. Prolonged superficial local cryotherapy attenuates microcirculatory impairment, regional inflammation, and muscle necrosis after closed soft tissue injury in rats. Am J Sports Med. 2007;35(1):93-102 [DOI] [PubMed] [Google Scholar]
- 59. Stevens KJ, Crain JM, Akizuki KH, Beaulieu CF. Imaging and ultrasound-guided steroid injection of internal oblique muscle strains in baseball pitchers. Am J Sports Med. 2010;38(3):581-585 [DOI] [PubMed] [Google Scholar]
- 60. Thaloor D, Miller KJ, Gephart J, Mitchell PO, Pavlath GK. Systemic administration of the NF-kappaB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am J Physiol. 1999;277(2 Pt 1):C320-C329 [DOI] [PubMed] [Google Scholar]
- 61. Thorsson O, Lilja B, Nilsson P, Westlin N. Immediate external compression in the management of an acute muscle injury. Scand J Med Sci Sports. 1997;7(3):182-190 [DOI] [PubMed] [Google Scholar]
- 62. Tidball JG. Force transmission across muscle cell membranes. J Biomech. 1991;24(suppl 1):43-52 [DOI] [PubMed] [Google Scholar]
- 63. Vaittinen S, Lukka R, Sahlgren C, et al. Specific and innervation-regulated expression of the intermediate filament protein nestin at neuromuscular and myotendinous junctions in skeletal muscle. Am J Pathol. 1999;154(2):591-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van der Windt DA, van der Heijden GJ, van den Berg SG, Riet ter G, de Winter AF, Bouter LM. Ultrasound therapy for musculoskeletal disorders: a systematic review. Pain. 1999;81(3):257-271 [DOI] [PubMed] [Google Scholar]
- 65. Wilkin LD, Merrick MA, Kirby TE, Devor ST. Influence of therapeutic ultrasound on skeletal muscle regeneration following blunt contusion. Int J Sports Med. 2004;25(1):73-77 [DOI] [PubMed] [Google Scholar]



